External Basic Hyperthermia Devices for Preclinical Studies in Small Animals
Abstract
:Simple Summary
Abstract
1. Introduction
2. Study Design—General Considerations
2.1. Tumor Model
2.2. Anesthesia and Analgesia Management
2.3. Temperature Monitoring
3. Hyperthermia Devices
3.1. Water Bath
| Tumor | Device | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Strain | Cell Line | Method | Location | tDevelop | VTumor * | TM | HTD | HTT | HTt | Ref. | |
| Days | mm3 | °C | °C | min | |||||||||
| Crile | 1961 | M | Swiss | S180 | Fragment | Foot | 7 | ~400 | TC | - | 41.0–49.0 | 0–240 | [58] |
| Bleehen | 1977 | M | BALB/c | EMT6 | Suspension | Hind leg | 8–10 | - | TC | 42.0–45.0 | 40.0–44.0 | 60 | [25] |
| Stone | 1978 | M | C3H/Mai | CSU-Mca | Fragment | Hind leg | - | ~200 | - | 42.5–43.0 | - | 60 | [59] |
| Overgaard | 1980 | M | C3D2F1/Bom | C3H/Tif | Fragment | Foot | 14 | 200 | TC | 40.7–45.7 | 40.5–45.5 | 60 | [60] |
| Gibbs | 1981 | M | C3H | C3H | Fragment | Flank/foot | - | ~125–300 | TC | 43.2 | 41.0–43.0 | 60 | [50] |
| Rofstad | 1982 | M | NMRI, Nu/Nu | PDX | Fragment | Hind leg | 21–28 | 200–675 | TC | 42.5 | 42.2–42.4 | 0–180 | [61] |
| Joiner | 1982 | M | C57BL/Cbi | B16 | Suspension | Hind leg | 10–14 | ~350–575 | TC | - | 43.0 | 60 | [62] |
| M | C57BL/Cbi | LLC | Suspension | Hind leg | 10–14 | ~350–575 | TC | - | 43.0 | 60 | |||
| O’Hara | 1985 | M | C3H | MCa | Fragment | Hind leg | 10–14 | ~125–300 | TC | 43.1 | 42.7 | 10 | [22] |
| Nishimura | 1988 | M | C3H/He | MCa | Suspension | Hind thigh | - | 500–800 | TC | 43.0 | 42.7 | 10 | [29] |
| M | C3H/He | SCC VII | Suspension | Hind thigh | - | 500–800 | TC | 43.0 | 42.7 | 10 | |||
| Cope | 1990 | M | BALB/c, Nu/Nu | D-54 MG | Suspension | Flank/leg | 8 | 100–200 | TC | - | 42.0 | 120/240 | [26] |
| Hauck | 1997 | M | BALB/c, athymic | D-54 MG | Suspension | Lateral thigh | 7–9 | 200 | TC | 42.2 | 41.8 | 240 | [32] |
| Locke | 2005 | M | Nu/nu, athymic | HeLa | Suspension | Proximal thigh | - | 350 | TC | - | 41.0/43.0 | 60 | [51] |
| Peller | 2008 | M | C57BL/6 | BFS-1 | Suspension | Hind leg | 14 | ~525 | FOP | 43.0 | - | 20–30 | [15] |
| Dicheva | 2015 | M | C57BL/6 | B16BL6 | Fragment | Hind leg | - | ~50 | TC | 43.0 | 42.0 | 60 | [45] |
| M | C57BL/6 | LLC | Fragment | Hind leg | - | ~50 | TC | 43.0 | 42.0 | 60 | |||
| Suzuki | 1967 | R | Donryu | Yoshida | Suspension | Feet | 3 | 180–575 | - | 40.0–42.0–44.0–46.0 | - | 30 | [63] |
| Calderwood | 1980 | R | Wistar | Yoshida | Suspension | Foot | 9/16 | 1000–3500 | TC | - | 42.0 | 60 | [64] |
| Dahl | 1982 | R | BD IX | TCL | Fragment | Thigh | - | - | TC | 42.2–45.8 | 42.0–45.0 | 0–120 | [65] |
| Wheldon | 1982 | R | Wistar/CFHB | SSBIa | Fragment | Dorsal | 125–400 | 43.5 | 43.0–43.3 | 60 | [66] | ||
| Van der Zee | 1995 | R | WAG/Rij | RIOS | Fragment | Thigh | 14 | ~300–1300 | TC | 43.0 | 42.0 | 60 | [67] |
| Moroi | 1996 | R | Fischer | T9 | Suspension | Hind Leg | - | 200 | TC | 43.0 | - | 30 | [68] |
| Willerding | 2016 | R | Brown Norway | BN175 | Suspension | Hind leg | - | 700–2200 | TC | 41.0–42.0 | 41.5 | 60 | [41] |
| Derieppe | 2019 | R | WAG/Rij | R-1 | Fragment | Hind leg | 21 | ~ 1500 | FOP | 43.0 | 43.0 | 10 | [69] |
| Leunig | 1992 | H | Syrian Golden | A-Mel-3 | Suspension | Dorsal S.C. | 7 | 100–150 | - | 43.0 | - | 30/60 | [70] |
| Dellian | 1993 | H | Syrian Golden | A-Mel-3 | Suspension | Dorsal S.C. | 5 | 90–140 | TC | 43.3 | - | 30 | [71] |
| Pahernik | 1999 | H | Syrian Golden | A-Mel-3 | Suspension | Dorsal S.C. | 5 | 90–140 | FOP | 37.0/44.0 | 36.2/43.8 | 20 | [72] |
3.2. Cold Light Source (CLS)

| Tumor | Device | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Strain | Cell Line | Method | Location | tDevelop | VTumor * | TM | λ | Intensity | HTT | HTt | Ref. | |
| Days | mm3 | nm | W/cm2 | °C | min | |||||||||
| Halogen | ||||||||||||||
| Ickenstein | 2003 | M | Rag2-M | MDA435 | Suspension | Dorsal S.C. | - | 20–30 | TC | - | - | 41.0 | 60 | [91] |
| Foxley | 2012 | M | Nu/nu | AT6 | Suspension | Hind leg | - | 250 | TC | - | - | 41.0 | 15 | [28] |
| Sien | 1980 | R | Sprague-Dawley | W256 | Suspension | Back | 7 | - | THM | - | - | 38.0 | 60 | [81] |
| Limmer | 2014 | R | Brown Norway | BN175 | Fragment | Hind leg | - | ~50 | TC | - | - | 41.0 | 60 | [80] |
| Willerding | 2016 | R | Brown Norway | BN175 | Suspension | Hind leg | - | 700–2200 | TC | 350–700 | - | 41.0 | 60 | [41] |
| wIRA | ||||||||||||||
| Kelleher | 1999 | R | Sprague-Dawley | DS | Suspension | Hind foot | 6 | - | TC | 665–800 | 0.80 | 43.0 | 60 | [90] |
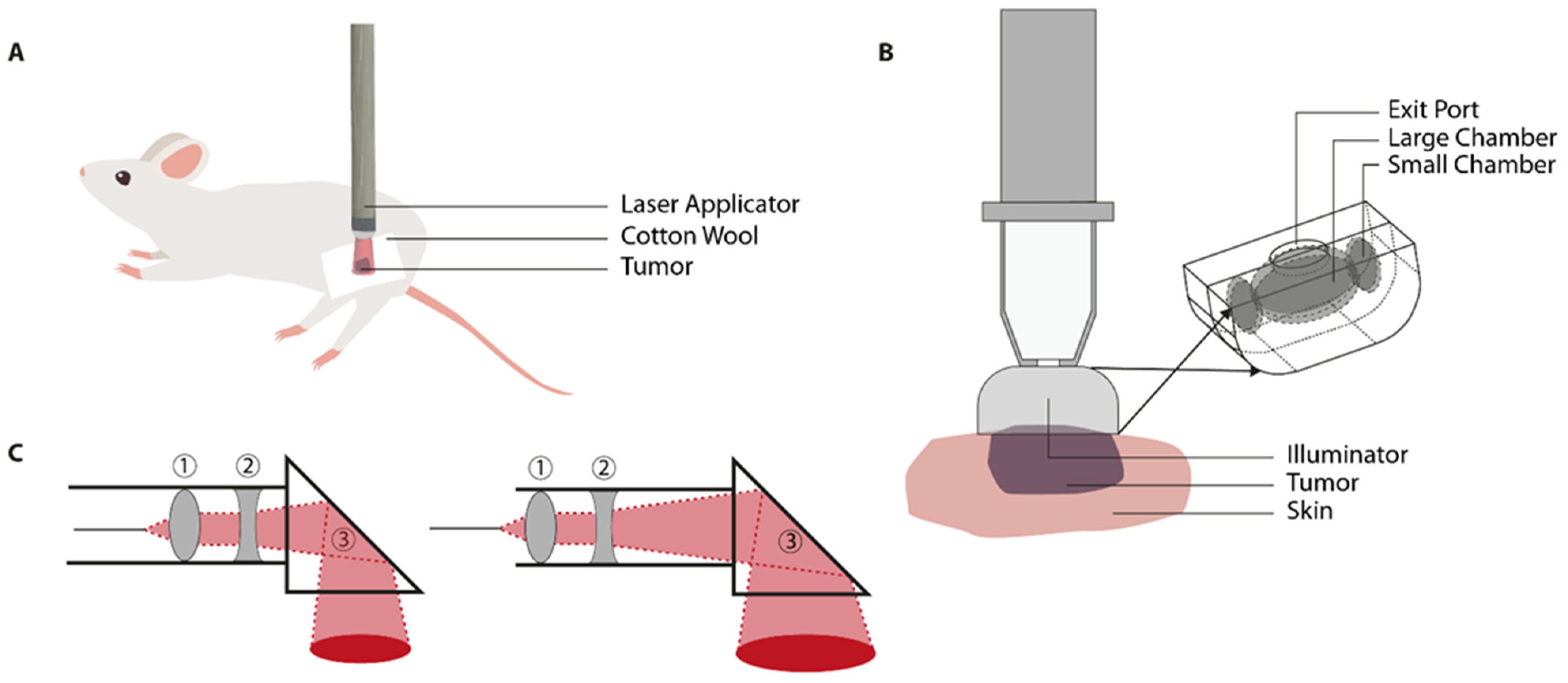
3.3. Near-Infrared (NIR) Laser Light
| Tumor | Device | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Strain | Cell Line | Method | Location | tDevelop | VTumor * | TM | λ | Beam | Intensity | HTT | HTt | Ref. | |
| Days | mm3 | nm | ø mm | W/cm2 | °C | min | |||||||||
| Waldow | 1988 | M | DBA/2J | SMT-F | Fragment | Axillary region | 7–10 | 120–675 | TC | 1064 | 15–20 | 0.08–0.19 | 41.0–46.0 | 25 | [105] |
| Barnes | 2013 | M | SCK | A/J | Suspension | Hind leg | ~30–200 | 755 | 8 | - | 42.5 | 60 | [108] | ||
| SCCVII | C3H | Suspension | Hind Leg | ~30–200 | 755 | 8 | - | 42.5 | 60 | ||||||
| Kirui | 2013 | M | Nu/nu | CAPAN-1 | Suspension | Flank | 5–7 | 290–350 | IRC | 810 | 4 | 1.00 | 42.0 | 20 | [109] |
| Zhou | 2013 | M | Nu/nu | HCT116 | Suspension | Dorsal | - | ~30–80 | IRC | 808 | - | 0.5 | 31.2–48.8 | 5 | [110] |
| Dou | 2014 | M | SCID | ME-180 | Fragment | Hind limb | - | ~80–300 | MRT | 763 | 10 | 0.50–1.70 | 42.0 | 25 | [102] |
| Dou | 2015 | M | SCID | ME-180 | Fragment | Hind limb | 14–21 | - | MRT | 763 | 10 | 0.10–0.80 | 42.0 | 5 | [101] |
| Panjehpour | 1991 | R | Sprague-Dawley | Spontaneous | - | MFP | <180 | ~1650 | TC | 1064 | 15 | 2.40 | 43.2–43.5 | 60 | [99] |
| Willerding | 2016 | R | Brown Norway | BN175 | Suspension | Hind leg | 9–13 | 700–1000 | TC | 940 | - | 1.00–3.00 | >40.0 | 60 | [41] |
3.4. Focused Ultrasound (FUS)
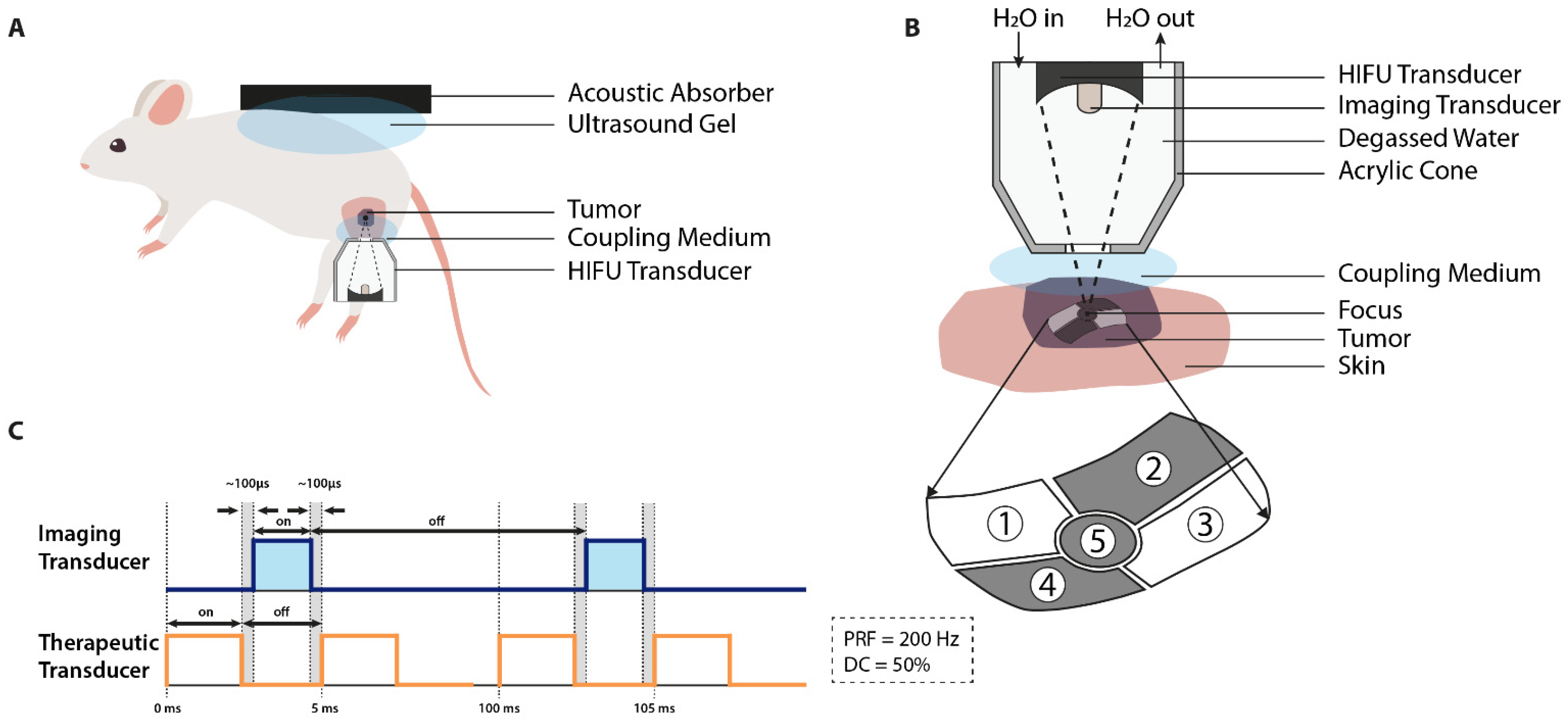
| Tumor | Device | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Strain | Cell Line | Methods | Location | tDevelop | VTumor * | TM | f | TAP | INT | DC | PRF | HTT | HTt | Ref. | |
