Tumour-Derived Cell Lines and Their Potential for Therapy Prediction in Patients with Metastatic Colorectal Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Line Establishment
2.2. Mice and Tumour Xenografting
2.3. Chemosensitivity In Vivo
2.4. Chemosensitivity In Vitro by Crystal Violet Staining
2.5. Colony Formation Assay
2.6. Spheroid Formation Assay
2.7. Next Generation Sequencing Analyses
3. Results
3.1. Patient Characteristics
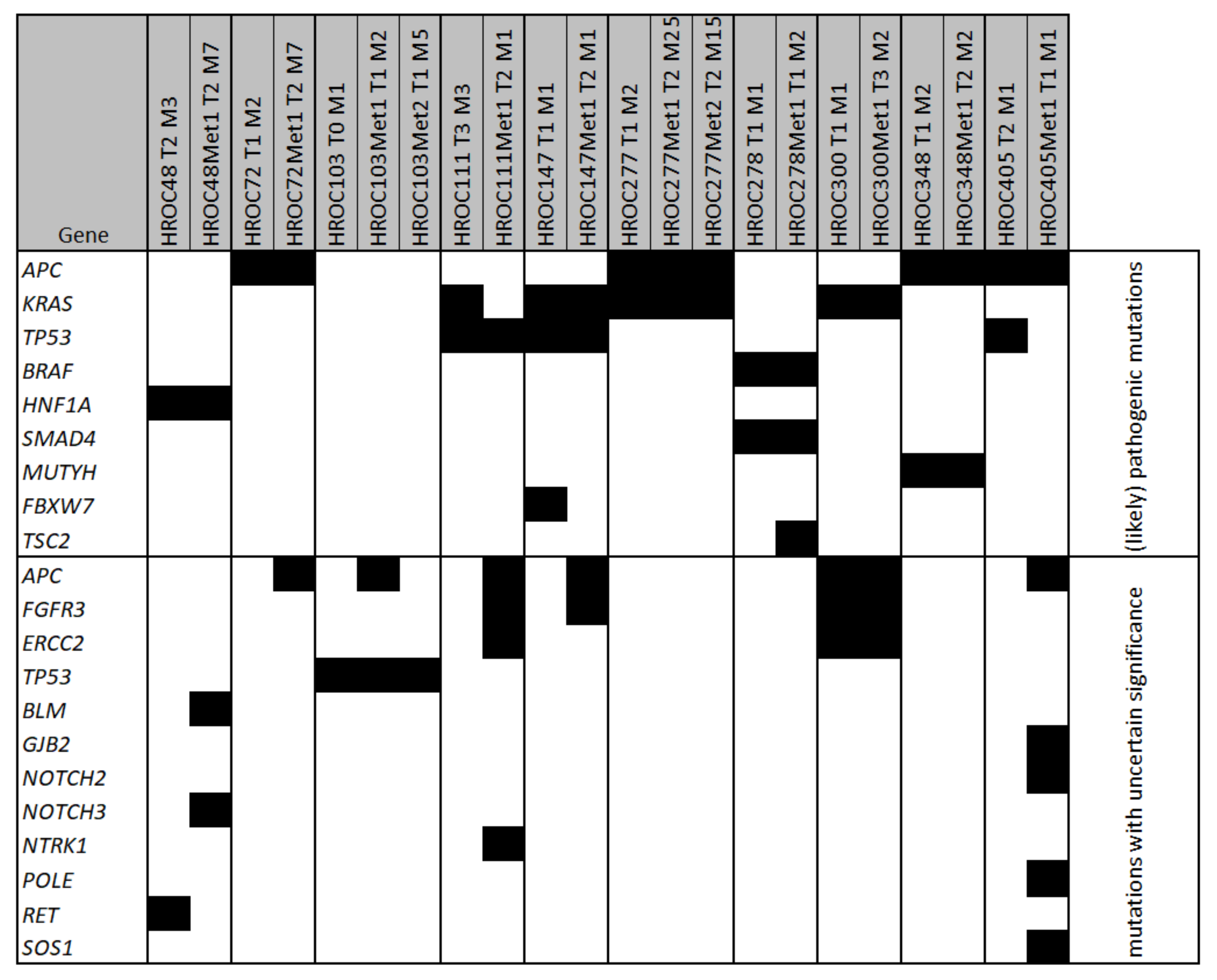
3.2. Molecular Characterisation of Tumour Material
3.3. Morphology and Cell Doubling Time of Selected Cell Lines
3.4. Colony Formation and Spheroid Formation
3.5. Chemosensitivity In Vitro
3.6. Patient HROC277
3.7. Chemosensitivity In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [Green Version]
- Pretzsch, E.; Bösch, F.; Neumann, J.; Ganschow, P.; Bazhin, A.; Guba, M.; Werner, J.; Angele, M. Mechanisms of metastasis in colorectal cancer and metastatic organotropism: Hematogenous versus peritoneal spread. J. Oncol. 2019, 2019, 7407190. [Google Scholar] [CrossRef]
- Leal, F.; Ferreira, F.P.; Sasse, A.D. FOLFOXIRI regimen for metastatic colorectal cancer: A systematic review and meta-analysis. Clin. Colorectal Cancer 2017, 16, 405–409. [Google Scholar] [CrossRef]
- Douillard, J.Y.; Cunningham, D.; Roth, A.D.; Navarro, M.; James, R.D.; Karasek, P.; Jandik, P.; Iveson, T.; Carmichael, J.; Alakl, M.; et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multicentre randomised trial. Lancet 2000, 355, 1041–1047. [Google Scholar] [CrossRef]
- Masi, G.; Allegrini, G.; Cupini, S.; Marcucci, L.; Cerri, E.; Brunetti, I.; Fontana, E.; Ricci, S.; Andreuccetti, M.; Falcone, A. First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): Results of a phase II study with a simplified biweekly schedule. Ann. Oncol. 2004, 15, 1766–1772. [Google Scholar] [CrossRef]
- Falcone, A.; Masi, G.; Allegrini, G.; Danesi, R.; Pfanner, E.; Brunetti, I.M.; Di Paolo, A.; Cupini, S.; Del Tacca, M.; Conte, P. Biweekly chemotherapy with oxaliplatin, irinotecan, infusional Fluorouracil, and leucovorin: A pilot study in patients with metastatic colorectal cancer. J. Clin. Oncol. 2002, 20, 4006–4014. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Goding Sauer, A.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef] [Green Version]
- Longley, D.B.; Johnston, P.G. Molecular mechanisms of drug resistance. J. Pathol. 2005, 205, 275–292. [Google Scholar] [CrossRef]
- Mullins, C.S.; Micheel, B.; Matschos, S.; Leuchter, M.; Bürtin, F.; Krohn, M.; Hühns, M.; Klar, E.; Prall, F.; Linnebacher, M. Integrated biobanking and tumor model establishment of human colorectal carcinoma provides excellent tools for preclinical research. Cancers 2019, 11. [Google Scholar] [CrossRef] [Green Version]
