Liver Stiffness-Based Risk Prediction Model for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
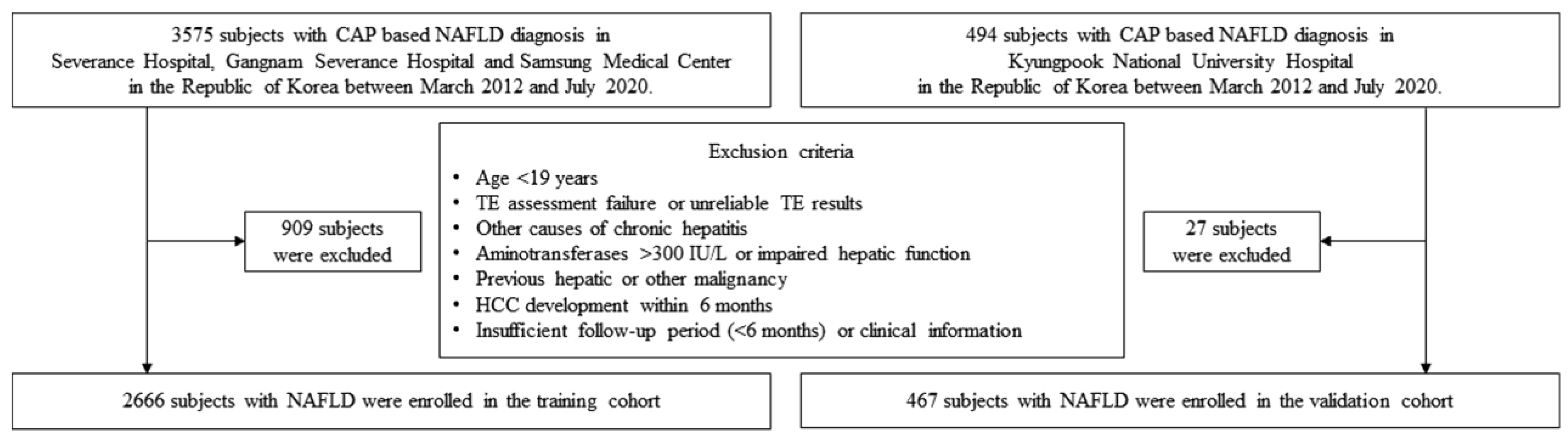
2.1. Patient Eligibility
2.2. Definition
2.3. TE Assessment
2.4. HCC
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Comparison between Subjects Who Developed HCC and Those Who Did Not
3.3. Risk Factors of HCC Development in the Training Cohort
3.4. Establishment of a New Risk Prediction Model for HCC
3.5. Internal and External Validation of a New Risk Prediction Model for HCC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.H.; Lee, H.W.; Yoo, J.J.; Cho, Y.; Kim, S.U.; Lee, T.H.; Jang, B.K.; Kim, S.G.; Ahn, S.B.; Kim, H.; et al. Kasl clinical practice guidelines: Management of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2021, 27, 363–401. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Plank, L.D.; Suk, K.T.; Park, Y.E.; Lee, J.; Choi, J.H.; Heo, N.Y.; Park, J.; Kim, T.O.; Moon, Y.S.; et al. Trends in the prevalence of chronic liver disease in the korean adult population, 1998–2017. Clin. Mol. Hepatol. 2020, 26, 209–215. [Google Scholar] [CrossRef]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755.e743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of nafld-related hcc: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Hainaut, P.; Gores, G.J.; Amadou, A.; Plymoth, A.; Roberts, L.R. A global view of hepatocellular carcinoma: Trends, risk, prevention and management. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 589–604. [Google Scholar] [CrossRef]
- Choi, J.; Han, S.; Kim, N.; Lim, Y.S. Increasing burden of liver cancer despite extensive use of antiviral agents in a hepatitis b virus-endemic population. Hepatology 2017, 66, 1454–1463. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.A.; Lee, Y.-R.; Park, J.; Oh, I.-H.; Kim, H.; Yoon, S.-J.; Park, K. Socioeconomic burden of cancer in korea from 2011 to 2015. Cancer Res. Treat. 2020, 52, 896–906. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.J.; Cheung, R.; Ahmed, A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology 2014, 59, 2188–2195. [Google Scholar] [CrossRef]
- Cho, E.J.; Kwack, M.S.; Jang, E.S.; You, S.J.; Lee, J.H.; Kim, Y.J.; Yoon, J.H.; Lee, H.S. Relative etiological role of prior hepatitis b virus infection and nonalcoholic fatty liver disease in the development of non-b non-c hepatocellular carcinoma in a hepatitis b-endemic area. Digestion 2011, 84 (Suppl. S1), 17–22. [Google Scholar] [CrossRef]
- Ascha, M.S.; Hanouneh, I.A.; Lopez, R.; Tamimi, T.A.; Feldstein, A.F.; Zein, N.N. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology 2010, 51, 1972–1978. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Mapakshi, S.; Natarajan, Y.; Chayanupatkul, M.; Richardson, P.A.; Li, L.; Desiderio, R.; Thrift, A.P.; Asch, S.M.; et al. Risk of hepatocellular cancer in patients with non-alcoholic fatty liver disease. Gastroenterology 2018, 155, 1828–1837.e1822. [Google Scholar] [CrossRef] [Green Version]