| Days | mm3 | MHz | W | W/cm2 | % | Hz | °C | min | |||||||||
| Frenkel | 2006 | M | BALB/c | JC | Suspension | Flank | 18–21 | 400–700 | TC | 1.00 | 20.5 | 124 | 9 | 1.0 | 41.5 | 2 | [136] |
| M | C3H | SCC7 | Suspension | Flank | 7–10 | 400–700 | TC | 1.00 | 20.5 | 124 | 9 | 1.0 | 41.5 | 2 | [136] | ||
| Patel | 2008 | M | C3H | SCC7 | Suspension | Flank | 7–10 | 700–800 | IRC/TC | 1.00 | 20–80 | - | 10–50 | 1.0 | 42 | 2 | [120] |
| Kheirolomoom | 2013 | M | FVB | NDL | Fragment | MFP | 14 | 30 | TC | 1.54 | - | - | - | - | 42 | 25 | [137] |
| Chae | 2014 | M | BALB/c | SCC7 | Suspension | Thigh | 1.00 | 12 | - | 50 | 5.0 | 42.9 | 40 | [126] | |||
| Cha | 2016 | M | BALB/c | EMT6 | Suspension | Thigh | 7–9 | 150–200 | TC | 1.50 | 20 | - | 15 | 15.0 | 42.0 | 40 | [128] |
| Farr | 2017 | M | KPC | Spontaneous | - | Pancreas | - | 400 | MRT | 1.20 | 7 | - | - | - | 42.5 | 15 | [134] |
| Centelles | 2018 | M | SHO | IGROV-1 | Suspension | Flank | 14 | ~50–80 | TC | 1.30 | 10–20 | 99.9 | 1.30 | 42.0 | 3–5 | [138] | |
| Jeong | 2016 | M | BALB/c | CT26 | Suspension | Dorsal | 11 | 473 | 1.5 | 10 | 84 | 10 | 10 | 42.0 | <4 | [139] | |
| Hijnen | 2012 | R | Fisher 344 | GS 9L | Suspension | Hind leg | - | 400 | MRT | 1.44 | 8 | 117 | - | - | 42 | 15 | [132] |
3.5. Capacitive Hyperthermia

| Tumor | Device | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Year | Strain | Cell Line | Methods | Location | tDevelop | VTumor * | TM | Type | UPS | f | P | HTT | HTt | Ref. | |
| Days | mm3 | ø cm | MHz | W | °C | min | ||||||||||
| Danics | 2020 | M | BALB/c | 4T1 | Suspension | MFP | 7 | - | FOP | LabEHY 100/200 | 2.5/1.8 | 13.56 | 0.7 ± 0.3 | 41.5 | 35 | [141] |
| Cohen | 2019 | M | Fox1nu | PC3-Luc | Suspension | Prostate | - | 150–250 | FOP | LabEHY 100 | 2.5 | 13.56 | 0.3–1.0 | 41.0 | 30 | [143] |
| Vancsik | 2018 | M | BALB/c | C26 | Suspension | Thigh | 14 | ~900 | FOP | LabEHY | 2.5 | 13.56 | 1.0–3.0 | 42.0 | 30 | [144] |
| Andocs | 2009 | M | BALB/c | HT29 | Suspension | Thigh | 18 | 500–800 | FOP | LabEHY | 2.5 | 13.56 | 4.0 | 42.0 | 30 | [156] |
| Uchibayashi | 1994 | M | BALB/c | KK-47 | Suspension | Dorsal | <14 | 200–300 | TC | Thermotron RF 8 | - | 8.00 | - | 42.5 | 30 | [157] |
| Marmor | 1977 | M | BALB/c | KHJJ | Suspension | Flank | 10–12 | 100 | THM | Critical Systems | 1.2 | 13.56 | 0.6–0.9 | 43.0 | 30 | [145] |
| BALB/c | EMT-6 | Suspension | Flank | 10–12 | 100 | THM | Critical Systems | 1.2 | 13.56 | 0.6–0.9 | 43.0 | 30 | [145] | |||
| R | ~ | 2.0 | ||||||||||||||
| Shinkai | 2002 | R | F344 | T9 | Suspension | Thigh | 11 | ~675–1300 | TC | Thermotron RF-IV | 2.0 | 8.00 | 40.0–60.0 | 41.0 | 20 | [150] |
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Scutigliani, E.M.; Liang, Y.; Crezee, J.; Kanaar, R.; Krawczyk, P.M. Modulating the Heat Stress Response to Improve Hyperthermia-Based Anticancer Treatments. Cancers 2021, 13, 1243. [Google Scholar] [CrossRef]
- Dunne, M.; Regenold, M.; Allen, C. Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy. Adv. Drug Deliv. Rev. 2020, 163–164, 98–124. [Google Scholar] [CrossRef]
- Lee, S.Y.; Fiorentini, G.; Szasz, A.M.; Szigeti, G.; Szasz, A.; Minnaar, C.A. Quo Vadis Oncological Hyperthermia (2020)? Front. Oncol. 2020, 10, 1690. [Google Scholar] [CrossRef] [PubMed]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; Ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020. [Google Scholar] [CrossRef]
- Haveman, J.; Sminia, P.; Wondergem, J.; van der Zee, J.; Hulshof, M.C. Effects of hyperthermia on the central nervous system: What was learnt from animal studies? Int. J. Hyperth. 2005, 21, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J. The mouse thermoregulatory system: Its impact on translating biomedical data to humans. Physiol. Behav. 2017, 179, 55–66. [Google Scholar] [CrossRef]
- Yarmolenko, P.S.; Moon, E.J.; Landon, C.; Manzoor, A.; Hochman, D.W.; Viglianti, B.L.; Dewhirst, M.W. Thresholds for thermal damage to normal tissues: An update. Int. J. Hyperth. 2011, 27, 320–343. [Google Scholar] [CrossRef] [PubMed]
- Hunter, K.W.; Amin, R.; Deasy, S.; Ha, N.H.; Wakefield, L. Genetic insights into the morass of metastatic heterogeneity. Nat. Rev. Cancer 2018, 18, 211–223. [Google Scholar] [CrossRef] [Green Version]
- Sant, S.; Johnston, P.A. The production of 3D tumor spheroids for cancer drug discovery. Drug Discov. Today Technol. 2017, 23, 27–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rofstad, E.K.; Midthjell, H.; Brustad, T. Heat Sensitivity and Thermotolerance in Cells from Five Human Melanoma Xenografts. Cancer Res. 1984, 44, 4347–4354. [Google Scholar] [PubMed]
- Kalamida, D.; Karagounis, I.V.; Mitrakas, A.; Kalamida, S.; Giatromanolaki, A.; Koukourakis, M.I. Fever-range hyperthermia vs. hypothermia effect on cancer cell viability, proliferation and HSP90 expression. PLoS ONE 2015, 10, e0116021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, D.; Mittal, V.; Ban, Y.; Lourenco, A.R.; Yomtoubian, S.; Lee, S. Metastatic tumor cells-genotypes and phenotypes. Front. Biol. 2018, 13, 277–286. [Google Scholar] [CrossRef]
- Santos, N.P.; Colaco, A.A.; Oliveira, P.A. Animal models as a tool in hepatocellular carcinoma research: A Review. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickson, J.A. The effects of hyperthermia in animal tumour systems. In Selective Heat Sensitivity of Cancer Cells; Springer: Berlin/Heidelberg, Germany, 1977. [Google Scholar]
