Simple Summary
The approval of the two antibody combinations trastuzumab/pertuzumab and ipilimumab/nivolumab in oncology has paved the way for novel antibody combinations or oligoclonal antibody mixtures to improve their efficacy in cancer. The underlying biological mechanisms and challenges of these strategies will be discussed using data from clinical trials listed in databases. These therapeutic combinations also lead to questions on how to optimize their formulation and delivery to induce a therapeutic polyclonal response in patients with cancer.
Abstract
Monoclonal antibodies have revolutionized the treatment of many diseases, but their clinical efficacy remains limited in some other cases. Pre-clinical and clinical trials have shown that combinations of antibodies that bind to the same target (homo-combinations) or to different targets (hetero-combinations) to mimic the polyclonal humoral immune response improve their therapeutic effects in cancer. The approval of the trastuzumab/pertuzumab combination for breast cancer and then of the ipilimumab/nivolumab combination for melanoma opened the way to novel antibody combinations or oligoclonal antibody mixtures as more effective biologics for cancer management. We found more than 300 phase II/III clinical trials on antibody combinations, with/without chemotherapy, radiotherapy, small molecules or vaccines, in the ClinicalTrials.gov database. Such combinations enhance the biological responses and bypass the resistance mechanisms observed with antibody monotherapy. Usually, such antibody combinations are administered sequentially as separate formulations. Combined formulations have also been developed in which separately produced antibodies are mixed before administration or are produced simultaneously in a single cell line or a single batch of different cell lines as a polyclonal master cell bank. The regulation, toxicity and injection sequence of these oligoclonal antibody mixtures still need to be addressed in order to optimize their delivery and their therapeutic effects.
1. Introduction
In the 19th century, the pioneering work of Shibasaburo Kitasato and Emil von Behring in Germany and Emile Roux in France paved the way for serotherapy. This treatment is based on the use of sera that originate from previously immunized animals or humans and contain pathogen-specific antibodies as the active substance. César Milstein and Georges Köhler revolutionized this concept by inventing the lymphocyte hybridization technique that led to the development of a new pharmacological class of biologics called “monoclonal” antibodies (mAbs). However, partial and short-lived responses, often associated with resistance phenomena (extensively studied in basic research), limit the clinical efficacy of mAbs. To overcome these obstacles, mAb combinations, most often evaluated separately, and oligoclonal antibody cocktails, considered as a single biologic, have been developed. Indeed, the immune system has naturally evolved to generate a polyclonal humoral response to optimize its ability to fight diseases, rather than the monoclonal strategy proposed by the currently approved antibody biologics. In this review, we first describe pre-clinical studies showing the potential of co-targeting tumor and/or immune checkpoint molecules with antibodies in oncology. Antibody mixtures can be made of antibodies against the same target (i.e., homo-combinations) or against different targets (i.e., hetero-combinations). The approval of two therapeutic antibody combinations, trastuzumab/pertuzumab and ipilimumab/nivolumab, validated this concept of “mimicking” the polyclonal humoral immune response for cancer treatment. We then list the antibody combinations that are currently tested in phase II and III clinical trials. Finally, we discuss how the technical improvements for the reproducible manufacturing of oligoclonal antibody mixtures, in which each antibody is selected on the basis of specific criteria (e.g., epitope specificity, affinity or intrinsic biological activity), now allow the natural polyclonal humoral immune response to be mimicked, paving the way for 21st century serotherapy.
2. Homo-Combinations and Hetero-Combinations of Antibodies in Preclinical Studies
2.1. Tumor Co-Targeting in Oncology
Around the year 2000, the notion of homo-combination of antibodies, involving distinct epitopes on the same receptor, was pioneered by Yosef Yarden (Weizmann Institute, Israel) and then by other research groups. For instance, homo-combinations of antibodies against epidermal growth factor receptor (EGFR) [1,2,3,4,5], human epidermal growth factor receptor-2 (HER2) [6,7,8,9] or hepatocyte growth factor (HGF) receptor (i.e., cMET) [10,11] induce synergistic anti-tumor activity due to accelerated degradation of the targeted receptors and enhanced antibody-dependent cell-mediated cytotoxicity (ADCC) (Figure 1). Moreover, these antibody combinations bypass the resistance to treatment induced by monotherapy with cetuximab (anti-EGFR mAb) in colorectal cancer [12] and with an anti-cMET antibody in gastric cancer [13]. They also maintain anti-tumor activity despite the presence of EGFR extracellular domain mutations that might impair antibody binding [14].
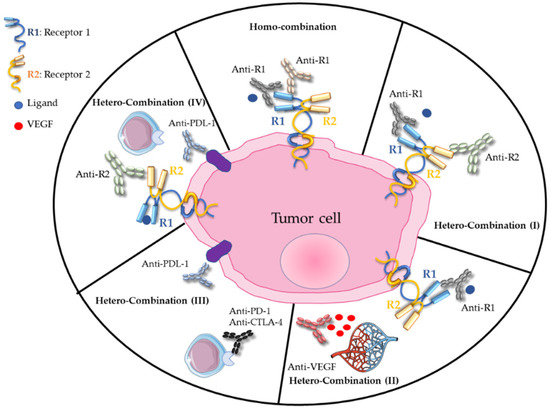
Figure 1.
Homo- and hetero-combinations of monoclonal antibodies, adapted from [15].
In 2007, our team demonstrated that the hetero-combination of antibodies against EGFR and HER2, two functionally collaborating receptors (Figure 1), has a higher anti-tumor effect by promoting ADCC, by reducing the expression of these receptors and homodimer formation [16,17,18] and also by inhibiting intracellular signaling pathways [19]. This preclinical work, confirmed by other research groups [20,21,22], led to the initiation of the THERAPY phase I/II clinical trial in patients with metastatic pancreatic cancer who progressed on gemcitabine. This trial showed that the combination of cetuximab and trastuzumab (targeting EGFR and HER2, respectively) stabilizes the disease in 27% of patients, without objective response but with a positive correlation between skin toxicity and progression-free survival [23]. The clinical trial was discontinued because of high toxicity, highlighting the need to rethink the active dose when using antibody combinations. The idea behind the administration of this hetero-combination was to avoid compensatory signaling phenomena related to the targeting of a single receptor. It was then extended to the dual targeting of EGFR and HER3 in cetuximab- and osimertinib-resistant tumors [12,24]. Such antibody hetero-combinations can also include antibodies against a ligand, for instance vascular endothelial growth factor (VEGF) or HGF, and a receptor (Figure 1), to target both the tumor microenvironment and a tumor-specific receptor [25,26,27].
Finally, oligoclonal cocktails of three [28,29] or six [30,31] antibodies against EGFR, HER2 and HER3 have an increased anti-tumor effect in experimental models, with blockade of the underlying extracellular signal-regulated kinase (ERK) and protein kinase B (AKT) signaling pathways and accelerated receptor degradation. The six-mAb cocktail PanHER (Sym013) demonstrated strong efficiency in gemcitabine-sensitive and also in chemotherapy-resistant pancreatic cancer by downregulating these three receptors [32]. Homo- or hetero-combinations of antibodies against CD20, CD22 or CD52 expressed by B lymphocytes (and T cells for CD52) have also been proposed for blood malignancies [33].
2.2. Co-Targeting of Immune Checkpoint Molecules (ICM): Awakening the Immune System
The importance of immune checkpoints, such as cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death 1 (PD-1), in modulating the anti-tumor T-cell response has been highlighted by the awarding of the 2018 Nobel Prize in Physiology or Medicine to James Allison and Tasuku Honjo. The understanding of their roles in regulating lymphocyte activation and tumor immune escape led to the development of antibody combinations against molecules of this functional family that are classified in co-inhibitory molecules (that must be blocked) and co-activating molecules (that must be stimulated). In 2010, it was shown that the hetero-combination of anti-CTLA-4/-PD-1 blocking antibodies (Figure 1) displays an increased anti-tumor efficacy in mouse models of colorectal cancer and melanoma. This effect is characterized by increased infiltration of cytotoxic T cells and inhibition of regulatory T cells and suppressive myeloid cells [34]. Other hetero-combinations of antibodies targeting the ICMs PD-1 and 4-1BBL (also known as tumor necrosis factor receptor superfamily member 9 or CD137), PD-1 and lymphocyte-activating gene 3 (LAG3) [35], or CD137 and T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) [36], among others, have been proposed to modulate the immune response in cancers. The link between the expression or overexpression of PD-L1 (the ligand of PD-1) and the efficacy of anti-HER2 antibodies in some patients [37,38,39] led to the testing of the combination of tumor-targeting mAbs (TTmAbs), for instance against HER family members, and of antibodies against ICMs in some cancers.
2.3. Pre-Clinical Studies to Understand the Mechanisms of Tumor-Targeting Antibodies in Combination with Immune Checkpoint Blockade
The first clinical successes with anti-CD20, anti-HER2 or anti-EGFR TTmAbs were mainly attributed to the interruption of their respective signaling pathways or their ability to induce ADCC; however, several data also suggested an essential role for the innate and adaptive immunity in the therapeutic outcome. Unfortunately, the use of these naked antibodies as monotherapy in advanced solid tumors, such as breast cancer, metastatic colorectal cancer and head and neck squamous cell carcinoma, results in a high proportion of tumors displaying primary and acquired resistance, and relatively low lasting therapeutic response rates. This suggested that TTmAbs should be associated with anti-ICM antibodies to obtain synergistic effects and sustained antitumor activity. Many clinical trials have been set up in recent years, but with variable success, depending on the cancer type and drugs used. To optimize these combinatorial approaches, preclinical animal models must be developed to better characterize and understand the mechanisms implicated in their effects.