- Mullins, C.S.; Bock, S.; Krohn, M.; Linnebacher, M. Generation of xenotransplants from human cancer biopsies to assess anti-cancer activities of HDACi. Methods Mol. Biol. (Clifton N.J.) 2017, 1510, 217–229. [Google Scholar] [CrossRef]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Ostwald, C.; Linnebacher, M.; Weirich, V.; Prall, F. Chromosomally and microsatellite stable colorectal carcinomas without the CpG island methylator phenotype in a molecular classification. Int. J. Oncol. 2009, 35, 321–327. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Cho, Y.B.; Hong, H.K.; Wu, S.; Ebert, P.J.; Bray, S.M.; Wong, S.S.; Ting, J.C.; Calley, J.N.; Whittington, C.F.; et al. Molecular dissection of CRC primary tumours and their matched liver metastases reveals critical role of immune microenvironment, EMT and angiogenesis in cancer metastasis. Sci. Rep. 2020, 10, 10725. [Google Scholar] [CrossRef]
- Hu, Z.; Ding, J.; Ma, Z.; Sun, R.; Seoane, J.A.; Scott Shaffer, J.; Suarez, C.J.; Berghoff, A.S.; Cremolini, C.; Falcone, A.; et al. Quantitative evidence for early metastatic seeding in colorectal cancer. Nat. Genet. 2019, 51, 1113–1122. [Google Scholar] [CrossRef]
- Wu, J.; Bryan, J.; Rubinstein, S.M.; Wang, L.; Lenoue-Newton, M.; Zuhour, R.; Levy, M.; Micheel, C.; Xu, Y.; Bhavnani, S.K.; et al. Opportunities and challenges for analyzing cancer data at the inter- and intra-institutional levels. JCO Precis. Oncol. 2020, 4, 743–756. [Google Scholar] [CrossRef]
- Giannakis, M.; Mu, X.J.; Shukla, S.A.; Qian, Z.R.; Cohen, O.; Nishihara, R.; Bahl, S.; Cao, Y.; Amin-Mansour, A.; Yamauchi, M.; et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016, 15, 857–865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Werner, B.; Traulsen, A.; Sottoriva, A.; Dingli, D. Detecting truly clonal alterations from multi-region profiling of tumours. Sci. Rep. 2017, 7, 44991. [Google Scholar] [CrossRef] [Green Version]
- Dentro, S.C.; Leshchiner, I.; Haase, K.; Tarabichi, M.; Wintersinger, J.; Deshwar, A.G.; Yu, K.; Rubanova, Y.; Macintyre, G.; Demeulemeester, J.; et al. Characterizing genetic intra-tumor heterogeneity across 2658 human cancer genomes. Cell 2021, 184, 2239–2254. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Nishisho, I.; Kinzler, K.W.; Vogelstein, B.; Miyoshi, Y.; Miki, Y.; Ando, H.; Horii, A.; Nagase, H. Mutations of the adenomatous polyposis coli gene in familial polyposis coli patients and sporadic colorectal tumours. Princess Takamatsu Symp. 1991, 22, 285–292. [Google Scholar]
- Bodmer, W.F.; Cottrell, S.; Frischauf, A.M.; Kerr, I.B.; Murday, V.A.; Rowan, A.J.; Smith, M.F.; Solomon, E.; Thomas, H.; Varesco, L. Genetic analysis of colorectal cancer. Princess Takamatsu Symp. 1989, 20, 49–59. [Google Scholar] [PubMed]