- Peller, M.; Schwerdt, A.; Hossann, M.; Reinl, H.M.; Wang, T.; Sourbron, S.; Ogris, M.; Lindner, L.H. MR Characterization of Mild Hyperthermia-Induced Gadodiamide Release from Thermosensitive Liposomes in Solid Tumors. Investig. Radiol. 2008, 43, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, T.; Wu, X.; Li, L.; Tan, L.; Chen, D.; Tang, F. Targeting gold nanoshells on silica nanorattles: A drug cocktail to fight breast tumors via a single irradiation with near-infrared laser light. Adv. Mater. 2012, 24, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, S.; Greco, A.; Gramanzini, M.; Esposito, S.; Affuso, A.; Brunetti, A.; Vesce, G. Mice Anesthesia, Analgesia, and Care, Part I: Anesthetic Considerations in Preclinical Research. ILAR J. 2012, 53, E55–E69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alieva, M.; Ritsma, L.; Giedt, R.J.; Weissleder, R.; van Rheenen, J. Imaging windows for long-term intravital imaging: General overview and technical insights. Intravital 2014, 3, e29917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tremoleda, J.L.; Kerton, A.; Gsell, W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: Considerations on experimental outcomes and animal welfare. EJNMMI Res. 2012, 2, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Hohlbaum, K.; Bert, B.; Dietze, S.; Palme, R.; Fink, H.; Thone-Reineke, C. Severity classification of repeated isoflurane anesthesia in C57BL/6JRj mice-Assessing the degree of distress. PLoS ONE 2017, 12, e0179588. [Google Scholar] [CrossRef]
- van Zutphen, L.F.M.; Baumans, V.; Beynen, A.C. Chapter 15—Anaesthesia, analgesia, and euthanasia. In Principles of Laboratory Animal Science; Elsevier: Amsterdam, The Netherlands, 2001; pp. 277–311. [Google Scholar]
- O’Hara, M.D.; Hetzel, F.W.; Frinak, S. Thermal distributions in a water bath heated mouse tumor. Int. J. Radiat. Oncol. Biol. Phys. 1985, 11, 817–822. [Google Scholar] [CrossRef]
- van Zutphen, L.F.M.; Baumans, V.; Beynen, A.C. Chapter 3—Biology and husbandry of laboratory animals. In Principles of Laboratory Animal Science; Elsevier: Amsterdam, The Netherlands, 2001; pp. 19–76. [Google Scholar]
- Yatvin, M.B.; Mühlensiepen, H.; Porschen, W.; Weinstein, J.N.; Feinendegen, L.E. Selective delivery of liposome-associated cis-dichlorodiammineplatinum(II) by heat and its influence on tumor drug uptake and growth. Cancer Res. 1981, 41, 1602–1607. [Google Scholar] [PubMed]
- Bleehen, N.M.; Honess, D.J.; Morgan, J.E. Interaction of hyperthermia and the hypoxic cell sensitizer Ro-07-0582 on the EMT6 mouse tumour. Br. J. Cancer 1977, 35, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cope, D.A.; Dewhirst, M.W.; Friedman, H.S.; Bigner, D.D.; Zalutsky, M.R. Enhanced delivery of a monoclonal antibody fragment to subcutaneous human glioma xenografts using local hyperthermia. Cancer Res. 1990, 50, 1803–1809. [Google Scholar] [PubMed]
- Lokerse, W.J.; Kneepkens, E.C.; ten Hagen, T.L.; Eggermont, A.M.; Grull, H.; Koning, G.A. In depth study on thermosensitive liposomes: Optimizing formulations for tumor specific therapy and in vitro to in vivo relations. Biomaterials 2016, 82, 138–150. [Google Scholar] [CrossRef]
- Foxley, S.; Fan, X.; River, J.; Zamora, M.; Markiewicz, E.; Sokka, S.; Karczmar, G.S. Hyperthermically induced changes in high spectral and spatial resolution MR images of tumor Tissue—A pilot study. Phys. Med. Biol. 2012, 57, 2653–2666. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, Y.; Hiraoka, M.; Jo, S.; Akuta, K.; Yukawa, Y.; Shibamoto, Y.; Takahashi, M.; Abe, M. Microangiographic and histologic analysis of the effects of hyperthermia on murine tumor vasculature. Int. J. Radiat. Oncol. 1988, 15, 411–420. [Google Scholar] [CrossRef]
- Masunaga, S.I.; Tano, K.; Nakamura, J.; Watanabe, M.; Kashino, G.; Suzuki, M.; Kinashi, Y.; Ono, K. Adverse effect of mild temperature hyperthermia combined with hexamethylenetetramine compared to its effect combined with tirapazamine in the treatment of solid tumors. Exp. Ther. Med. 2010, 1, 169–174. [Google Scholar] [CrossRef]
- Ware, M.J.; Krzykawska-Serda, M.; Chak-Shing Ho, J.; Newton, J.; Suki, S.; Law, J.; Nguyen, L.; Keshishian, V.; Serda, M.; Taylor, K.; et al. Optimizing non-invasive radiofrequency hyperthermia treatment for improving drug delivery in 4T1 mouse breast cancer model. Sci. Rep. 2017, 7, 43961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauck, M.L.; Dewhirst, M.W.; Bigner, D.D.; Zalutsky, M.R. Local Hyperthermia Improves Uptake of a Chimeric Monoclonal Antibody in a Subcutaneous Xenograft Model. Clin. Cancer Res. 1997, 3, 63–70. [Google Scholar]
- Schuster, J.M.; Zalutsky, M.R.; Noska, M.A.; Dodge, R.; Friedman, H.S.; Bigner, D.D.; Dewhirst, M.W. Hyperthermic modulation of radiolabelled antibody uptake in a human glioma xenograft and normal tissues. Int. J. Hyperth. 1995, 11, 59–72. [Google Scholar] [CrossRef]
- Zaltieri, M.; Massaroni, C.; Cauti, F.M.; Schena, E. Techniques for Temperature Monitoring of Myocardial Tissue Undergoing Radiofrequency Ablation Treatments: An Overview. Sensors 2021, 21, 1453. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Brezovich, I. Error Sources Affecting Thermocouple Thermometry in RF Electromagnetic Fields. J. Microw. Power 1982, 17, 17–28. [Google Scholar] [CrossRef]
- Schena, E.; Saccomandi, P.; Massaroni, C.; Frauenfelder, G.; Giurazza, F.; Peroglio, G.M.; Polimadei, A. Thermocouples for temperature monitoring during pancreatic laser ablation: Analysis of the measurement error. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, Turin, Italy, 7–9 May 2015; pp. 219–223. [Google Scholar]
- Curto, S.; Faridi, P.; Shrestha, T.B.; Pyle, M.; Maurmann, L.; Troyer, D.; Bossmann, S.H.; Prakash, P. An integrated platform for small-animal hyperthermia investigations under ultra-high-field MRI guidance. Int. J. Hyperth. 2018, 34, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Dicheva, B.M.K.; Koning, G.A. Targeted thermosensitive liposomes: An attractive novel approach for increased drug delivery to solid tumors. Expert Opin. Drug Deliv. 2014, 11, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Priest, J. Temperature and Its Measurement. In Encyclopedia of Energy; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 45–54. [Google Scholar]
- Kok, H.P.; Cressman, E.N.K.; Ceelen, W.; Brace, C.L.; Ivkov, R.; Grull, H.; Ter Haar, G.; Wust, P.; Crezee, J. Heating technology for malignant tumors: A review. Int. J. Hyperth. 2020, 37, 711–741. [Google Scholar] [CrossRef]
- Willerding, L.; Limmer, S.; Hossann, M.; Zengerle, A.; Wachholz, K.; Ten Hagen, T.L.; Koning, G.A.; Sroka, R.; Lindner, L.H.; Peller, M. Method of hyperthermia and tumor size influence effectiveness of doxorubicin release from thermosensitive liposomes in experimental tumors. J. Control. Release 2016, 222, 47–55. [Google Scholar] [CrossRef]