We and others recently demonstrated, using several immunocompetent mice models of solid and hematological tumors, that TTmAbs can overcome immune tolerance and induce the development of an adaptive immune memory, leading to long-lasting effects [40,41,42,43]. It is now clear that TTmAbs have immunomodulatory effects via the Fc fragment, through the recruitment of antigen-presenting cells at the tumor site, better antigen presentation and stronger adaptive immunity with consequences for both the memory cytotoxic and humoral responses [44,45]. Therefore, antitumor therapeutic approaches in which TTmAbs and anti-ICM antibodies are combined to reinforce this antitumor response are interesting for awakening the exhausted antitumor immune response and to reach long-term remission. We demonstrated in the mouse B16F10 melanoma model that anti-PD1 antibodies synergize with the TA99 mAb against TYRP-1 expressed at the surface of malignant melanocytes. In mice treated with this combination, CD8+ T cells, natural killer (NK) and γδ T cells with cytolytic activity were increased as well as plasma antitumor IgGs, leading to better overall survival [40]. Similar results were described recently by another group using the TA99 mAb with the anti-CTLA-4 mAb or the agonist anti-CD137 mAb in the B16F10 mouse melanoma model [46]. In both cases, treatment with the TA99 mAb resulted in an increased expression of the secondary targets CTLA-4, PD1 or CD137, leading to an optimal combinatorial effect of the anti-PD1 or anti-CD137 mAbs.
In the past ten years, other groups have shown that TTmAbs in combination with anti-ICM antibodies might have synergistic effects on the host adaptive anti-immune response and on tumor eradication. For example, anti-angiogenic therapy using anti-VEGFR antibodies can elicit or enhance the anti-tumor immune response, and reciprocally, the immune system can support angiogenesis. Yasuda et al. demonstrated, in a mouse model of colorectal cancer, that the simultaneous blockade of PD1 and VEGFR2 induces a synergistic anti-tumor effect. This might occur through different mechanisms that may not be mutually exclusive [47]. More recently, Allen et al. investigated the efficacy of the anti-PDL-1 and anti-VEGFR2 antibody combination in mice bearing pancreatic neuroendocrine tumor, mammary carcinoma or glioblastoma [48]. They found that the anti-VEGFR2 antibody treatment is associated with lymphocyte homing into the tumor, whereas the anti-PD-L1 antibody induces activation of infiltrated CD4+ and CD8+ T cells that produce IFNγ. Another study showed that in head and neck cancer, the anti-EGFR mAb cetuximab combined with an anti-CD137 agonist antibody leads to tumor regression and prolonged survival. This might be dependent on enhanced NK cell degranulation and cytotoxicity, dendritic cell presentation and tumor antigen cross-presentation [49]. Moreover, it is known that blocking VEGF induces ICM expression, and that the combination of anti-PD-L1 and anti-VEGF antibodies synergistically suppresses tumor growth in small cell lung cancer [50]. Another example illustrates the capacity of TTmAbs to modify the tumor microenvironment and increase the efficacy of anti-ICM antibodies. Indeed, the anti-HER2 mAb trastuzumab can increase IFNγ production through the MyD88 pathway, and IFNγ induces PD-L1 expression on tumor cells. Consequently, anti-PD1/anti-PD-L1 antibodies can strengthen the antitumor activity of anti-HER2 TTmAbs [51].
The CD47 ligand and its receptor SIRPα are other ICMs targeted by antibodies in several clinical trials in combination with TTmAbs. In mouse models of hematological or solid cancer, rituximab synergizes with the humanized anti-CD47 antibody HU5F9-G4 to promote phagocytosis and to eliminate non-Hodgkin lymphoma and solid tumors in xenografted mice [52]. Interestingly, the combination of HU5F9-G4 with cetuximab or panitumumab (anti-EGFR mAbs) reduces tumor burden more than any of the monotherapies in immunodeficient mice harboring patient-derived xenografts [53].
Altogether, these data show that TTmAbs can modulate the tumor microenvironment (vasculature, cytokine profiles, innate immunity activation, increase of the adaptive immune repertoire) and therefore reinforce the antitumor response of anti-ICM antibodies to achieve tumor regression. However, drug combination protocols are complex and require data obtained in preclinical studies to find the optimal conditions for the combined delivery (administered doses, concomitant vs. sequential administration, formulations, pharmacokinetics). Understanding the mechanisms involved in their synergistic effects will allow optimal clinical therapeutic approaches to be developed to counteract treatment resistance in patients with cancer [54].
3. The Initial Clinical Proof of Concept about Antibody Combinations
In 2012, the first TTmAb homo-combination (the anti-HER2 mAbs trastuzumab and pertuzumab) was approved with docetaxel for the treatment of patients with HER2-amplified metastatic breast cancer. The phase III trial CLEOPATRA (n = 808 patients) found a mean progression-free survival of 18.7 months in the antibody homo-combination + docetaxel arm compared with 12.4 months in the trastuzumab alone + docetaxel arm [55]. Cardiac toxicity was comparable in the two arms. This antibody homo-combination with docetaxel was subsequently approved for neo-adjuvant treatment of newly diagnosed patients (APHINITY and NeoSphere trials [56,57]). However, the NeoSphere trial reported increased toxicity in the trastuzumab/pertuzumab + docetaxel arm. Recently, a subcutaneous formulation of the TTmAbs trastuzumab and pertuzumab with recombinant hyaluronidase in one ready-to-use, fixed-dose vial plus chemotherapy was approved by the U.S. Food and Drug Administration (FDA) and European Medicines Agency (EMA) for patients with HER2-positive early and metastatic breast cancer (FeDeriCa trial [58], MetaPHER trial [59]). In these open-label phase III trials, non-inferiority, safety and tolerability were satisfactorily addressed. Therefore, they paved the way to improve the patients’ quality of life by significantly reducing the treatment time for patients, physicians, nurses and pharmacy staff. This sub-cutaneous formulation brings opportunities for more flexible home management of patients with cancer.
However, not all phase III clinical trials on TTmAb combinations produced positive results. For example, in HER2-positive gastric cancer (JACOB study), the combination of pertuzumab and trastuzumab with cisplatin or 5-fluorouracil did not improve patient survival [60]. Similarly, the CAIRO2 study [61] on the hetero-combination of bevacizumab/cetuximab with oxaliplatin and capecitabine, the PACCE study [26] on bevacizumab/panitumumab combined with oxaliplatin and irinotecan in metastatic colorectal cancer, and the AVEREL [27] study on trastuzumab and bevacizumab in HER2-amplified breast cancer also were unsuccessful. Therefore, the co-targeting of VEGF and of EGFR or HER2 does not seem to be relevant in terms of synergy or additivity, possibly due to negative interactions between signaling pathways, or pharmacodynamic interactions (lack of tumor vascularization, inhibition of the expression of one of the two receptors) [62,63].
Only 20–30% of patients with metastatic melanoma responds to monotherapy with anti-CTLA-4 or anti-PD-1 antibodies to block immune checkpoints. In 2013, a phase I clinical trial in which ipilimumab (anti-CTLA-4 mAb) was combined with nivolumab (anti-PD1 mAb) reported tumor regression in 50% of treated patients [64]. In the phase III Checkmate 067 clinical trial (n = 945 patients with metastatic melanoma), progression-free survival was longer in the arm treated with the ipilimumab/nivolumab hetero-combination (11.5 months) than in the arms treated with nivolumab (6.9 months) or ipilimumab (2.9 months) alone [65,66]. However, this survival benefit was associated with increased toxicity in the nivolumab/ipilimumab hetero-combination arm compared with the two monotherapy arms (55% of patients with grade 3 and 4 adverse events in the hetero-combination arm vs. 16% in the nivolumab and 27% in the ipilimumab arms) [66]. This trial led the FDA to approve this hetero-combination of anti-ICM antibodies for metastatic melanoma. Since then, phase III clinical trials on anti-CTLA-4 antibodies combined with anti-PD-1 or anti-PD-L1 antibodies have shown positive results in lung cancer and renal cell carcinoma. In lung cancer, the combination of the anti-PD-L1 antibody atezolizumab with the anti-VEGF TTmAb bevacizumab, associated with chemotherapy, has shown a benefit in terms of progression-free survival compared with the arm without atezolizumab (8.3 months vs. 6.8 months) [67]. Similarly, the phase I/II trial PANACEA, which tested the anti-ICM pembrolizumab combined with the TTmAb trastuzumab in patients with HER2-amplified breast cancer, showed an improved clinical benefit in the subset of patients with PD-L1-positive tumors [68].