- Andreyev, H.J.; Norman, A.R.; Cunningham, D.; Oates, J.R.; Clarke, P.A. Kirsten ras mutations in patients with colorectal cancer: The multicenter “RASCAL” study. J. Natl. Cancer Inst. 1998, 90, 675–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogelstein, B.; Fearon, E.R.; Hamilton, S.R.; Kern, S.E.; Preisinger, A.C.; Leppert, M.; Nakamura, Y.; White, R.; Smits, A.M.; Bos, J.L. Genetic alterations during colorectal-tumor development. N. Engl. J. Med. 1988, 319, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, H.; Bardelli, A.; Lengauer, C.; Kinzler, K.W.; Vogelstein, B.; Velculescu, V.E. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature 2002, 418, 934. [Google Scholar] [CrossRef]
- Yuen, S.T.; Davies, H.; Chan, T.L.; Ho, J.W.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Tsui, W.W.; Chan, A.S.; et al. Similarity of the phenotypic patterns associated with BRAF and KRAS mutations in colorectal neoplasia. Cancer Res. 2002, 62, 6451–6455. [Google Scholar]
- Fleming, N.I.; Jorissen, R.N.; Mouradov, D.; Christie, M.; Sakthianandeswaren, A.; Palmieri, M.; Day, F.; Li, S.; Tsui, C.; Lipton, L.; et al. SMAD2, SMAD3 and SMAD4 mutations in colorectal cancer. Cancer Res. 2013, 73, 725–735. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Sun, W.; Zhou, Y.; Li, P.; Chen, F.; Chen, H.; Xia, D.; Xu, E.; Lai, M.; Wu, Y.; et al. Mutations of key driver genes in colorectal cancer progression and metastasis. Cancer Metastasis Rev. 2018, 37, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, F.M.; Tigges, A.J.; Schipper, M.E.; den Hartog-Jager, F.C.; Kroes, W.G.; Walboomers, J.M. Expression of the nuclear oncogene p53 in colon tumours. J. Pathol. 1989, 157, 193–199. [Google Scholar] [CrossRef]
- Baker, S.J.; Fearon, E.R.; Nigro, J.M.; Hamilton, S.R.; Preisinger, A.C.; Jessup, J.M.; van Tuinen, P.; Ledbetter, D.H.; Barker, D.F.; Nakamura, Y.; et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science 1989, 244, 217–221. [Google Scholar] [CrossRef]
- Zhu, W.; Zhuang, P.; Song, W.; Duan, S.; Xu, Q.; Peng, M.; Zhou, J. Knockdown of lncRNA HNF1A-AS1 inhibits oncogenic phenotypes in colorectal carcinoma. Mol. Med. Rep. 2017, 16, 4694–4700. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Babaei-Jadidi, R.; Lorenzi, F.; Spencer-Dene, B.; Clarke, P.; Domingo, E.; Tulchinsky, E.; Vries, R.G.J.; Kerr, D.; Pan, Y.; et al. An FBXW7-ZEB2 axis links EMT and tumour microenvironment to promote colorectal cancer stem cells and chemoresistance. Oncogenesis 2019, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.-C.; Lin, H.-H.; Lin, J.-K.; Lin, C.-C.; Lan, Y.-T.; Wang, H.-S.; Yang, S.-H.; Chen, W.-S.; Lin, T.-C.; Jiang, J.-K.; et al. FBXW7 mutation analysis and its correlation with clinicopathological features and prognosis in colorectal cancer patients. Int. J. Biol. Markers 2015, 30, 88–95. [Google Scholar] [CrossRef]
- Nielsen, M.; Infante, E.; Brand, R. GeneReviews®: MUTYH Polyposis; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Gock, M.; Mullins, C.S.; Bergner, C.; Prall, F.; Ramer, R.; Göder, A.; Krämer, O.H.; Lange, F.; Krause, B.J.; Klar, E.; et al. Establishment, functional and genetic characterization of three novel patient-derived rectal cancer cell lines. World J. Gastroenterol. 2018, 24, 4880–4892. [Google Scholar] [CrossRef]