- Copelan, A.; Hartman, J.; Chehab, M.; Venkatesan, A.M. High-Intensity Focused Ultrasound: Current Status for Image-Guided Therapy. Semin. Interv. Radiol 2015, 32, 398–415. [Google Scholar] [CrossRef] [Green Version]
- Lokerse, W.J.; Bolkestein, M.; Ten Hagen, T.L.; de Jong, M.; Eggermont, A.M.; Grull, H.; Koning, G.A. Investigation of Particle Accumulation, Chemosensitivity and Thermosensitivity for Effective Solid Tumor Therapy Using Thermosensitive Liposomes and Hyperthermia. Theranostics 2016, 6, 1717–1731. [Google Scholar] [CrossRef]
- Lu, T.; Lokerse, W.J.M.; Seynhaeve, A.L.B.; Koning, G.A.; Ten Hagen, T.L.M. Formulation and optimization of idarubicin thermosensitive liposomes provides ultrafast triggered release at mild hyperthermia and improves tumor response. J. Control. Release 2015, 220, 425–437. [Google Scholar] [CrossRef]
- Dicheva, B.M.; Seynhaeve, A.L.; Soulie, T.; Eggermont, A.M.; Ten Hagen, T.L.; Koning, G.A. Pharmacokinetics, Tissue Distribution and Therapeutic Effect of Cationic Thermosensitive Liposomal Doxorubicin Upon Mild Hyperthermia. Pharm. Res. 2015, 33, 627–638. [Google Scholar] [CrossRef] [Green Version]
- Manzoor, A.A.; Lindner, L.H.; Landon, C.D.; Park, J.Y.; Simnick, A.J.; Dreher, M.R.; Das, S.; Hanna, G.; Park, W.; Chilkoti, A.; et al. Overcoming limitations in nanoparticle drug delivery: Triggered, intravascular release to improve drug penetration into tumors. Cancer Res. 2012, 72, 5566–5575. [Google Scholar] [CrossRef] [Green Version]
- Kong, G.; Anyarambhatla, G.; Petros, W.P.; Braun, R.D.; Colvin, O.M.; Needham, D.; Dewhirst, M.W. Efficacy of liposomes and hyperthermia in a human tumor xenograft model: Importance of triggered drug release. Cancer Res. 2000, 60, 6950–6957. [Google Scholar]
- Rofstad, E.K.; Falkvoll, K.H.; Oftedal, P. Micronucleus Formation in Human Melanoma Xenografts Following Exposure to Hyperthermia. Radiat. Environ. Biophys. 1984, 23, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Ono, K.; Hiraoka, M.; Masunaga, S.-i.; Jo, S.; Shibamoto, Y.; Sasai, K.; Abe, M.; Iga, K.; Ogawa, Y. Treatment of Murine SCC VII Tumors with Localized Hyperthermia and Temperature-Sensitive Liposomes Containing Cisplatin. Radiat. Res. 1990, 122, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, F.A., Jr.; Peck, J.W.; Dethlefsen, L.A. The Importance of Intratumor Temperature Uniformity in the Study of Radiosensitizing Effects of Hyperthermia in Vivo. Radiat. Res. 1981, 87, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Locke, J.; Zeug, A.; Thompson, D., Jr.; Allan, J.; Mazzarella, K.; Novak, P.; Hanson, D.; Singh, A.K.; Moros, E.G.; Pandita, T.K. Localized versus regional hyperthermia: Comparison of xenotransplants treated with a small animal ultrasound system and waterbath limb immersion. Int. J. Hyperth. 2005, 21, 271–281. [Google Scholar] [CrossRef]
- Adibzadeh, F.; Paulides, M.M.; van Rhoon, G.C. SAR thresholds for electromagnetic exposure using functional thermal dose limits. Int. J. Hyperth. 2018, 34, 1248–1254. [Google Scholar] [CrossRef]
- Tungjitkusolmun, S.; Staelin, S.T.; Haemmerich, D.; Tsai, J.-Z.; Cao, H.; Webster, J.G.; Lee, F.T., Jr.; Mahvi, D.M.; Vorperian, V.R. Three-Dimensional Finite-Element Analyses for Radio-Frequency Hepatic Tumor Ablation. IEEE Trans. Biomed. Eng. 2002, 49, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Ware, M.J.; Nguyen, L.P.; Law, J.J.; Krzykawska-Serda, M.; Taylor, K.M.; Cao, H.S.T.; Anderson, A.O.; Pulikkathara, M.; Newton, J.M.; Ho, J.C.; et al. A new mild hyperthermia device to treat vascular involvement in cancer surgery. Sci. Rep. 2017, 7, 11299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horsman, M.R.; Vaupel, P. Pathophysiological Basis for the Formation of the Tumor Microenvironment. Front. Oncol. 2016, 6, 66. [Google Scholar] [CrossRef] [Green Version]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance induced at a mild temperature of 40 degrees C alleviates heat shock-induced ER stress and apoptosis in HeLa cells. Biochim. Biophys. Acta 2015, 1853, 52–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumser, K.; Bellizzi, G.G.; van Rhoon, G.C.; Paulides, M.M. The Potential of Adjusting Water Bolus Liquid Properties for Economic and Precise MR Thermometry Guided Radiofrequency Hyperthermia. Sensors 2020, 20, 2946. [Google Scholar] [CrossRef] [PubMed]
- Crile, G., Jr. Heat as an adjunct to the treatment of cancer: Experimental Studies. Clevel. Clin. Quart 1961, 28, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Stone, H.B. Enhancement of local tumour control by misonidazole and hyperthermia. Br. J. Cancer 1978, 3, 178–183. [Google Scholar]
- Overgaard, J. Simultaneous and sequential hyperthermia and radiation treatment of an experimental tumor and its surrounding normal tissue in vivo. Radiat. Oncol. Biol. Phys. 1980, 6, 1507–1517. [Google Scholar] [CrossRef]
- Rofstad, E.K.; Brustad, T. Effect of hyperthermia on human melanoma cells heated either as solid tumors in athymic nude mice or in vitro. Cancer 1982, 50, 1304–1308. [Google Scholar] [CrossRef] [Green Version]
- Joiner, M.C.; Steel, G.G.; Stephens, T.C. Response of two mouse tumours to hyperthermia with CCNU or melphalan. Br. J. Cancer 1982, 45, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, K. Application of heat to cancer chemotherapy. Nagoya J. Med. Sci. 1967, 30, 1–21. [Google Scholar]
- Calderwood, S.K.; Dickson, J.A. Influence of tumour volume and cell kinetics on the response of the solid Yoshida sarcoma to hyperthermia. Br. J. Cancer 1980, 41, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Dahl, O. Effect of hyperthermia on a neurogenic rat cell line (BT4A) in vivo. Acta Radiol Oncol. 1982, 21, 67–77. [Google Scholar] [CrossRef]
- Wheldon, T.E.; Hingston, E.C. Differential effect of hyperthermia and x-irradiation on regrowth rate and tumour-bed effect for a rat sarcoma. Br. J. Cancer 1982, 45, 265–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Zee, J.; Van den Aardweg, G.J.M.J.; Van Rhoon, G.C.; Van den Berg, A.P.; De Wit, R. Thermal enhancement of both tumour necrosis factor alpha-induced systemic toxicity and tumour cure in rats. Br. J. Cancer 1995, 71, 1158–1162. [Google Scholar] [CrossRef] [Green Version]