To date, the cocktail of nivolumab (anti-PD1 antibody) and ipilimumab (anti-CTLA-4 antibody) was the first approved and remains the only anti-ICM antibody combination approved in the clinic as first-line treatment for untreated patients with metastatic melanoma. The latest clinical data for melanoma showed up to 4 years of survival in 53% of patients receiving this hetero-combination [69]. Its use has been extended also to patients with low-risk renal carcinoma [70] and mismatch repair-deficient colorectal cancer [71]. The optimism for these mAb-based combination treatments in overcoming therapeutic resistance in different malignancies is very high. They also improve survival compared with platinum-based chemotherapy in advanced non-small cell lung cancer [72]. The combination of ipilimumab and nivolumab has been approved by the FDA for all patients with tumor displaying ≥1% of PD-L1 expression. Although these antibodies are currently used in clinical practice, some questions remain unanswered, such as the best-treatment strategy, the role of different biomarkers for patient selection and the effectiveness of immunotherapy according to specific clinical characteristics.
4. Antibody Combination in Phase II and Phase III Clinical Trials: A 2021 Update in Oncology
More than 300 phase II/III clinical trials in patients with cancer to test combinations of antibodies, mainly against ICMs, angiogenic factors, CD20 and receptor tyrosine kinases (RTKs), combined or not with chemotherapy, radiotherapy, small molecules or vaccines, are currently registered and in progress [15,73,74,75,76] (www.clinicaltrials.gov (accessed on 3 May 2021)). Most of these studies, completed, active or recruiting (as listed in Table 1), involve the combination of ipilimumab (anti-CTLA-4 antibody) and nivolumab (anti-PD-1 antibody) (more than 80 trials [76]), tremelimumab (anti-CTLA-4 antibody) and durvalumab (anti-PD-L1 antibody) (more than 25 trials), trastuzumab or anti-CD20 antibodies combined with anti-ICM antibodies (more than 10 and 15 trials, respectively), bevacizumab (anti-VEGF-A antibody) and atezolizumab (anti-PD-L1 antibody) (more than 10 trials) and trastuzumab combined with pertuzumab (anti-HER2 antibody) (more than 5 trials [75]). It is worth noting that most of the listed phase II/III clinical trials concern antibodies targeting ICMs (CTLA-4, PD1/PD-L1, LAG-3, TIM-3, GITR, TIGIT, CD73, ICOS, PVRIG) combined or not with TTmAbs. Conversely, fewer than 25 trials concern only TTmAb combinations.

Table 1.
Antibody-based drug combinations currently examined by clinical trials at the date of 3 May 2021, updated from [73].
In addition to “classical” naked antibodies, new formats, such as bispecific antibodies, probodies, antibody–drug conjugates, immunocytokines, immune-stimulating antibody conjugates or chimeric antigen receptor T (CAR-T) cells, also are included in the antibody combinations tested in phase II/III clinical trials (Table 1). Interestingly, triple antibody combinations are emerging (more than 15 trials), mainly using anti-PD1/anti-CTLA-4 antibodies with antibodies against RTKs, other ICMs (GITR, TIGIT), killer-cell immunoglobulin-like receptor, antibody–drug conjugates or immunocytokines. Triple combinations of anti-PD-L1 antibodies and TTmAbs (against RTKs, and anti-CD20 and anti-CD79b antibody-drug conjugates), or ICM inhibitors (anti-CD137, anti-CTLA-4 antibodies) also have been assessed for cancer management, as well as the anti-PD1/TIGIT/PVRIG and anti-PD1/CD40/CFS1 combinations.
Most of these antibody combinations are administered sequentially, using antibodies developed individually as active substances and initially licensed as single-agent mAbs. The treatment sequence has not been optimized yet, especially when TTmAb and anti-ICM antibody combinations are proposed, or when a chemotherapeutic agent also is added. The delivery of antibody combinations (schedule, dose and timing) has to be carefully addressed to improve the pharmacokinetics and pharmacodynamics in patients and to avoid toxicities and side effects.
5. Optimization of the Formulation and Delivery of Antibody Combinations
In a few cases, new industrial manufacturing/formulation strategies, developed by some pharmaceutical companies, have allowed the production of antibody combinations to be rationalized and optimized (Figure 2). Currently, antibodies for therapeutic combinations are produced and administered following four main strategies (Figure 2) that have led to the clinical development of oligoclonal antibody mixtures (Table 1 and Table 2).
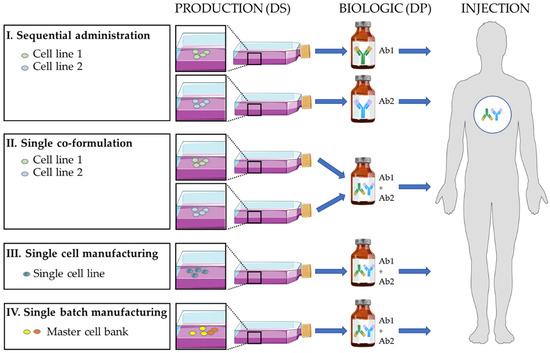
Figure 2.
Different strategies to produce a biologic as a drug product (DP) that is a mixture of two antibody drug substances (DS). The single-cell manufacturing allows a mixture of mono- and bi-specific antibodies to be produced. Ab: antibody.

Table 2.
Antibody mixtures in oncology.
5.1. Sequential Administration
The “antibody” active substances are produced separately (one cell line for each antibody; mainly CHO cells), the pharmaceutical formulations are made individually, and the antibody biologics are injected sequentially. This is the most classical pharmaceutical strategy when using already approved “mAb” biologics. It is the basis for the approval of the trastuzumab/pertuzumab and nivolumab/ipilimumab combinations by regulatory authorities and is used in most ongoing clinical trials in oncology (Table 1).
5.2. Single Co-Formulation
The “antibody” active substances are produced separately (one cell line for each antibody; mainly CHO cells), and during the pharmaceutical formulation step, the active ingredients are mixed to obtain to the “combination” biologics. The single co-formulation has been used to develop the approved combination of trastuzumab and pertuzumab (two TTmAbs) plus recombinant hyaluronidase for sub-cutaneous delivery in breast cancer, with a 1:1 or 1:2 stoichiometry depending on the formulations [58,59]. This strategy has also been employed to produce the Sym004 combination (1:1 stoichiometry) of two anti-EGFR antibodies (futuximab and modotuximab) [77,78,79] that is currently evaluated in phase II clinical trials in metastatic colorectal cancer and glioblastoma. This strategy has also been used to produce MM-151 [14,80,81], a 2:2:1 stoichiometric mixture of three anti-EGFR antibodies. This mixture has been evaluated in phase I trials in combination with chemotherapy or the anti-HER3 MM-121 antibody in colorectal and lung cancer (Table 2). In infectious diseases, a combination of three antibodies against the Clostridium botulinum neurotoxin has been developed and tested in a phase I trial [82]. Similarly, the single formulation of three antibodies (atoltivimab + maftivimab + odesivimab) has been approved by the FDA in 2020 for Ebola hemorrhagic fever. As demonstrated for the co-formulation of six approved antibodies [83], it seems that therapeutic monoclonal antibodies of the IgG1 subclass can be combined without severe detrimental effects to the stability of these proteins in binary mixtures.
5.3. Single-Cell Line Manufacturing
The active “antibody” substances are produced together in a single cell line. A single pharmaceutical formulation leads to the “combination” biologics. This Oligoclonics® process [84,85] uses the PER.C6 cell line transfected with a construct that encodes a single kappa light chain, and two constructs, each encoding a heavy H chain of different specificity (“common light chain” technology). This approach has been used to produce mixtures of mono- and bi-specific antibodies. Moreover, this technology has been combined with CH3 domain engineering to force the preferential production of bispecific antibodies (Biclonics®). Some bispecific antibodies are currently in clinical development, such as antibodies targeting HER2/HER3 [86,87] in breast, pancreatic and gastric cancer, EGFR/leucine-rich repeat containing G protein-coupled receptor 5 (LGR5) in solid tumors, CD3/C-type lectin domain family 12 member A (CLEC12A) in acute myeloid leukemia [88], PD-L1/CD137 and EGFR/cMET in solid tumors.
5.4. Single Batch Manufacturing
The cell lines producing the active drug substances are initially mixed to generate a polyclonal master-cell bank. A unique pharmaceutical formulation leads to the “combination” biologics. The process allows the site-specific integration of each antibody construct on the same chromosomal locus in each cell line (Flp-In, CHO, CHO-DG44) [89,90] to standardize the expression level of each antibody after mixing the transformed cell lines. For instance, a controlled mixture of 25 anti-rhesus D antibodies (rozrolimupab or Sym001 [90]) was produced and tested in a phase II trial in patients with thrombocytopenic purpura [50]. Moreover, a mixture of two anti-cMET antibodies (1:1 stoichiometry; Sym015 [10,11,13]) was assessed in cMET-amplified tumors (phase II trial), and an oligoclonal mixture of two anti-EGFR antibodies, two anti-HER2 antibodies and two anti-HER3 antibodies (PanHER or Sym013 [12,30,31,32,91,92,93]) in epithelial cancers (phase I trials).
6. Challenges and Regulation of Antibody Combinations
Homo- and hetero-combinations of antibodies have many advantages compared with antibody monotherapy (Table 3). Antibody combinations allow several well-defined epitopes on one or more antigens to be targeted with a perfectly controlled and adjustable antibody stoichiometric ratio. Within an antibody cocktail, the affinity, epitope, isotype, or glycosylation of each antibody can be tailored.

Table 3.
Challenges of antibody combinations.