- Kawamura, Y.; Arase, Y.; Ikeda, K.; Seko, Y.; Imai, N.; Hosaka, T.; Kobayashi, M.; Saitoh, S.; Sezaki, H.; Akuta, N.; et al. Large-scale long-term follow-up study of japanese patients with non-alcoholic fatty liver disease for the onset of hepatocellular carcinoma. Am. J. Gastroenterol. 2012, 107, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.; Jun, D.W.; Saeed, W.K.; Nguyen, M.H. Non-alcoholic fatty liver diseases: Update on the challenge of diagnosis and treatment. Clin. Mol. Hepatol. 2016, 22, 327–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.A.; Lee, H.C.; Choe, J.; Kim, M.J.; Lee, M.J.; Chang, H.S.; Bae, I.Y.; Kim, H.K.; An, J.; Shim, J.H.; et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J. Hepatol. 2018, 68, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Park, S.Y.; Kim, S.U.; Jang, J.Y.; Park, H.; Kim, J.K.; Lee, C.K.; Chon, Y.E.; Han, K.H. Discrimination of nonalcoholic steatohepatitis using transient elastography in patients with nonalcoholic fatty liver disease. PLoS ONE 2016, 11, e0157358. [Google Scholar] [CrossRef]
- Zhang, X.; Wong, G.L.; Wong, V.W. Application of transient elastography in nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2020, 26, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Yoo, E.J.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H. Prediction of development of liver-related events by transient elastography in hepatitis b patients with complete virological response on antiviral therapy. Am. J. Gastroenterol. 2014, 109, 1241–1249. [Google Scholar] [CrossRef]
- Sasso, M.; Beaugrand, M.; de Ledinghen, V.; Douvin, C.; Marcellin, P.; Poupon, R.; Sandrin, L.; Miette, V. Controlled attenuation parameter (cap): A novel vcte™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: Preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med. Biol. 2010, 36, 1825–1835. [Google Scholar] [CrossRef] [PubMed]
- Chon, Y.E.; Jung, K.S.; Kim, S.U.; Park, J.Y.; Park, Y.N.; Kim, D.Y.; Ahn, S.H.; Chon, C.Y.; Lee, H.W.; Park, Y.; et al. Controlled attenuation parameter (cap) for detection of hepatic steatosis in patients with chronic liver diseases: A prospective study of a native korean population. Liver Int. 2014, 34, 102–109. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Dong, N.L.; Qu, Q.; Zhao, X.F.; Yang, H.J. The correlation between controlled attenuation parameter and metabolic syndrome and its components in middle-aged and elderly nonalcoholic fatty liver disease patients. Medicine 2018, 97, e12931. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.W.; Chon, Y.E.; Kim, S.U.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Jung, K.S.; Park, Y.N.; Han, K.H. Predicting liver-related events using transient elastography in chronic hepatitis c patients with sustained virological response. Gut Liver 2016, 10, 429–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.S.; Kim, S.U. Clinical applications of transient elastography. Clin. Mol. Hepatol. 2012, 18, 163–173. [Google Scholar] [CrossRef]
- Lee, Y.H.; Jung, K.S.; Kim, S.U.; Yoon, H.J.; Yun, Y.J.; Lee, B.W.; Kang, E.S.; Han, K.H.; Lee, H.C.; Cha, B.S. Sarcopaenia is associated with nafld independently of obesity and insulin resistance: Nationwide surveys (knhanes 2008–2011). J. Hepatol. 2015, 63, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Heagerty, P.J.; Zheng, Y. Survival model predictive accuracy and roc curves. Biometrics 2005, 61, 92–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlas, T.; Petroff, D.; Sasso, M.; Fan, J.G.; Mi, Y.Q.; de Lédinghen, V.; Kumar, M.; Lupsor-Platon, M.; Han, K.H.; Cardoso, A.C.; et al. Individual patient data meta-analysis of controlled attenuation parameter (cap) technology for assessing steatosis. J. Hepatol. 2017, 66, 1022–1030. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Loomis, A.K.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed nafld: Real-world study of 18 million patients in four european cohorts. BMC Med. 2019, 17, 95. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.; Lowy, E.; Mun, E.J.; Berry, K. Differences in hepatocellular carcinoma risk, predictors and trends over time according to etiology of cirrhosis. PLoS ONE 2018, 13, e0204412. [Google Scholar] [CrossRef] [Green Version]