- Moroi, J.; Kashiwagi, S.; Kim, S.; Urakawa, M.; Ito, H.; Yamaguchi, K. Regional differences in apoptosis in murine gliosarcoma (T9) induced by mild hyperthermia. Int. J. Hyperth. 1996, 12, 345–354. [Google Scholar] [CrossRef]
- Derieppe, M.; Escoffre, J.M.; Denis de Senneville, B.; van Houtum, Q.; Rijbroek, A.B.; van der Wurff-Jacobs, K.; Dubois, L.; Bos, C.; Moonen, C. Assessment of Intratumoral Doxorubicin Penetration after Mild Hyperthermia-Mediated Release from Thermosensitive Liposomes. Contrast Media Mol. Imaging 2019, 2019, 2645928. [Google Scholar] [CrossRef]
- Leunig, M.; Goetz, A.E.; Dellian, M.; Zetterer, G.; Gamarra, F.; Jain, R.K.; Messmer, K. Interstitial Fluid Pressure in Solid Tumors following Hyperthermia: Possible Correlation with Therapeutic Response. Cancer Res. 1992, 52, 487–490. [Google Scholar]
- Dellian, M.; Walenta, S.; Kuhnle, G.E.; Gamarra, F.; Mueller-Klieser, W.; Goetz, A.E. Relation between autoradiographically measured blood flow and ATP concentrations obtained from imaging bioluminescence in tumors following hyperthermia. Int. J. Cancer 1993, 53, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Pahernik, S.A.; Peller, M.; Dellian, M.; Loeffler, R.; Issels, R.; Reiser, M.; Messmer, K.; Goetz, A.E. Validation of MR Thermometry Technology: A Small Animal Model for Hyperthermic Treatment of Tumours. Res. Exp. Med. 1999, 199, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A.; Iluri, N.; Szasz, O. Local Hyperthermia in Oncology—To Choose or not to Choose? In Hyperthermia; Huilgol, N., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Von Ardenne, A.; Wehner, H. Extreme Whole-Body Hyperthermia with Water-Filtered Infrared-A Radiation. In Hyperthermia in Cancer Treatment: A Primer; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Kelleher, D.K.; Engel, T.; Vaupel, P.W. Changes in microregional perfusion, oxygenation, ATP and lactate distribution in subcutaneous rat tumours upon water-filtered IR-A hyperthermia. Int. J. Hyperth. 1995, 11, 241–255. [Google Scholar] [CrossRef]
- Vaupel, P.; Piazena, H.; Muller, W.; Notter, M. Biophysical and photobiological basics of water-filtered infrared-A hyperthermia of superficial tumors. Int. J. Hyperth. 2018, 35, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.; Lambert, C.; Biesalski, H.K.; Thews, O.; Vaupel, P.; Kelleher, D.K. Intensified oxidative and nitrosative stress following combined ALA-based photodynamic therapy and local hyperthermia in rat tumors. Int. J. Cancer 2003, 107, 941–948. [Google Scholar] [CrossRef]
- Cabuy, E. Hyperthermia in cancer treatment. Reliab. Cancer Ther. Energy-Based Ther. 2011, 1, 1–48. [Google Scholar]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, 5007–5008. [Google Scholar] [CrossRef]
- Limmer, S.; Hahn, J.; Schmidt, R.; Wachholz, K.; Zengerle, A.; Lechner, K.; Eibl, H.; Issels, R.D.; Hossann, M.; Lindner, L.H. Gemcitabine treatment of rat soft tissue sarcoma with phosphatidyldiglycerol-based thermosensitive liposomes. Pharm. Res. 2014, 31, 2276–2286. [Google Scholar] [CrossRef] [PubMed]
- Sien, H.P.; Jain, R.K. Intratumour temperature distributions during hyperthermia. J. Therm. Biol. 1980, 5, 127–130. [Google Scholar] [CrossRef]
- Lilge, L.; Tierney, K.; Nussbaum, E. Low-Level Laser Therapy for Wound Healing: Feasibility of Wound Dressing Transillumination. J. Clin. Laser Med. Surg. 2000, 18, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Avraham Dilmanian, F.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomedicine 2014, 10, 1609–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piazena, H.; Muller, W.; Vaupel, P. wIRA-heating of piglet skin and subcutis in vivo: Proof of accordance with ESHO criteria for superficial hyperthermia. Int. J. Hyperth. 2020, 37, 887–896. [Google Scholar] [CrossRef]
- Martens, A.; Moor, A.D.E.; Waelkens, E.; Merlevede, W.; de Witte, P. In vitro and in vivo evaluation of hypericin for photodynamic therapy of equine sarcoids. Vet. J. 2000, 159, 77–84. [Google Scholar] [CrossRef]
- Mallidi, S.; Anbil, S.; Bulin, A.L.; Obaid, G.; Ichikawa, M.; Hasan, T. Beyond the Barriers of Light Penetration: Strategies, Perspectives and Possibilities for Photodynamic Therapy. Theranostics 2016, 6, 2458–2487. [Google Scholar] [CrossRef] [Green Version]
- Mandel, L.; Wolf, E. Optical Coherence and Quantum Optics; Cambridge University Press: Cambridge, UK, 1995. [Google Scholar]
- Sciammarella, C.A.; Sciammarella, F.M. Optical Methods—Interference and Diffraction of Light. In Experimtental Mechanics of Solids; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Piazena, H.; Muller, W.; Pendl, W.; von Ah, S.; Cap, V.H.; Hug, P.J.; Sidler, X.; Pluschke, G.; Vaupel, P. Thermal field formation during wIRA-hyperthermia: Temperature measurements in skin and subcutis of piglets as a basis for thermotherapy of superficial tumors and local skin infections caused by thermosensitive microbial pathogens. Int. J. Hyperth. 2019, 36, 938–952. [Google Scholar] [CrossRef]
- Kelleher, D.K.; Thews, O.; Rzeznik, J.; Scherz, A.; Salomon, Y.; Vaupel, P. Water-filtered infrared-A radiation: A novel technique for localized hyperthermia in combination with bacteriochlorophyll-based photodynamic therapy. Int. J. Hyperth. 1999, 15, 467–474. [Google Scholar]
- Ickenstein, L.M. Triggered Drug Release from Thermosensitive Liposomes; The University of British Columbia: Vancouver, BC, Canada, 2003. [Google Scholar]
- Di Costanzo, G.G.; Francica, G.; Pacella, C.M. Laser ablation for small hepatocellular carcinoma: State of the art and future perspectives. World J. Hepatol. 2014, 6, 704–715. [Google Scholar] [CrossRef]
- Tang, F.; Zhang, Y.; Zhang, J.; Guo, J.; Liu, R. Assessment of the efficacy of laser hyperthermia and nanoparticle-enhanced therapies by heat shock protein analysis. AIP Adv. 2014, 4, 031334. [Google Scholar] [CrossRef]