Antibodies are glycoproteins that contain a glycosylation site at position 297 in the Fc region. TTmAbs need to be N-glycosylated to display effector functions. Moreover, the presence or absence of terminal sugars on the glycans in the Fc region strongly influence the antibody pharmacokinetics (e.g., high mannose content decreases the antibody half-life), pharmacodynamics, stability, safety (immunogenicity, specifically when the antibody is derived from non-human cells) and efficacy. The effector functions (ADCC and complement-dependent cytotoxicity) can also be affected. Therefore, glycoforms should be thoroughly analyzed in each antibody batch produced to offer stable and safe antibodies, an essential step for their successful clinical translation. Glycoengineering strategies have been developed to produce antibodies harboring the desired glycoforms in order to enhance their efficacy and safety [94,95].
Targeting several epitopes on the same receptor or on several receptors allows the number of targets recruited to be increased and thus an increased number of antibodies bound per cell. The biological responses induced by these antibodies are enhanced, such as the Fc-dependent immune effector mechanisms of IgGs (ADCC, complement-dependent cytotoxicity, antibody-dependent cell phagocytosis), and the inhibition of compensatory cell signaling (possibly through the target elimination or internalization/degradation) based on the Fab portion of IgGs. In hetero-combinations of antibodies against immune checkpoints or their ligands (e.g., PD-L1), the combination simultaneously counteracts the redundant negative regulatory mechanisms exploited by tumors to escape the immune system. Therefore, an oligoclonal mixture of antibodies allows their spectrum of activity to be broadened by anticipating and avoiding possible resistance to the treatment (pre-existing or acquired through the emergence of resistant clones under the effect of one of the combination components). Finally, in oncology, hetero-combinations to target cancer cells and/or the tumor immune microenvironment and vessels might implicate synergistic mechanisms of action in vivo, the modalities of which remain to be clarified. In addition, antibody combinations could also pose unique intellectual property challenges [96].
The production of antibody mixtures is generally regulated by the good manufacturing practices conventionally used to produce single therapeutic antibodies [97]. Currently, the strategy of “sequential administration” is still predominant for combinations of two mAbs, in approved formulations and also in clinical trials. However, if mixtures of three to six antibodies are going to be developed and tested, the cost of this strategy will progressively increase, and regulatory procedures and constraints will become more cumbersome and complicated. Indeed, according to the EMA and FDA regulations, the toxicity, efficacy and pharmacokinetics of each component of an oligoclonal mixture must be evaluated individually and in combination. Therefore, the regulatory procedures are duplicated for each active substance, because each antibody is considered as a single biologic in the combination. Faced with these difficulties, the “single co-formulation”, or the “single cell” and “single batch” manufacturing should allow the production processes and costs to be controlled and the regulatory and registration procedures to be simplified [98]. Concretely, the FDA has already authorized oligoclonal antibody mixtures, prepared according to the “single co-formulation” strategy, for use in phase I and II clinical trials [82]. Moreover, the FDA has approved phase I trials with Sym004 [99] and MM-151 [100], obtained using the “single co-formulation” strategy, to be tested as a “single biologic” in oncology. The combination of trastuzumab and pertuzumab plus hyaluronidase, produced by single co-formulation, has been approved by the FDA and EMA. Therefore, it is necessary to choose, already at the preclinical stage, the production strategy of oligoclonal mixtures to reduce risks and costs. It remains to be seen how the FDA and EMA will consider, from a regulatory point of view, the new oligoclonal antibody mixtures prepared using “single cell” and “single batch” manufacturing.
Compared with single-agent therapies, determining the optimal dose and schedule of each mAb is crucial in combination regimens. An oligoclonal mixture is formulated according to a stoichiometric ratio of antibodies defined at the time of the initial regulatory application for an investigational new drug. Due to the specific pharmacokinetics of each antibody (absorption, distribution, metabolism and excretion), the initial stoichiometric formulation is unlikely to be maintained in the patient during treatment. This problem must be addressed in preclinical studies. The dose and treatment sequence choice should take into account the specific pharmacokinetic features of each antibody in the mixture. For example, the stoichiometric ratio of the six antibodies in the Sym013 mixture varies in vivo over time in function of target exposure, ranging from high for EGFR to medium or low for HER2 and HER3. The mechanisms of action of the oligoclonal mixture, compared with each antibody in the mixture, must be determined in relevant preclinical models to support clinical development.
The challenge today is to better understand signaling networks with the ultimate aim of developing combination regimens or adaptive sequential strategies that translate high partial response rates to durable complete responses. Clinical observations highlight the importance of flexible approaches to optimize the dose and schedule of mAb-based combinations [101]. The toxicity potentiation observed in some combination trials [23] underscores the need of careful dose titration in phase I clinical trials. The two clinically approved combinations use the antibody doses identified in the monotherapy clinical trials. However, the dose chosen in phase I clinical trials of new antibody combinations may not necessarily be the same as the dose approved for monotherapy. For instance, a recent follow-on study evaluated the combination of vemurafenib and ipilimumab using a sequential schedule of administration [102]. This regimen demonstrated a substantially improved safety profile, with marked reduction of hepatotoxicity compared with the previous study in which ipilimumab and vemurafenib were administered concurrently. This study clearly highlights the clinical development challenges and risks of combining anti-cancer antibodies at standard doses and schedules. In addition, it is clear that the optimal dose and schedule for a given combination may differ in function from the indication due to differences in disease biology and/or co-morbidities in the various patient populations. Although pre-clinical animal models have limitations, they can be useful to assess the therapeutic potential of specific combinations by unraveling their mechanisms of action and providing insights into the underlying biology of various therapeutic strategies. Moreover, in the context of cancer therapies, mathematical and computational approaches are becoming more and more relevant to overcome the various challenges related to the optimization of combined protocols to obtain synergistic effects [103,104,105].
7. Conclusions: Towards a Therapeutic Polyclonal Immune Response?
The immune system has naturally evolved towards a polyclonal humoral response. The development of oligoclonal antibody combinations and mixtures to improve the existing targeted therapies is approaching the goal of mimicking the natural humoral immune response. However, practical and regulatory biological constraints must be overcome to enrich this pharmacological class of antibodies.
Author Contributions
C.L., L.G. and T.C. collected literature and wrote the manuscript. T.C. collected information about clinical trials. C.L. and T.C. designed the figures and tables. A.P. checked and corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Ligue contre le Cancer, the GSO Canceropole, the GEFLUC, the FUI13 UmAbHER3 program (BPI France), SIRIC Montpellier Cancer, Inserm Transfert and the LabEx MAbImprove ANR-10-LABX-53-01.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors thank Isabelle Navarro-Teulon, Bruno Robert, Nadège Gaborit, Yassamine Lazrek, Gaëlle Thomas and Emilia Rabia for their contribution to the team’s work referred to in this article; they also thank Véronique Garambois, Geneviève Heintz, Sabine Bousquié and the entire IRCM animal facility team for their technical assistance.