- Hsiang, J.C.; Bai, W.W.; Raos, Z.; Stableforth, W.; Upton, A.; Selvaratnam, S.; Gane, E.J.; Gerred, S.J. Epidemiology, disease burden and outcomes of cirrhosis in a large secondary care hospital in south auckland, new zealand. Intern. Med. J. 2015, 45, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Lymp, J.F.; St Sauver, J.; Sanderson, S.O.; Lindor, K.D.; Feldstein, A.; Angulo, P. The natural history of nonalcoholic fatty liver disease: A population-based cohort study. Gastroenterology 2005, 129, 113–121. [Google Scholar] [CrossRef]
- Seko, Y.; Sumida, Y.; Tanaka, S.; Mori, K.; Taketani, H.; Ishiba, H.; Hara, T.; Okajima, A.; Umemura, A.; Nishikawa, T.; et al. Development of hepatocellular carcinoma in japanese patients with biopsy-proven non-alcoholic fatty liver disease: Association between pnpla3 genotype and hepatocarcinogenesis/fibrosis progression. Hepatol. Res. 2017, 47, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Ishigami, M.; Ishizu, Y.; Kuzuya, T.; Honda, T.; Hayashi, K.; Nishimura, D.; Toyoda, H.; Kumada, T.; Goto, H.; et al. Utility and limitations of noninvasive fibrosis markers for predicting prognosis in biopsy-proven japanese non-alcoholic fatty liver disease patients. J. Gastroenterol. Hepatol. 2019, 34, 207–214. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.W.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, K.J.; Han, K.H. Prevalence and predictors of significant fibrosis among subjects with transient elastography-defined nonalcoholic fatty liver disease. Dig. Dis. Sci. 2017, 62, 2150–2158. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.S.; Kim, S.U.; Song, K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Kim, B.K.; Han, K.H. Validation of hepatitis b virus-related hepatocellular carcinoma prediction models in the era of antiviral therapy. Hepatology 2015, 62, 1757–1766. [Google Scholar] [CrossRef]
- Loomba, R.; Lim, J.K.; Patton, H.; El-Serag, H.B. Aga clinical practice update on screening and surveillance for hepatocellular carcinoma in patients with nonalcoholic fatty liver disease: Expert review. Gastroenterology 2020, 158, 1822–1830. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Variable | Total (n = 2666) | Non-HCC (n = 2644, 99.2%) | HCC (n = 22, 0.8%) | p Value |
|---|---|---|---|---|
| Age (years) | 52.0 (41.0–60.0) | 52.0 (41.0–60.0) | 70.0 (61.0–72.3) | <0.001 |
| Male sex | 1524 (57.2) | 1509 (57.1) | 15 (68.2) | 0.204 |
| Diabetes mellitus | 1029 (38.6) | 1016 (38.4) | 13 (59.1) | 0.041 |
| Hypertension | 1080 (40.5) | 1065 (40.3) | 15 (68.2) | 0.008 |
| BMI (kg/m2) | 26.18 (24.17–28.73) | 26.2 (24.2–28.7) | 27.3 (24.4–29.6) | 0.573 |
| Cirrhosis | 171 (6.4) | 153 (5.8) | 18 (81.8) | <0.001 |
| Transient elastography | ||||
| LS (kPa) | 5.9 (4.6–7.9) | 5.9 (4.6–7.8) | 21.5 (13.4–33.0) | <0.001 |
| <7.5 | 1903 (71.4) | 1902 (71.9) | 1 (4.5) | |
| 7.5–9.3 | 333 (12.4) | 332 (12.6) | 1 (4.5) | |
| 9.3–11.0 | 127 (4.8) | 125 (4.7) | 2 (9.1) | |
| 11.0–14.0 | 112 (4.2) | 110 (4.2) | 2 (9.1) | |
| ≥14.0 | 191 (7.2) | 175 (6.6) | 16 (72.8) | |
| CAP (dB/m) | 303 (273–331) | 303 (273–331) | 290 (256–325) | 0.122 |