- Huff, T.B.; Tong, L.; Zhao, Y.; Hansen, M.N.; Cheng, J.X.; Wei, A. Hyperthermic effects of gold nanorods on tumor cells. Nanomedicine 2007, 2, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, E.; Alonso-de Castro, S.; Habtemariam, A.; Salassa, L. Upconverting nanoparticles for the near infrared photoactivation of transition metal complexes: New opportunities and challenges in medicinal inorganic photochemistry. Dalton Trans. 2016, 45, 13012–13020. [Google Scholar] [CrossRef] [Green Version]
- Stolik, S.; Delgado, J.A.; Pérez, A.; Anasagasti, L. Measurement of the penetration depths of red and near infrared light in human “ex vivo” tissues. J. Photochem. Photobiol. B Biol. 2000, 57, 90–93. [Google Scholar] [CrossRef]
- Teraphongphom, N.; Kong, C.S.; Warram, J.M.; Rosenthal, E.L. Specimen mapping in head and neck cancer using fluorescence imaging. Laryngoscope Investig. Otolaryngol. 2017, 2, 447–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morscher, S.; Driessen, W.H.; Claussen, J.; Burton, N.C. Semi-quantitative Multispectral Optoacoustic Tomography (MSOT) for volumetric PK imaging of gastric emptying. Photoacoustics 2014, 2, 103–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panjehpour, M.; Wilke, A.V.; Frazier, D.L.; Overholt, B.F. Nd:YAG Laser Hyperthermia Treatment of Rat Mammary Adenocarcinoma in Conjunction With Surface Cooling. Lasers Surg. Med. 1991, 11, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Peller, M.; Willerding, L.; Limmer, S.; Hossann, M.; Dietrich, O.; Ingrisch, M.; Sroka, R.; Lindner, L.H. Surrogate MRI markers for hyperthermia-induced release of doxorubicin from thermosensitive liposomes in tumors. J. Control. Release 2016, 237, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.N.; Weersink, R.A.; Foltz, W.D.; Zheng, J.; Chaudary, N.; Jaffray, D.A.; Allen, C. Custom-designed Laser-based Heating Apparatus for Triggered Release of Cisplatin from Thermosensitive Liposomes with Magnetic Resonance Image Guidance. J. Vis. Exp. 2015, 106, e53055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dou, Y.N.; Zheng, J.; Foltz, W.D.; Weersink, R.; Chaudary, N.; Jaffray, D.A.; Allen, C. Heat-activated thermosensitive liposomal cisplatin (HTLC) results in effective growth delay of cervical carcinoma in mice. J. Control. Release 2014, 178, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, S.; Dunne, M.; Milosevic, M.; Tran, C.W.; Gold, M.J.; Vedadi, A.; McKee, T.D.; Ohashi, P.S.; Allen, C.; Jaffray, D.A. Radiation and Heat Improve the Delivery and Efficacy of Nanotherapeutics by Modulating Intratumoral Fluid Dynamics. ACS Nano 2018, 12, 7583–7600. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.; Epp-Ducharme, B.; Sofias, A.M.; Regenold, M.; Dubins, D.N.; Allen, C. Heat-activated drug delivery increases tumor accumulation of synergistic chemotherapies. J. Control. Release 2019, 308, 197–208. [Google Scholar] [CrossRef]
- Waldow, S.M.; Morrison, P.R.; Grossweiner, L.I. Nd:YAG Laser-Induced Hyperthermia in a Mouse Tumor Model. Lasers Surg. Med. 1988, 8, 510–514. [Google Scholar] [CrossRef]
- Brace, C. Thermal tumor ablation in clinical use. IEEE Pulse 2011, 2, 28–38. [Google Scholar] [CrossRef] [Green Version]
- Pereira Gomes, I.; Aparecida Duarte, J.; Chaves Maia, A.L.; Rubello, D.; Townsend, D.M.; Branco de Barros, A.L.; Leite, E.A. Thermosensitive Nanosystems Associated with Hyperthermia for Cancer Treatment. Pharmaceuticals 2019, 12, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnes, K.D.; Shafirstein, G.; Webber, J.S.; Koonce, N.A.; Harris, Z.; Griffin, R.J. Hyperthermia-enhanced indocyanine green delivery for laser-induced thermal ablation of carcinomas. Int. J. Hyperth. 2013, 29, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Kirui, D.K.; Koay, E.J.; Guo, X.; Cristini, V.; Shen, H.; Ferrari, M. Tumor vascular permeabilization using localized mild hyperthermia to improve macromolecule transport. Nanomedicine 2014, 10, 1487–1496. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Lu, Z.; Zhu, X.; Wang, X.; Liao, Y.; Ma, Z.; Li, F. NIR photothermal therapy using polyaniline nanoparticles. Biomaterials 2013, 34, 9584–9592. [Google Scholar] [CrossRef]
- Grull, H.; Langereis, S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J. Control. Release 2012, 161, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Poorman, M.E.; Chaplin, V.L.; Wilkens, K.; Dockery, M.D.; Giorgio, T.D.; Grissom, W.A.; Caskey, C.F. Open-source, small-animal magnetic resonance-guided focused ultrasound system. J. Ther. Ultrasound 2016, 4, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haemmerich, D. Non-invasive image-guided targeted drug delivery. Lancet Oncol. 2018, 19, 1000–1001. [Google Scholar] [CrossRef] [Green Version]
- Staruch, R.; Chopra, R.; Hynynen, K. Localised drug release using MRI-controlled focused ultrasound hyperthermia. Int. J. Hyperth. 2011, 27, 156–171. [Google Scholar] [CrossRef]
- Tempany, C.M.; McDannold, N.J.; Hynynen, K.; Jolesz, F.A. Focused ultrasound surgery in oncology: Overview and principles. Radiology 2011, 259, 39–56. [Google Scholar] [CrossRef]
- Khokhlova, T.D.; Wang, Y.N.; Simon, J.C.; Cunitz, B.W.; Starr, F.; Paun, M.; Crum, L.A.; Bailey, M.R.; Khokhlova, V.A. Ultrasound-guided tissue fractionation by high intensity focused ultrasound in an in vivo porcine liver model. Proc. Natl. Acad Sci. USA 2014, 111, 8161–8166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.C.; Chen, W.S.; Ho, M.C.; Huang, K.W.; Chen, C.N.; Yen, J.Y.; Lee, P.H. Minimizing abdominal wall damage during high-intensity focused ultrasound ablation by inducing artificial ascites. J. Acoust. Soc. Am. 2008, 124, 674–679. [Google Scholar] [CrossRef]
- Shung, K.K. The principle of multidimensional arrays. Eur. J. Echocardiogr. 2002, 3, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Dromi, S.; Frenkel, V.; Luk, A.; Traughber, B.; Angstadt, M.; Bur, M.; Poff, J.; Xie, J.; Libutti, S.K.; Li, K.C.; et al. Pulsed-high intensity focused ultrasound and low temperature-sensitive liposomes for enhanced targeted drug delivery and antitumor effect. Clin. Cancer Res. 2007, 13, 2722–2727. [Google Scholar] [CrossRef] [Green Version]