Conflicts of Interest
C.L., A.P. and T.C. declare having participated in occasional interventions (collaboration for scientific works) with the companies Roche, LFB, CisBio, Surgimab, Biomunex and GamaMabs. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Friedman, L.M.; Rinon, A.; Schechter, B.; Lyass, L.; Lavi, S.; Bacus, S.S.; Sela, M.; Yarden, Y. Synergistic Down-Regulation of Receptor Tyrosine Kinases by Combinations of MAbs: Implications for Cancer Immunotherapy. Proc. Natl. Acad. Sci. USA 2005, 102, 1915–1920. [Google Scholar] [CrossRef] [PubMed]
- Perera, R.M.; Narita, Y.; Furnari, F.B.; Gan, H.K.; Murone, C.; Ahlkvist, M.; Luwor, R.B.; Burgess, A.W.; Stockert, E.; Jungbluth, A.A.; et al. Treatment of Human Tumor Xenografts with Monoclonal Antibody 806 in Combination with a Prototypical Epidermal Growth Factor Receptor-Specific Antibody Generates Enhanced Antitumor Activity. Clin. Cancer Res. 2005, 11, 6390–6399. [Google Scholar] [CrossRef] [PubMed]
- Spangler, J.B.; Neil, J.R.; Abramovitch, S.; Yarden, Y.; White, F.M.; Lauffenburger, D.A.; Wittrup, K.D. Combination Antibody Treatment Down-Regulates Epidermal Growth Factor Receptor by Inhibiting Endosomal Recycling. Proc. Natl. Acad. Sci. USA 2010, 107, 13252–13257. [Google Scholar] [CrossRef]
- Ferraro, D.A.; Gaborit, N.; Maron, R.; Cohen-Dvashi, H.; Porat, Z.; Pareja, F.; Lavi, S.; Lindzen, M.; Ben-Chetrit, N.; Sela, M.; et al. Inhibition of Triple-Negative Breast Cancer Models by Combinations of Antibodies to EGFR. Proc. Natl. Acad. Sci. USA 2013, 110, 1815–1820. [Google Scholar] [CrossRef]
- Kol, A.; Terwisscha van Scheltinga, A.; Pool, M.; Gerdes, C.; de Vries, E.; de Jong, S. ADCC Responses and Blocking of EGFR-Mediated Signaling and Cell Growth by Combining the Anti-EGFR Antibodies Imgatuzumab and Cetuximab in NSCLC Cells. Oncotarget 2017, 8, 45432–45446. [Google Scholar] [CrossRef] [PubMed]
- Ben-Kasus, T.; Schechter, B.; Lavi, S.; Yarden, Y.; Sela, M. Persistent Elimination of ErbB-2/HER2-Overexpressing Tumors Using Combinations of Monoclonal Antibodies: Relevance of Receptor Endocytosis. Proc. Natl. Acad. Sci. USA 2009, 106, 3294–3299. [Google Scholar] [CrossRef] [PubMed]
- Drebin, J.A.; Link, V.C.; Greene, M.I. Monoclonal Antibodies Reactive with Distinct Domains of the Neu Oncogene-Encoded P185 Molecule Exert Synergistic Anti-Tumor Effects in Vivo. Oncogene 1988, 2, 273–277. [Google Scholar]
- Kasprzyk, P.G.; Song, S.U.; Di Fiore, P.P.; King, C.R. Therapy of an Animal Model of Human Gastric Cancer Using a Combination of Anti-ErbB-2 Monoclonal Antibodies. Cancer Res. 1992, 52, 2771–2776. [Google Scholar]
- Spiridon, C.I.; Ghetie, M.-A.; Uhr, J.; Marches, R.; Li, J.-L.; Shen, G.-L.; Vitetta, E.S. Targeting Multiple Her-2 Epitopes with Monoclonal Antibodies Results in Improved Antigrowth Activity of a Human Breast Cancer Cell Line in Vitro and in Vivo. Clin. Cancer Res. 2002, 8, 1720–1730. [Google Scholar]
- Poulsen, T.T.; Grandal, M.M.; Skartved, N.J.Ø.; Hald, R.; Alifrangis, L.; Koefoed, K.; Lindsted, T.; Fröhlich, C.; Pollmann, S.E.; Eriksen, K.W.; et al. Sym015: A Highly Efficacious Antibody Mixture against MET-Amplified Tumors. Clin. Cancer Res. 2017, 23, 5923–5935. [Google Scholar] [CrossRef]
- Grandal, M.M.; Havrylov, S.; Poulsen, T.T.; Koefoed, K.; Dahlman, A.; Galler, G.R.; Conrotto, P.; Collins, S.; Eriksen, K.W.; Kaufman, D.; et al. Simultaneous Targeting of Two Distinct Epitopes on MET Effectively Inhibits MET- and HGF-Driven Tumor Growth by Multiple Mechanisms. Mol. Cancer Ther. 2017, 16, 2780–2791. [Google Scholar] [CrossRef]
- Iida, M.; Brand, T.M.; Starr, M.M.; Huppert, E.J.; Luthar, N.; Bahrar, H.; Coan, J.P.; Pearson, H.E.; Salgia, R.; Wheeler, D.L. Overcoming Acquired Resistance to Cetuximab by Dual Targeting HER Family Receptors with Antibody-Based Therapy. Mol. Cancer 2014, 13, 242. [Google Scholar] [CrossRef] [PubMed]
- Pollmann, S.E.; Calvert, V.S.; Rao, S.; Boca, S.M.; Madhavan, S.; Horak, I.D.; Kjaer, A.; Petricoin, E.F.; Kragh, M.; Poulsen, T.T. Acquired Resistance to a MET Antibody In Vivo Can Be Overcome by the MET Antibody Mixture Sym015. Mol. Cancer Ther. 2018, 17, 1259–1270. [Google Scholar] [CrossRef]
- Arena, S.; Siravegna, G.; Mussolin, B.; Kearns, J.D.; Wolf, B.B.; Misale, S.; Lazzari, L.; Bertotti, A.; Trusolino, L.; Adjei, A.A.; et al. MM-151 Overcomes Acquired Resistance to Cetuximab and Panitumumab in Colorectal Cancers Harboring EGFR Extracellular Domain Mutations. Sci. Transl. Med. 2016, 8, 324ra14. [Google Scholar] [CrossRef] [PubMed]
- Zahavi, D.; Weiner, L. Monoclonal Antibodies in Cancer Therapy. Antibodies 2020, 9, 34. [Google Scholar] [CrossRef]
- Larbouret, C.; Robert, B.; Navarro-Teulon, I.; Thèzenas, S.; Ladjemi, M.-Z.; Morisseau, S.; Campigna, E.; Bibeau, F.; Mach, J.-P.; Pèlegrin, A.; et al. In Vivo Therapeutic Synergism of Anti-Epidermal Growth Factor Receptor and Anti-HER2 Monoclonal Antibodies against Pancreatic Carcinomas. Clin. Cancer Res. 2007, 13, 3356–3362. [Google Scholar] [CrossRef] [PubMed]
- Larbouret, C.; Robert, B.; Bascoul-Mollevi, C.; Penault-Llorca, F.; Ho-Pun-Cheung, A.; Morisseau, S.; Navarro-Teulon, I.; Mach, J.-P.; Pèlegrin, A.; Azria, D. Combined Cetuximab and Trastuzumab Are Superior to Gemcitabine in the Treatment of Human Pancreatic Carcinoma Xenografts. Ann. Oncol. 2010, 21, 98–103. [Google Scholar] [CrossRef]
- Larbouret, C.; Gaborit, N.; Chardès, T.; Coelho, M.; Campigna, E.; Bascoul-Mollevi, C.; Mach, J.-P.; Azria, D.; Robert, B.; Pèlegrin, A. In Pancreatic Carcinoma, Dual EGFR/HER2 Targeting with Cetuximab/Trastuzumab Is More Effective than Treatment with Trastuzumab/Erlotinib or Lapatinib Alone: Implication of Receptors’ down-Regulation and Dimers’ Disruption. Neoplasia 2012, 14, 121–130. [Google Scholar] [CrossRef]
- Thomas, G.; Chardès, T.; Gaborit, N.; Mollevi, C.; Leconet, W.; Robert, B.; Radosevic-Robin, N.; Penault-Llorca, F.; Gongora, C.; Colombo, P.-E.; et al. HER3 as Biomarker and Therapeutic Target in Pancreatic Cancer: New Insights in Pertuzumab Therapy in Preclinical Models. Oncotarget 2014, 5, 7138–7148. [Google Scholar] [CrossRef]
- Maron, R.; Schechter, B.; Mancini, M.; Mahlknecht, G.; Yarden, Y.; Sela, M. Inhibition of Pancreatic Carcinoma by Homo- and Heterocombinations of Antibodies against EGF-Receptor and Its Kin HER2/ErbB-2. Proc. Natl. Acad. Sci. USA 2013, 110, 15389–15394. [Google Scholar] [CrossRef]
- Romaniello, D.; Mazzeo, L.; Mancini, M.; Marrocco, I.; Noronha, A.; Kreitman, M.; Srivastava, S.; Ghosh, S.; Lindzen, M.; Salame, T.M.; et al. A Combination of Approved Antibodies Overcomes Resistance of Lung Cancer to Osimertinib by Blocking Bypass Pathways. Clin. Cancer Res. 2018, 24, 5610–5621. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Romaniello, D.; Vaknin, I.; Drago-Garcia, D.; Oren, R.; Uribe, M.L.; Belugali Nataraj, N.; Ghosh, S.; Eilam, R.; Salame, T.-M.; et al. Upfront Admixing Antibodies and EGFR Inhibitors Preempts Sequential Treatments in Lung Cancer Models. EMBO Mol. Med. 2021, 13, e13144. [Google Scholar] [CrossRef] [PubMed]
- Assenat, E.; Azria, D.; Mollevi, C.; Guimbaud, R.; Tubiana-Mathieu, N.; Smith, D.; Delord, J.-P.; Samalin, E.; Portales, F.; Larbouret, C.; et al. Dual Targeting of HER1/EGFR and HER2 with Cetuximab and Trastuzumab in Patients with Metastatic Pancreatic Cancer after Gemcitabine Failure: Results of the “THERAPY”Phase 1-2 Trial. Oncotarget 2015, 6, 12796–12808. [Google Scholar] [CrossRef]