- Bocci, G.; Danesi, R.; Di Paolo, A.D.; Innocenti, F.; Allegrini, G.; Falcone, A.; Melosi, A.; Battistoni, M.; Barsanti, G.; Conte, P.F.; et al. Comparative pharmacokinetic analysis of 5-fluorouracil and its major metabolite 5-fluoro-5,6-dihydrouracil after conventional and reduced test dose in cancer patients. Clin. Cancer Res. 2000, 6, 3032–3037. [Google Scholar]
- Ehrsson, H.; Wallin, I.; Yachnin, J. Pharmacokinetics of oxaliplatin in humans. Med Oncol. 2002, 19, 261–265. [Google Scholar] [CrossRef]
- Graham, M.A.; Lockwood, G.F.; Greenslade, D.; Brienza, S.; Bayssas, M.; Gamelin, E. Clinical pharmacokinetics of oxaliplatin: A critical review. Clin. Cancer Res. 2000, 6, 1205–1218. [Google Scholar]
- Shirao, K.; Matsumura, Y.; Yamada, Y.; Muro, K.; Gotoh, M.; Boku, N.; Ohtsu, A.; Nagashima, F.; Sano, Y.; Mutoh, M.; et al. Phase I study of single-dose oxaliplatin in Japanese patients with malignant tumours. Jpn. J. Clin. Oncol. 2006, 36, 295–300. [Google Scholar] [CrossRef] [Green Version]
- Mechetner, E.; Brünner, N.; Parker, R.J. In vitro drug responses in primary and metastatic colorectal cancers. Scand. J. Gastroenterol. 2011, 46, 70–78. [Google Scholar] [CrossRef]
- Takebayashi, K.; Mekata, E.; Sonoda, H.; Shimizu, T.; Shiomi, H.; Naka, S.; Endo, Y.; Tani, T. Differences in chemosensitivity between primary and metastatic tumours in colorectal cancer. PLoS ONE 2013, 8, e73215. [Google Scholar] [CrossRef]
- Chabot, G.G. Clinical pharmacokinetics of irinotecan. Clin. Pharmacokinet. 1997, 33. [Google Scholar] [CrossRef]
- Kaneda, N.; Nagata, H.; Furuta, T.; Yokokura, T. Metabolism and pharmacokinetics of the camptothecin analogue CPT-11 in the mouse. Cancer Res. 1990, 50, 1715–1720. [Google Scholar] [PubMed]
- Guichard, S.; Terret, C.; Hennebelle, I.; Lochon, I.; Chevreau, P.; Frétigny, E.; Selves, J.; Chatelut, E.; Bugat, R.; Canal, P. CPT-11 converting carboxylesterase and topoisomerase activities in tumour and normal colon and liver tissues. Br. J. Cancer 1999, 80, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.F.; Zamboni, W.C.; Crom, W.R.; Houghton, P.J. Disposition of irinotecan and SN-38 following oral and intravenous irinotecan dosing in mice. Cancer Chemother. Pharmacol. 1997, 40, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef] [Green Version]


| Tumour ID | Collection Site | Metastasis Appearance | Pretreatment | Molecular Subtype |
|---|---|---|---|---|
| HROC48 | Transverse Colon | - | no | spMSI |
| HROC48Met1 | Multivisceral (small intestine, stomach, pancreas) | metachronous (56 months) | no | - |
| HROC72 | Transverse Colon | - | no | spStd |
| HROC72Met1 | Liver | synchronous | no | - |
| HROC103 | Rectum | - | no | spStd |
| HROC103Met1 | Liver | metachronous (48 months) | no | - |
| HROC103Met2 | Liver | metachronous (67 months) | no | - |
| HROC111 | Left Colon | - | no | spStd |
| HROC111Met1 | Brain | metachronous (30 months) | no | - |