- Patel, P.R.; Luk, A.; Durrani, A.; Dromi, S.; Cuesta, J.; Angstadt, M.; Dreher, M.R.; Wood, B.J.; Frenkel, V. In vitro and in vivo evaluations of increased effective beam width for heat deposition using a split focus high intensity ultrasound (HIFU) transducer. Int. J. Hyperth. 2008, 24, 537–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seip, R.; Sanghvi, N.T.; Uchida, T.; Umemura, S.I. Comparison of split-beam transducer geometries and excitation configurations for transrectal prostate HIFU treatments. In Proceedings of the 2001 IEEE Ultrasonics Symposium Proceedings an International Symposium (Cat. No.01CH37263), Atlanta, GA, USA, 7–10 October 2001; pp. 1343–1346. [Google Scholar]
- Lee, F.F.; He, Q.; Gao, J.; Pan, A.; Sun, S.; Liang, X.; Luo, J. Evaluating HIFU-mediated local drug release using thermal strain imaging: Phantom and preliminary in-vivo studies. Med. Phys. 2019, 46, 3864–3876. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Swanevelder, J. Resolution in ultrasound imaging. Contin. Educ. Anaesth. Crit. Care Pain 2011, 11, 186–192. [Google Scholar] [CrossRef]
- Karmacharya, M.B.; Sultan, L.R.; Hunt, S.J.; Sehgal, C.M. Hydralazine augmented ultrasound hyperthermia for the treatment of hepatocellular carcinoma. Sci. Rep. 2021, 11, 15553. [Google Scholar] [CrossRef]
- van Rhoon, G.C.; Franckena, M.; Ten Hagen, T.L.M. A moderate thermal dose is sufficient for effective free and TSL based thermochemotherapy. Adv. Drug Deliv. Rev. 2020, 163–164, 145–156. [Google Scholar] [CrossRef]
- Chae, S.Y.; Kim, Y.S.; Park, M.J.; Yang, J.; Park, H.; Namgung, M.S.; Rhim, H.; Lim, H.K. High-intensity focused ultrasound-induced, localized mild hyperthermia to enhance anti-cancer efficacy of systemic doxorubicin: An experimental study. Ultrasound Med. Biol. 2014, 40, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Yonetsuji, T.; Ando, T.; Wang, J.; Fujiwara, K.; Itani, K.; Azuma, T.; Yoshinaka, K.; Sasaki, A.; Takagi, S.; Kobayashi, E.; et al. A novel high intensity focused ultrasound robotic system for breast cancer treatment. Med. Image Comput. Comput. Assist Interv. 2013, 16, 388–395. [Google Scholar] [PubMed]
- Cha, J.M.; You, D.G.; Choi, E.J.; Park, S.J.; Um, W.; Jeon, J.; Kim, K.; Kwon, I.C.; Park, J.C.; Kim, H.R.; et al. Improvement of Antitumor Efficacy by Combination of Thermosensitive Liposome with High-Intensity Focused Ultrasound. J. Biomed. NanoTechnol. 2016, 12, 1724–1733. [Google Scholar] [CrossRef]
- Rizzitelli, S.; Giustetto, P.; Faletto, D.; Delli Castelli, D.; Aime, S.; Terreno, E. The release of Doxorubicin from liposomes monitored by MRI and triggered by a combination of US stimuli led to a complete tumor regression in a breast cancer mouse model. J. Control. Release 2016, 230, 57–63. [Google Scholar] [CrossRef] [Green Version]
- de Smet, M.; Heijman, E.; Langereis, S.; Hijnen, N.M.; Grull, H. Magnetic resonance imaging of high intensity focused ultrasound mediated drug delivery from temperature-sensitive liposomes: An in vivo proof-of-concept study. J. Control. Release 2011, 150, 102–110. [Google Scholar] [CrossRef]
- Hijnen, N.; Kneepkens, E.; de Smet, M.; Langereis, S.; Heijman, E.; Grull, H. Thermal combination therapies for local drug delivery by magnetic resonance-guided high-intensity focused ultrasound. Proc. Natl. Acad Sci. USA 2017, 114, E4802–E4811. [Google Scholar] [CrossRef] [Green Version]
- Hijnen, N.M.; Heijman, E.; Kohler, M.O.; Ylihautala, M.; Ehnholm, G.J.; Simonetti, A.W.; Grull, H. Tumour hyperthermia and ablation in rats using a clinical MR-HIFU system equipped with a dedicated small animal set-up. Int. J. Hyperth. 2012, 28, 141–155. [Google Scholar] [CrossRef]
- Park, S.M.; Kim, M.S.; Park, S.J.; Park, E.S.; Choi, K.S.; Kim, Y.S.; Kim, H.R. Novel temperature-triggered liposome with high stability: Formulation, in vitro evaluation, and in vivo study combined with high-intensity focused ultrasound (HIFU). J. Control. Release 2013, 170, 373–379. [Google Scholar] [CrossRef]
- Farr, N.; Wang, Y.N.; D’Andrea, S.; Starr, F.; Partanen, A.; Gravelle, K.M.; McCune, J.S.; Risler, L.J.; Whang, S.G.; Chang, A.; et al. Hyperthermia-enhanced targeted drug delivery using magnetic resonance-guided focussed ultrasound: A pre-clinical study in a genetic model of pancreatic cancer. Int. J. Hyperth. 2018, 34, 284–291. [Google Scholar] [CrossRef] [Green Version]
- de Smet, M.; Hijnen, N.M.; Langereis, S.; Elevelt, A.; Heijman, E.; Dubois, L.; Grüll, H. Magnetic Resonance Guided High-Intensity Focused Ultrasound Mediated Hyperthermia Improves the Intratumoral Distribution of Temperature-Sensitive Liposomal Doxorubicin. Investig. Radiol. 2013, 48, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, V.; Etherington, A.; Greene, M.; Quijano, J.; Xie, J.; Hunter, F.; Dromi, S.; Li, K.C. Delivery of liposomal doxorubicin (Doxil) in a breast cancer tumor model: Investigation of potential enhancement by pulsed-high intensity focused ultrasound exposure. Acad. Radiol. 2006, 13, 469–479. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Lai, C.Y.; Tam, S.M.; Mahakian, L.M.; Ingham, E.S.; Watson, K.D.; Ferrara, K.W. Complete regression of local cancer using temperature-sensitive liposomes combined with ultrasound-mediated hyperthermia. J. Control. Release 2013, 172, 266–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centelles, M.N.; Wright, M.; So, P.W.; Amrahli, M.; Xu, X.Y.; Stebbing, J.; Miller, A.D.; Gedroyc, W.; Thanou, M. Image-guided thermosensitive liposomes for focused ultrasound drug delivery: Using NIRF-labelled lipids and topotecan to visualise the effects of hyperthermia in tumours. J. Control. Release 2018, 280, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.S.; Hwang, H.; Oh, P.S.; Kim, E.M.; Lee, T.K.; Kim, M.; Kim, H.S.; Lim, S.T.; Sohn, M.H.; Jeong, H.J. Effect of High-Intensity Focused Ultrasound on Drug Release from Doxorubicin-Loaded PEGylated Liposomes and Therapeutic Effect in Colorectal Cancer Murine Models. Ultrasound Med. Biol. 2016, 42, 947–955. [Google Scholar] [CrossRef]
- Krenacs, T.; Meggyeshazi, N.; Forika, G.; Kiss, E.; Hamar, P.; Szekely, T.; Vancsik, T. Modulated Electro-Hyperthermia-Induced Tumor Damage Mechanisms Revealed in Cancer Models. Int. J. Mol. Sci. 2020, 21, 6270. [Google Scholar] [CrossRef]