- Romaniello, D.; Marrocco, I.; Belugali Nataraj, N.; Ferrer, I.; Drago-Garcia, D.; Vaknin, I.; Oren, R.; Lindzen, M.; Ghosh, S.; Kreitman, M.; et al. Targeting HER3, a Catalytically Defective Receptor Tyrosine Kinase, Prevents Resistance of Lung Cancer to a Third-Generation EGFR Kinase Inhibitor. Cancers 2020, 12, 2394. [Google Scholar] [CrossRef] [PubMed]
- Van Cutsem, E.; Eng, C.; Nowara, E.; Swieboda-Sadlej, A.; Tebbutt, N.C.; Mitchell, E.; Davidenko, I.; Stephenson, J.; Elez, E.; Prenen, H.; et al. Randomized Phase Ib/II Trial of Rilotumumab or Ganitumab with Panitumumab versus Panitumumab Alone in Patients with Wild-Type KRAS Metastatic Colorectal Cancer. Clin. Cancer Res. 2014, 20, 4240–4250. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Mitchell, E.; Chidiac, T.; Scroggin, C.; Hagenstad, C.; Spigel, D.; Marshall, J.; Cohn, A.; McCollum, D.; Stella, P.; et al. A Randomized Phase IIIB Trial of Chemotherapy, Bevacizumab, and Panitumumab Compared with Chemotherapy and Bevacizumab Alone for Metastatic Colorectal Cancer. J. Clin. Oncol. 2009, 27, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Gianni, L.; Romieu, G.H.; Lichinitser, M.; Serrano, S.V.; Mansutti, M.; Pivot, X.; Mariani, P.; Andre, F.; Chan, A.; Lipatov, O.; et al. AVEREL: A Randomized Phase III Trial Evaluating Bevacizumab in Combination with Docetaxel and Trastuzumab as First-Line Therapy for HER2-Positive Locally Recurrent/Metastatic Breast Cancer. J. Clin. Oncol. 2013, 31, 1719–1725. [Google Scholar] [CrossRef]
- Mancini, M.; Gal, H.; Gaborit, N.; Mazzeo, L.; Romaniello, D.; Salame, T.M.; Lindzen, M.; Mahlknecht, G.; Enuka, Y.; Burton, D.G.; et al. An Oligoclonal Antibody Durably Overcomes Resistance of Lung Cancer to Third-Generation EGFR Inhibitors. EMBO Mol. Med. 2018, 10, 294–308. [Google Scholar] [CrossRef]
- Mancini, M.; Gaborit, N.; Lindzen, M.; Salame, T.M.; Dall’Ora, M.; Sevilla-Sharon, M.; Abdul-Hai, A.; Downward, J.; Yarden, Y. Combining Three Antibodies Nullifies Feedback-Mediated Resistance to Erlotinib in Lung Cancer. Sci. Signal. 2015, 8, ra53. [Google Scholar] [CrossRef]
- Jacobsen, H.J.; Poulsen, T.T.; Dahlman, A.; Kjær, I.; Koefoed, K.; Sen, J.W.; Weilguny, D.; Bjerregaard, B.; Andersen, C.R.; Horak, I.D.; et al. Pan-HER, an Antibody Mixture Simultaneously Targeting EGFR, HER2, and HER3, Effectively Overcomes Tumor Heterogeneity and Plasticity. Clin. Cancer Res. 2015, 21, 4110–4122. [Google Scholar] [CrossRef]
- Schwarz, L.J.; Hutchinson, K.E.; Rexer, B.N.; Estrada, M.V.; Gonzalez Ericsson, P.I.; Sanders, M.E.; Dugger, T.C.; Formisano, L.; Guerrero-Zotano, A.; Red-Brewer, M.; et al. An ERBB1-3 Neutralizing Antibody Mixture With High Activity Against Drug-Resistant HER2+ Breast Cancers With ERBB Ligand Overexpression. J. Natl. Cancer Inst. 2017, 109, 65. [Google Scholar] [CrossRef]
- Rabia, E.; Garambois, V.; Hubert, J.; Bruciamacchie, M.; Pirot, N.; Delpech, H.; Broyon, M.; Theillet, C.; Colombo, P.-E.; Vie, N.; et al. Anti-Tumoral Activity of the Pan-HER (Sym013) Antibody Mixture in Gemcitabine-Resistant Pancreatic Cancer Models. MAbs 2021, 13, 1914883. [Google Scholar] [CrossRef] [PubMed]
- Strauss, S.J.; Morschhauser, F.; Rech, J.; Repp, R.; Solal-Celigny, P.; Zinzani, P.L.; Engert, A.; Coiffier, B.; Hoelzer, D.F.; Wegener, W.A.; et al. Multicenter Phase II Trial of Immunotherapy with the Humanized Anti-CD22 Antibody, Epratuzumab, in Combination with Rituximab, in Refractory or Recurrent Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2006, 24, 3880–3886. [Google Scholar] [CrossRef]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 Combination Blockade Expands Infiltrating T Cells and Reduces Regulatory T and Myeloid Cells within B16 Melanoma Tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Lee, L.-F.; Fisher, T.S.; Jessen, B.; Elliott, M.; Evering, W.; Logronio, K.; Tu, G.H.; Tsaparikos, K.; Li, X.; et al. Combination of 4-1BB Agonist and PD-1 Antagonist Promotes Antitumor Effector/Memory CD8 T Cells in a Poorly Immunogenic Tumor Model. Cancer Immunol. Res. 2015, 3, 149–160. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, D.; Xia, Z.; Luan, M.; Wu, L.; Wang, G.; Zhang, S. Combined TIM-3 Blockade and CD137 Activation Affords the Long-Term Protection in a Murine Model of Ovarian Cancer. J. Transl. Med. 2013, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Chaganty, B.K.R.; Qiu, S.; Gest, A.; Lu, Y.; Ivan, C.; Calin, G.A.; Weiner, L.M.; Fan, Z. Trastuzumab Upregulates PD-L1 as a Potential Mechanism of Trastuzumab Resistance through Engagement of Immune Effector Cells and Stimulation of IFNγ Secretion. Cancer Lett. 2018, 430, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.N.; Ishii, C.; Ishida, S.; Ogitani, Y.; Wada, T.; Agatsuma, T. A HER2-Targeting Antibody-Drug Conjugate, Trastuzumab Deruxtecan (DS-8201a), Enhances Antitumor Immunity in a Mouse Model. Mol. Cancer Ther. 2018, 17, 1494–1503. [Google Scholar] [CrossRef]
- Junttila, T.T.; Li, J.; Johnston, J.; Hristopoulos, M.; Clark, R.; Ellerman, D.; Wang, B.-E.; Li, Y.; Mathieu, M.; Li, G.; et al. Antitumor Efficacy of a Bispecific Antibody That Targets HER2 and Activates T Cells. Cancer Res. 2014, 74, 5561–5571. [Google Scholar] [CrossRef]
- They, L.; Michaud, H.-A.; Becquart, O.; Lafont, V.; Guillot, B.; Boissière-Michot, F.; Jarlier, M.; Mollevi, C.; Eliaou, J.-F.; Bonnefoy, N.; et al. PD-1 Blockade at the Time of Tumor Escape Potentiates the Immune-Mediated Antitumor Effects of a Melanoma-Targeting Monoclonal Antibody. Oncoimmunology 2017, 6, e1353857. [Google Scholar] [CrossRef]
- Ren, Z.; Guo, J.; Liao, J.; Luan, Y.; Liu, Z.; Sun, Z.; Liu, X.; Liang, Y.; Peng, H.; Fu, Y.-X. CTLA-4 Limits Anti-CD20-Mediated Tumor Regression. Clin. Cancer Res. 2017, 23, 193–203. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, X.; Mortenson, E.D.; Radkevich-Brown, O.; Wang, Y.; Fu, Y.-X. Cetuximab-Mediated Tumor Regression Depends on Innate and Adaptive Immune Responses. Mol. Ther. 2013, 21, 91–100. [Google Scholar] [CrossRef]
- Mortenson, E.D.; Park, S.; Jiang, Z.; Wang, S.; Fu, Y.-X. Effective Anti-Neu-Initiated Antitumor Responses Require the Complex Role of CD4+ T Cells. Clin. Cancer Res. 2013, 19, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Michaud, H.-A.; Eliaou, J.-F.; Lafont, V.; Bonnefoy, N.; Gros, L. Tumor Antigen-Targeting Monoclonal Antibody-Based Immunotherapy: Orchestrating Combined Strategies for the Development of Long-Term Antitumor Immunity. Oncoimmunology 2014, 3, e955684. [Google Scholar] [CrossRef]
- DiLillo, D.J.; Ravetch, J.V. Differential Fc-Receptor Engagement Drives an Anti-Tumor Vaccinal Effect. Cell 2015, 161, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Lorenzo, R.; Erjavec, S.O.; Christiano, A.M.; Clynes, R. Improved Therapeutic Efficacy of Unmodified Anti-Tumor Antibodies by Immune Checkpoint Blockade and Kinase Targeted Therapy in Mouse Models of Melanoma. Oncotarget 2021, 12, 66–80. [Google Scholar] [CrossRef]
- Yasuda, S.; Sho, M.; Yamato, I.; Yoshiji, H.; Wakatsuki, K.; Nishiwada, S.; Yagita, H.; Nakajima, Y. Simultaneous Blockade of Programmed Death 1 and Vascular Endothelial Growth Factor Receptor 2 (VEGFR2) Induces Synergistic Anti-Tumour Effect in Vivo. Clin. Exp. Immunol. 2013, 172, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Allen, E.; Jabouille, A.; Rivera, L.B.; Lodewijckx, I.; Missiaen, R.; Steri, V.; Feyen, K.; Tawney, J.; Hanahan, D.; Michael, I.P.; et al. Combined Antiangiogenic and Anti-PD-L1 Therapy Stimulates Tumor Immunity through HEV Formation. Sci. Transl. Med. 2017, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.M.; Trivedi, S.; Concha-Benavente, F.; Gibson, S.P.; Reeder, C.; Ferrone, S.; Ferris, R.L. CD137 Stimulation Enhances Cetuximab-Induced Natural Killer: Dendritic Cell Priming of Antitumor T-Cell Immunity in Patients with Head and Neck Cancer. Clin. Cancer Res. 2017, 23, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Meder, L.; Schuldt, P.; Thelen, M.; Schmitt, A.; Dietlein, F.; Klein, S.; Borchmann, S.; Wennhold, K.; Vlasic, I.; Oberbeck, S.; et al. Combined VEGF and PD-L1 Blockade Displays Synergistic Treatment Effects in an Autochthonous Mouse Model of Small Cell Lung Cancer. Cancer Res. 2018, 78, 4270–4281. [Google Scholar] [CrossRef]