| Laboratory test results | ||||
| Platelet count (×103/μL) | 236.0 (200.0–279.0) | 237.0 (201.0–279.0) | 142.5 (114.8–163.8) | <0.001 |
| AST (IU/L) | 33 (24–49) | 33 (24–49) | 47 (32–62) | 0.004 |
| ALT (IU/L) | 41 (24–68) | 41 (24–69) | 32 (20–51) | 0.200 |
| Total bilirubin (mg/dL) | 0.7 (0.5–0.9) | 0.7 (0.5–0.9) | 1.0 (0.6–1.5) | 0.009 |
| Serum albumin (g/dL) | 4.5 (4.3–4.7) | 4.5 (4.3–4.7) | 4.1 (3.7–4.4) | 0.001 |
| Prothrombin time (INR) | 0.95 (0.91–1.00) | 0.95 (0.91–1.00) | 1.08 (1.02–1.19) | 0.041 |
| Serum creatinine (mg/dL) | 0.78 (0.66–0.92) | 0.78 (0.66–0.92) | 0.76 (0.69–0.92) | 0.837 |
| Gamma-GT (mg/dL) | 45 (28–73) | 44 (28–73) | 75 (53–124) | 0.010 |
| ALP (IU/L) | 64 (53–79) | 64 (52–79) | 84 (66–103) | 0.001 |
| Triglyceride (mg/dL) | 155 (110–208) | 155 (110–208) | 149 (106–168) | 0.499 |
| LDL-cholesterol (mg/dL) | 121 (94–148) | 121 (94–148) | 94 (80–115) | 0.002 |
| HDL-cholesterol (mg/dL) | 45 (39–52) | 45 (39–52) | 42 (32–49) | 0.131 |
| Total cholesterol (mg/dL) | 189 (162–216) | 189 (163–216) | 150 (134.8–191) | <0.001 |
| Variable | Univariate p Value | Multivariate Analysis | |
|---|---|---|---|
| p Value | Hazard Ratio (95% CI) | ||
| Age ≥ 60 (vs. <60) years | <0.001 | 0.004 | 9.143 (2.055, 40.684) |
| Liver stiffness ≥ 9.3 (vs. <9.3) kPa | <0.001 | 0.001 | 13.757 (2.826, 66.955) |
| Platelet count < 150 (vs. ≥150) × 103/μL | <0.001 | 0.014 | 3.670 (1.295, 10.402) |
| AST ≥ 34 (vs. <34) IU/L | 0.068 | 0.395 | 1.583 (0.549, 4.560) |
| Serum albumin < 3.4 (vs. ≥3.4) g/dL | <0.001 | 0.161 | 2.699 (0.674, 10.803) |
| Total bilirubin ≥ 2.0 (vs. <2.0) mg/dL | <0.001 | 0.566 | 1.485 (0.385, 5.737) |
| Total cholesterol ≥ 168 (vs. <168) mg/dL | <0.001 | 0.259 | 0.560 (0.204, 1.534) |
| AUC | Training Cohort | Internal Validation (Bootstrap) | Validation Cohort |
|---|---|---|---|
| iAUC | 0.944 (0.909, 0.979) | 0.954 (0.916, 0.982) | 0.782 (0.610, 0.954) |
| 2 yr AUC | 0.948 (0.917, 0.979) | 0.956 (0.922, 0.982) | 0.777 (0.606, 0.948) |
| 3 yr AUC | 0.947 (0.914, 0.980) | 0.955 (0.920, 0.983) | 0.781 (0.614, 0.948) |
| 5 yr AUC | 0.939 (0.900, 0.978) | 0.950 (0.909, 0.983) | 0.784 (0.619, 0.949) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.S.; Sinn, D.H.; Park, S.Y.; Shin, H.J.; Lee, H.W.; Kim, B.K.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Oh, J.H.; et al. Liver Stiffness-Based Risk Prediction Model for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Cancers 2021, 13, 4567. https://doi.org/10.3390/cancers13184567
Lee JS, Sinn DH, Park SY, Shin HJ, Lee HW, Kim BK, Park JY, Kim DY, Ahn SH, Oh JH, et al. Liver Stiffness-Based Risk Prediction Model for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Cancers. 2021; 13(18):4567. https://doi.org/10.3390/cancers13184567
Chicago/Turabian StyleLee, Jae Seung, Dong Hyun Sinn, Soo Young Park, Hye Jung Shin, Hye Won Lee, Beom Kyung Kim, Jun Yong Park, Do Young Kim, Sang Hoon Ahn, Joo Hyun Oh, and et al. 2021. "Liver Stiffness-Based Risk Prediction Model for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease" Cancers 13, no. 18: 4567. https://doi.org/10.3390/cancers13184567
APA StyleLee, J. S., Sinn, D. H., Park, S. Y., Shin, H. J., Lee, H. W., Kim, B. K., Park, J. Y., Kim, D. Y., Ahn, S. H., Oh, J. H., Lee, J. I., & Kim, S. U. (2021). Liver Stiffness-Based Risk Prediction Model for Hepatocellular Carcinoma in Patients with Nonalcoholic Fatty Liver Disease. Cancers, 13(18), 4567. https://doi.org/10.3390/cancers13184567