- Danics, L.; Schvarcz, C.A.; Viana, P.; Vancsik, T.; Krenacs, T.; Benyo, Z.; Kaucsar, T.; Hamar, P. Exhaustion of Protective Heat Shock Response Induces Significant Tumor Damage by Apoptosis after Modulated Electro-Hyperthermia Treatment of Triple Negative Breast Cancer Isografts in Mice. Cancers 2020, 12, 2581. [Google Scholar] [CrossRef] [PubMed]
- Schvarcz, C.A.; Danics, L.; Krenacs, T.; Viana, P.; Beres, R.; Vancsik, T.; Nagy, A.; Gyenesei, A.; Kun, J.; Fonovic, M.; et al. Modulated Electro-Hyperthermia Induces a Prominent Local Stress Response and Growth Inhibition in Mouse Breast Cancer Isografts. Cancers 2021, 13, 1744. [Google Scholar] [CrossRef]
- Cohen, J.; Anvari, A.; Samanta, S.; Poirier, Y.; Soman, S.; Alexander, A.; Ranjbar, M.; Pavlovic, R.; Zodda, A.; Jackson, I.L.; et al. Mild hyperthermia as a localized radiosensitizer for deep-seated tumors: Investigation in an orthotopic prostate cancer model in mice. Br. J. Radiol. 2019, 92, 20180759. [Google Scholar] [CrossRef]
- Vancsik, T.; Kovago, C.; Kiss, E.; Papp, E.; Forika, G.; Benyo, Z.; Meggyeshazi, N.; Krenacs, T. Modulated electro-hyperthermia induced loco-regional and systemic tumor destruction in colorectal cancer allografts. J. Cancer 2018, 9, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Marmor, J.B.; Hahn, N.; Hahn, G.M. Tumor Cure and Cell Survival after Localized Radiofrequency Heating. Cancer Res. 1977, 37, 879–883. [Google Scholar]
- Andocs, G.; Renner, H.; Balogh, L.; Fonyad, L.; Jakab, C.; Szasz, A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther Onkol. 2009, 185, 120–126. [Google Scholar] [CrossRef]
- McDonald, M.; Corde, S.; Lerch, M.; Rosenfeld, A.; Jackson, M.; Tehei, M. First in vitro evidence of modulated electro-hyperthermia treatment performance in combination with megavoltage radiation by clonogenic assay. Sci. Rep. 2018, 8, 16608. [Google Scholar] [CrossRef] [Green Version]
- Uda, M.; Osborn, J.L.; Lee, C.K.; Nakhleh, R.E.; Song, C.W. Pathophysiological changes after local heating of rat liver. Int. J. Radiat. Oncol. Biol. Phys. 1990, 18, 587–594. [Google Scholar] [CrossRef]
- Habash, R.W.; Bansal, R.; Krewski, D.; Alhafid, H.T. Thermal Therapy, Part 2: Hyperthermia Techniques. Crit. Rev. Biomed. Eng. 2006, 34, 491–542. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, M.; Ueda, K.; Ohtsu, S.; Honda, H.; Kohri, K.; Kobayashi, T. Effect of Functional Magnetic Particles on Radiofrequency Capacitive Heating: An in vivo Study. Jpn. J. Cancer Res. 2002, 93, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Szasz, A.; Szasz, N.; Szasz, O. Oncothermia—A New Kind of Oncologic Hyperthermia. In Oncothermia: Principles and Practices; Springer: Berlin/Heidelberg, Germany, 2011; pp. 173–392. [Google Scholar]
- Salahi, S.; Maccarini, P.F.; Rodrigues, D.B.; Etienne, W.; Landon, C.D.; Inman, B.A.; Dewhirst, M.W.; Stauffer, P.R. Miniature microwave applicator for murine bladder hyperthermia studies. Int. J. Hyperth. 2012, 28, 456–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raaijmakers, E.A.L.; Mestrom, R.M.C.; Sumser, K.; Salim, G.; van Rhoon, G.C.; Essers, J.; Paulides, M.M. An MR-compatible antenna and application in a murine superficial hyperthermia applicator. Int. J. Hyperth. 2018, 34, 697–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulides, M.M.; Dobsicek Trefna, H.; Curto, S.; Rodrigues, D.B. Recent technological advancements in radiofrequency-andmicrowave-mediated hyperthermia for enhancing drug delivery. Adv. Drug Deliv. Rev. 2020, 163, 3–18. [Google Scholar] [CrossRef]
- Szasz, O.; Andocs, G.; Meggyeshazi, N. Modulation Effect in Oncothermia. Conf. Pap. Med. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Andocs, G.; Szasz, O.; Szasz, A. Oncothermia treatment of cancer: From the laboratory to clinic. Electromagn Biol. Med. 2009, 28, 148–165. [Google Scholar] [CrossRef]
- Uchibayashi, T.; Mihara, S.; Hirata, A.; Kinimi, K. Experimental study of radiofrequency capacitive hyperthermia on transplanted tumor. Int. J. Oncol. 1994, 4, 1301–1304. [Google Scholar] [CrossRef]
- Dou, Y.; Hynynen, K.; Allen, C. To heat or not to heat: Challenges with clinical translation of thermosensitive liposomes. J. Control. Release 2017, 249, 63–73. [Google Scholar] [CrossRef] [PubMed]

| Water Bath | Cold-Light Source | Near-Infrared Laser | Focused Ultrasound | Capacitive Hyperthermia | |
|---|---|---|---|---|---|
| Method | Tissue heating via convection and conduction by immersion in water | Visible Light via halogen or wIRA device | High energy laser light | Ultrasound waves heat tissue noninvasively via transducer | Capacitive heating though generation of an electromagnetic field |
| Tumor Position | Superficial (Flank, Breast, or Hind Limb) | Superficial (Flank or Hind Limb) | Superficial (Flank, Hind Limb, or Mammary Fat Pad) | Superficial and Deep (Flank, Abdominal Wall, or Pancreas) | Superficial and Deep (Flank, Mammary Fat Pad, or Prostate) |
| Setting | Water bath temperature | 350–1180 nm | 763–1064 nm | 1.00–1.54 MHz | 8.00 or 13.56 MHz |
| Device |
|
|
|
|
|
| Positioning |
|
| - |
| - |
| Protection | Skin | Skin | Skin | Skin | Skin |
|
|
|
|
| |
| Stage | Stage | Stage | Stage | Stage | |
| - | - |
|
| |
| Side-Effects |
|
|
|
|
|
| Advantages |
|
|
|
|
|
| Disadvantages |
|
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Priester, M.I.; Curto, S.; van Rhoon, G.C.; ten Hagen, T.L.M. External Basic Hyperthermia Devices for Preclinical Studies in Small Animals. Cancers 2021, 13, 4628. https://doi.org/10.3390/cancers13184628
Priester MI, Curto S, van Rhoon GC, ten Hagen TLM. External Basic Hyperthermia Devices for Preclinical Studies in Small Animals. Cancers. 2021; 13(18):4628. https://doi.org/10.3390/cancers13184628
Chicago/Turabian StylePriester, Marjolein I., Sergio Curto, Gerard C. van Rhoon, and Timo L. M. ten Hagen. 2021. "External Basic Hyperthermia Devices for Preclinical Studies in Small Animals" Cancers 13, no. 18: 4628. https://doi.org/10.3390/cancers13184628
APA StylePriester, M. I., Curto, S., van Rhoon, G. C., & ten Hagen, T. L. M. (2021). External Basic Hyperthermia Devices for Preclinical Studies in Small Animals. Cancers, 13(18), 4628. https://doi.org/10.3390/cancers13184628