- Stagg, J.; Loi, S.; Divisekera, U.; Ngiow, S.F.; Duret, H.; Yagita, H.; Teng, M.W.; Smyth, M.J. Anti-ErbB-2 MAb Therapy Requires Type I and II Interferons and Synergizes with Anti-PD-1 or Anti-CD137 MAb Therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Zhao, F.; Tseng, S.; Narayanan, C.; Shura, L.; Willingham, S.; Howard, M.; Prohaska, S.; Volkmer, J.; et al. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PLoS ONE 2015, 10, e0137345. [Google Scholar] [CrossRef]
- Krampitz, G.W.; George, B.M.; Willingham, S.B.; Volkmer, J.-P.; Weiskopf, K.; Jahchan, N.; Newman, A.M.; Sahoo, D.; Zemek, A.J.; Yanovsky, R.L.; et al. Identification of Tumorigenic Cells and Therapeutic Targets in Pancreatic Neuroendocrine Tumors. Proc. Natl. Acad. Sci. USA 2016, 113, 4464–4469. [Google Scholar] [CrossRef] [PubMed]
- Delou, J.M.A.; Souza, A.S.O.; Souza, L.C.M.; Borges, H.L. Highlights in Resistance Mechanism Pathways for Combination Therapy. Cells 2019, 8, E1013. [Google Scholar] [CrossRef]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; de la Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): A Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.R.; Im, S.-A.; Mattar, A.; Colomer, R.; Stroyakovskii, D.; Nowecki, Z.; De Laurentiis, M.; Pierga, J.-Y.; Jung, K.H.; Schem, C.; et al. Fixed-Dose Combination of Pertuzumab and Trastuzumab for Subcutaneous Injection plus Chemotherapy in HER2-Positive Early Breast Cancer (FeDeriCa): A Randomised, Open-Label, Multicentre, Non-Inferiority, Phase 3 Study. Lancet Oncol. 2021, 22, 85–97. [Google Scholar] [CrossRef]
- Kuemmel, S.; Tondini, C.A.; Abraham, J.; Nowecki, Z.; Itrych, B.; Hitre, E.; Karaszewska, B.; Juárez-Ramiro, A.; Morales-Vásquez, F.; Pérez-García, J.M.; et al. Subcutaneous Trastuzumab with Pertuzumab and Docetaxel in HER2-Positive Metastatic Breast Cancer: Final Analysis of MetaPHER, a Phase IIIb Single-Arm Safety Study. Breast Cancer Res. Treat. 2021, 187, 467–476. [Google Scholar] [CrossRef]
- Tabernero, J.; Hoff, P.M.; Shen, L.; Ohtsu, A.; Shah, M.A.; Cheng, K.; Song, C.; Wu, H.; Eng-Wong, J.; Kim, K.; et al. Pertuzumab plus Trastuzumab and Chemotherapy for HER2-Positive Metastatic Gastric or Gastro-Oesophageal Junction Cancer (JACOB): Final Analysis of a Double-Blind, Randomised, Placebo-Controlled Phase 3 Study. Lancet Oncol. 2018, 19, 1372–1384. [Google Scholar] [CrossRef]
- Tol, J.; Koopman, M.; Cats, A.; Rodenburg, C.J.; Creemers, G.J.M.; Schrama, J.G.; Erdkamp, F.L.G.; Vos, A.H.; van Groeningen, C.J.; Sinnige, H.A.M.; et al. Chemotherapy, Bevacizumab, and Cetuximab in Metastatic Colorectal Cancer. N. Engl. J. Med. 2009, 360, 563–572. [Google Scholar] [CrossRef]
- Heskamp, S.; Boerman, O.C.; Molkenboer-Kuenen, J.D.M.; Sweep, F.C.G.J.; Geurts-Moespot, A.; Engelhardt, M.S.; van der Graaf, W.T.A.; Oyen, W.J.G.; van Laarhoven, H.W.M. Cetuximab Reduces the Accumulation of Radiolabeled Bevacizumab in Cancer Xenografts without Decreasing VEGF Expression. Mol. Pharm. 2014, 11, 4249–4257. [Google Scholar] [CrossRef]
- Heskamp, S.; Boerman, O.C.; Molkenboer-Kuenen, J.D.M.; Oyen, W.J.G.; van der Graaf, W.T.A.; van Laarhoven, H.W.M. Bevacizumab Reduces Tumor Targeting of Antiepidermal Growth Factor and Anti-Insulin-like Growth Factor 1 Receptor Antibodies. Int. J. Cancer 2013, 133, 307–314. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Kluger, H.; Callahan, M.K.; Postow, M.A.; Rizvi, N.A.; Lesokhin, A.M.; Segal, N.H.; Ariyan, C.E.; Gordon, R.-A.; Reed, K.; et al. Nivolumab plus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2013, 369, 122–133. [Google Scholar] [CrossRef]
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Rutkowski, P.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Wagstaff, J.; Schadendorf, D.; Ferrucci, P.F.; et al. Overall Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2017, 377, 1345–1356. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus Trastuzumab in Trastuzumab-Resistant, Advanced, HER2-Positive Breast Cancer (PANACEA): A Single-Arm, Multicentre, Phase 1b-2 Trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Nivolumab plus Ipilimumab or Nivolumab Alone versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef]
- Sheng, I.Y.; Ornstein, M.C. Ipilimumab and Nivolumab as First-Line Treatment of Patients with Renal Cell Carcinoma: The Evidence to Date. Cancer Manag. Res. 2020, 12, 4871–4881. [Google Scholar] [CrossRef]
- Overman, M.J.; Lonardi, S.; Wong, K.Y.M.; Lenz, H.-J.; Gelsomino, F.; Aglietta, M.; Morse, M.A.; Van Cutsem, E.; McDermott, R.; Hill, A.; et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018, 36, 773–779. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, R.; Imbimbo, M.; Malouf, R.; Paget-Bailly, S.; Calais, F.; Marchal, C.; Westeel, V. Single or Combined Immune Checkpoint Inhibitors Compared to First-Line Platinum-Based Chemotherapy with or without Bevacizumab for People with Advanced Non-Small Cell Lung Cancer. Cochrane Database Syst. Rev. 2021, 4, CD013257. [Google Scholar] [CrossRef] [PubMed]
- Marrocco, I.; Romaniello, D.; Yarden, Y. Cancer Immunotherapy: The Dawn of Antibody Cocktails. Methods Mol. Biol. 2019, 1904, 11–51. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Rauth, S.; Aithal, A.; Kaur, S.; Ganguly, K.; Orzechowski, C.; Varshney, G.C.; Jain, M.; Batra, S.K. The Current Landscape of Antibody-Based Therapies in Solid Malignancies. Theranostics 2021, 11, 1493–1512. [Google Scholar] [CrossRef]
- Henricks, L.M.; Schellens, J.H.M.; Huitema, A.D.R.; Beijnen, J.H. The Use of Combinations of Monoclonal Antibodies in Clinical Oncology. Cancer Treat. Rev. 2015, 41, 859–867. [Google Scholar] [CrossRef]
- Chae, Y.K.; Arya, A.; Iams, W.; Cruz, M.R.; Chandra, S.; Choi, J.; Giles, F. Current Landscape and Future of Dual Anti-CTLA4 and PD-1/PD-L1 Blockade Immunotherapy in Cancer; Lessons Learned from Clinical Trials with Melanoma and Non-Small Cell Lung Cancer (NSCLC). J. Immunother. Cancer 2018, 6, 39. [Google Scholar] [CrossRef]
- Pedersen, M.W.; Jacobsen, H.J.; Koefoed, K.; Hey, A.; Pyke, C.; Haurum, J.S.; Kragh, M. Sym004: A Novel Synergistic Anti-Epidermal Growth Factor Receptor Antibody Mixture with Superior Anticancer Efficacy. Cancer Res. 2010, 70, 588–597. [Google Scholar] [CrossRef]
- Alifrangis, L.; Schoemaker, R.; Skartved, N.J.; Hald, R.; Montagut, C.; Kopetz, S.; Tabernero, J.; Kragh, M.; Wade, J.R. Population Pharmacokinetics and Covariate Analysis of Sym004, an Antibody Mixture against the Epidermal Growth Factor Receptor, in Subjects with Metastatic Colorectal Cancer and Other Solid Tumors. J. Pharmacokinet. Pharmacodyn. 2020, 47, 5–18. [Google Scholar] [CrossRef]
- Jones, S.; King, P.J.; Antonescu, C.N.; Sugiyama, M.G.; Bhamra, A.; Surinova, S.; Angelopoulos, N.; Kragh, M.; Pedersen, M.W.; Hartley, J.A.; et al. Targeting of EGFR by a Combination of Antibodies Mediates Unconventional EGFR Trafficking and Degradation. Sci. Rep. 2020, 10, 663. [Google Scholar] [CrossRef]