| HROC147 | Sigmoid colon | - | no | CIMP-H, non-MSI |
| HROC147Met1 | Liver | synchronous | no | - |
| HROC277 | Right colon/Appendix | - | no | spStd |
| HROC277Met1 | Liver | synchronous | no | - |
| HROC277Met2 | Liver | metachronous (14 months) | yes | - |
| HROC278 | Right colon | - | no | CIMP-H, non-MSI |
| HROC278Met1 | Peritoneum | synchronous | no | - |
| HROC300 | Rectum | - | no | CIMP-H, non MSI |
| HROC300Met1 | Liver | synchronous | no | - |
| HROC348 | Sigmoid | - | yes | spStd |
| HROC348Met1 | Liver | synchronous | yes | - |
| HROC405 | Right colon | - | no | spStd |
| HROC405Met1 | Liver | synchronous | no | - |
| Cell Line | 2D Cell Culture | 3D Cell Culture | Doubling Time (h) | Colony Formation (%) |
|---|---|---|---|---|
| HROC147 T0 M1 |  |  | 62.6 (±4.2) | 6.7 (±4.0) |
| HROC147Met1 |  |  | 57.8 (±3.2) | 5.5 (±2.2) |
| HROC277 T0 M1 |  |  | 61.6 (±11.3) | 17.6 (±5.1) |
| HROC277Met1 T0 M2 |  |  | 66.3 (±3.6) | 3.3 (±1.7) |
| HROC277Met2 |  |  | 61.6 (±3.7) | 11.9 (±3.0) |
| HROC278 T0 M1 |  |  | 61.0 (±9.8) | 3.2 (±3.1) |
| HROC278Met1 T2 M2 |  |  | 54.1 (±4.4) | 4.2 (±4.1) |
| HROC348 |  |  | 74.2 (±7.2) | 1.1 (±1.5) |
| HROC348Met1 |  |  | 66.1 (±6.7) | 3.2 (±2.3) |
| Cell Line | 5-FU [µM] | Irinotecan [µM] | Oxaliplatin [µM] |
|---|---|---|---|
| HROC147 T0 M1 | 2.5 | 0.8 | 4.5 |
| HROC147Met1 | 0.5 | 2.2 | 3.9 |
| HROC277 T0 M1 | 9.2 | 0.1 | 2.9 |
| HROC277Met1 T0 M2 | 1.6 | 9.2 | 2.6 |
| HROC277Met2 | 3.7 | 15.6 | 4.9 |
| HROC278 T0 M1 | 3.2 | 0.8 | 4.1 |
| HROC278Met1 T2 M2 | 3.2 | 0.3 | 2.8 |
| HROC348 | 4.4 | 2.1 | 2.2 |
| HROC348Met | 4.2 | 16.8 | 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, S.; Beger, N.T.; Matschos, S.; Szymanski, A.; Przybylla, R.; Bürtin, F.; Prall, F.; Linnebacher, M.; Mullins, C.S. Tumour-Derived Cell Lines and Their Potential for Therapy Prediction in Patients with Metastatic Colorectal Cancer. Cancers 2021, 13, 4717. https://doi.org/10.3390/cancers13184717
Wagner S, Beger NT, Matschos S, Szymanski A, Przybylla R, Bürtin F, Prall F, Linnebacher M, Mullins CS. Tumour-Derived Cell Lines and Their Potential for Therapy Prediction in Patients with Metastatic Colorectal Cancer. Cancers. 2021; 13(18):4717. https://doi.org/10.3390/cancers13184717
Chicago/Turabian StyleWagner, Sandra, Nicola T. Beger, Stephanie Matschos, Antonia Szymanski, Randy Przybylla, Florian Bürtin, Friedrich Prall, Michael Linnebacher, and Christina S. Mullins. 2021. "Tumour-Derived Cell Lines and Their Potential for Therapy Prediction in Patients with Metastatic Colorectal Cancer" Cancers 13, no. 18: 4717. https://doi.org/10.3390/cancers13184717
APA StyleWagner, S., Beger, N. T., Matschos, S., Szymanski, A., Przybylla, R., Bürtin, F., Prall, F., Linnebacher, M., & Mullins, C. S. (2021). Tumour-Derived Cell Lines and Their Potential for Therapy Prediction in Patients with Metastatic Colorectal Cancer. Cancers, 13(18), 4717. https://doi.org/10.3390/cancers13184717