- Napolitano, S.; Martini, G.; Martinelli, E.; Della Corte, C.M.; Morgillo, F.; Belli, V.; Cardone, C.; Matrone, N.; Ciardiello, F.; Troiani, T. Antitumor Efficacy of Triple Monoclonal Antibody Inhibition of Epidermal Growth Factor Receptor (EGFR) with MM151 in EGFR-Dependent and in Cetuximab-Resistant Human Colorectal Cancer Cells. Oncotarget 2017, 8, 82773–82783. [Google Scholar] [CrossRef][Green Version]
- Kearns, J.D.; Bukhalid, R.; Sevecka, M.; Tan, G.; Gerami-Moayed, N.; Werner, S.L.; Kohli, N.; Burenkova, O.; Sloss, C.M.; King, A.M.; et al. Enhanced Targeting of the EGFR Network with MM-151, an Oligoclonal Anti-EGFR Antibody Therapeutic. Mol. Cancer Ther. 2015, 14, 1625–1636. [Google Scholar] [CrossRef]
- Nayak, S.U.; Griffiss, J.M.; McKenzie, R.; Fuchs, E.J.; Jurao, R.A.; An, A.T.; Ahene, A.; Tomic, M.; Hendrix, C.W.; Zenilman, J.M. Safety and Pharmacokinetics of XOMA 3AB, a Novel Mixture of Three Monoclonal Antibodies against Botulinum Toxin A. Antimicrob. Agents Chemother. 2014, 58, 5047–5053. [Google Scholar] [CrossRef]
- Krieg, D.; Berner, C.; Winter, G.; Svilenov, H.L. Biophysical Characterization of Binary Therapeutic Monoclonal Antibody Mixtures. Mol. Pharm. 2020, 17, 2971–2986. [Google Scholar] [CrossRef]
- de Kruif, J.; Kramer, A.; Nijhuis, R.; van der Zande, V.; den Blanken, R.; Clements, C.; Visser, T.; Keehnen, R.; den Hartog, M.; Throsby, M.; et al. Generation of Stable Cell Clones Expressing Mixtures of Human Antibodies. Biotechnol. Bioeng. 2010, 106, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Logtenberg, T. Antibody Cocktails: Next-Generation Biopharmaceuticals with Improved Potency. Trends Biotechnol. 2007, 25, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Geuijen, C.A.W.; De Nardis, C.; Maussang, D.; Rovers, E.; Gallenne, T.; Hendriks, L.J.A.; Visser, T.; Nijhuis, R.; Logtenberg, T.; de Kruif, J.; et al. Unbiased Combinatorial Screening Identifies a Bispecific IgG1 That Potently Inhibits HER3 Signaling via HER2-Guided Ligand Blockade. Cancer Cell 2018, 33, 922–936.e10. [Google Scholar] [CrossRef]
- De Nardis, C.; Hendriks, L.J.A.; Poirier, E.; Arvinte, T.; Gros, P.; Bakker, A.B.H.; de Kruif, J. A New Approach for Generating Bispecific Antibodies Based on a Common Light Chain Format and the Stable Architecture of Human Immunoglobulin G1. J. Biol. Chem. 2017, 292, 14706–14717. [Google Scholar] [CrossRef]
- van Loo, P.F.; Hangalapura, B.N.; Thordardottir, S.; Gibbins, J.D.; Veninga, H.; Hendriks, L.J.A.; Kramer, A.; Roovers, R.C.; Leenders, M.; de Kruif, J.; et al. MCLA-117, a CLEC12AxCD3 Bispecific Antibody Targeting a Leukaemic Stem Cell Antigen, Induces T Cell-Mediated AML Blast Lysis. Expert Opin. Biol. Ther. 2019, 19, 721–733. [Google Scholar] [CrossRef]
- Rasmussen, S.K.; Nielsen, L.S.; Müller, C.; Bouquin, T.; Næsted, H.; Mønster, N.T.; Nygaard, F.; Weilguny, D.; Frandsen, T.P.; Tolstrup, A.B. Recombinant Antibody Mixtures; Optimization of Cell Line Generation and Single-Batch Manufacturing Processes. BMC Proc. 2011, 5 (Suppl. 8), O2. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, F.C.; Rasmussen, S.K.; Frandsen, T.P.; Rasmussen, L.K.; Tengbjerg, K.; Coljee, V.W.; Sharon, J.; Yang, C.-Y.; Bregenholt, S.; Nielsen, L.S.; et al. Production of Target-Specific Recombinant Human Polyclonal Antibodies in Mammalian Cells. Biotechnol. Bioeng. 2006, 94, 396–405. [Google Scholar] [CrossRef]
- Francis, D.M.; Huang, S.; Armstrong, E.A.; Werner, L.R.; Hullett, C.; Li, C.; Morris, Z.S.; Swick, A.D.; Kragh, M.; Lantto, J.; et al. Pan-HER Inhibitor Augments Radiation Response in Human Lung and Head and Neck Cancer Models. Clin. Cancer Res. 2016, 22, 633–643. [Google Scholar] [CrossRef]
- Ellebaek, S.; Brix, S.; Grandal, M.; Lantto, J.; Horak, I.D.; Kragh, M.; Poulsen, T.T. Pan-HER-An Antibody Mixture Targeting EGFR, HER2 and HER3 Abrogates Preformed and Ligand-Induced EGFR Homo- and Heterodimers. Int. J. Cancer 2016, 139, 2095–2105. [Google Scholar] [CrossRef] [PubMed]
- Reddy, T.P.; Choi, D.S.; Anselme, A.C.; Qian, W.; Chen, W.; Lantto, J.; Horak, I.D.; Kragh, M.; Chang, J.C.; Rosato, R.R. Simultaneous Targeting of HER Family Pro-Survival Signaling with Pan-HER Antibody Mixture Is Highly Effective in TNBC: A Preclinical Trial with PDXs. Breast Cancer Res. 2020, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Boune, S.; Hu, P.; Epstein, A.L.; Khawli, L.A. Principles of N-Linked Glycosylation Variations of IgG-Based Therapeutics: Pharmacokinetic and Functional Considerations. Antibodies 2020, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H. Characterization of Glycosylation in Monoclonal Antibodies and Its Importance in Therapeutic Antibody Development. Crit. Rev. Biotechnol. 2021, 41, 300–315. [Google Scholar] [CrossRef]
- Feng, L.; Chen, L.; Murphy, A.; Jacobstein, J.; Lewis, S. Patenting Antibody Combination Therapies. Antib. Ther. 2020, 3, 265–270. [Google Scholar] [CrossRef]
- Chon, J.H.; Zarbis-Papastoitsis, G. Advances in the Production and Downstream Processing of Antibodies. N. Biotechnol. 2011, 28, 458–463. [Google Scholar] [CrossRef]
- Rasmussen, S.K.; Næsted, H.; Müller, C.; Tolstrup, A.B.; Frandsen, T.P. Recombinant Antibody Mixtures: Production Strategies and Cost Considerations. Arch. Biochem. Biophys. 2012, 526, 139–145. [Google Scholar] [CrossRef]
- Kojima, T.; Yamazaki, K.; Kato, K.; Muro, K.; Hara, H.; Chin, K.; Goddemeier, T.; Kuffel, S.; Watanabe, M.; Doi, T. Phase I Dose-Escalation Trial of Sym004, an Anti-EGFR Antibody Mixture, in Japanese Patients with Advanced Solid Tumors. Cancer Sci. 2018, 109, 3253–3262. [Google Scholar] [CrossRef]
- Lieu, C.H.; Harb, W.A.; Beeram, M.; Power, L.; Kearns, J.D.; Nering, R.; Moyo, V.M.; Wolf, B.B.; Adjei, A.A. Phase 1 Trial of MM-151, a Novel Oligoclonal Anti-EGFR Antibody Combination in Patients with Refractory Solid Tumors. JCO 2014, 32, 2518. [Google Scholar] [CrossRef]
- Corraliza-Gorjón, I.; Somovilla-Crespo, B.; Santamaria, S.; Garcia-Sanz, J.A.; Kremer, L. New Strategies Using Antibody Combinations to Increase Cancer Treatment Effectiveness. Front. Immunol. 2017, 8, 1804. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Lawson, D.H.; Salama, A.K.S.; Koon, H.B.; Guthrie, T.; Thomas, S.S.; O’Day, S.J.; Shaheen, M.F.; Zhang, B.; Francis, S.; et al. Phase II Study of Vemurafenib Followed by Ipilimumab in Patients with Previously Untreated BRAF-Mutated Metastatic Melanoma. J. Immunother. Cancer 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Tosi, D.; Pérez-Gracia, E.; Atis, S.; Vié, N.; Combès, E.; Gabanou, M.; Larbouret, C.; Jarlier, M.; Mollevi, C.; Torro, A.; et al. Rational Development of Synergistic Combinations of Chemotherapy and Molecular Targeted Agents for Colorectal Cancer Treatment. BMC Cancer 2018, 18, 812. [Google Scholar] [CrossRef] [PubMed]
- Tosi, D.; Laghzali, Y.; Vinches, M.; Alexandre, M.; Homicsko, K.; Fasolo, A.; Del Conte, G.; Durigova, A.; Hayaoui, N.; Gourgou, S.; et al. Clinical Development Strategies and Outcomes in First-in-Human Trials of Monoclonal Antibodies. J. Clin. Oncol. 2015, 33, 2158–2165. [Google Scholar] [CrossRef]
- Viala, M.; Vinches, M.; Alexandre, M.; Mollevi, C.; Durigova, A.; Hayaoui, N.; Homicsko, K.; Cuenant, A.; Gongora, C.; Gianni, L.; et al. Strategies for Clinical Development of Monoclonal Antibodies beyond First-in-Human Trials: Tested Doses and Rationale for Dose Selection. Br. J. Cancer 2018, 118, 679–697. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).