Dynamic Interplay between Lower-Grade Glioma Instability and Brain Metaplasticity: Proposal of an Original Model to Guide the Therapeutic Strategy
Abstract
:Simple Summary
Abstract
1. Introduction
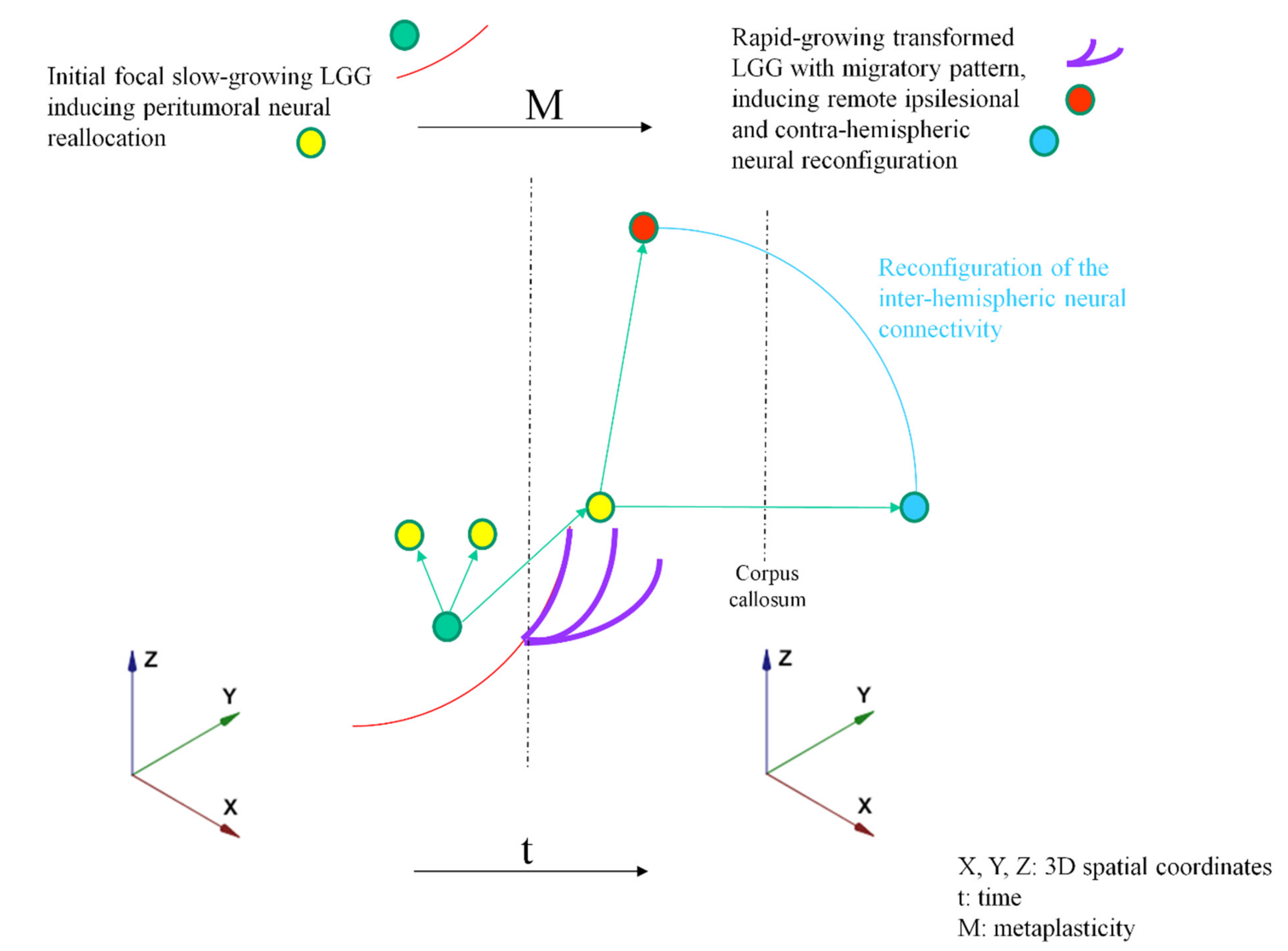
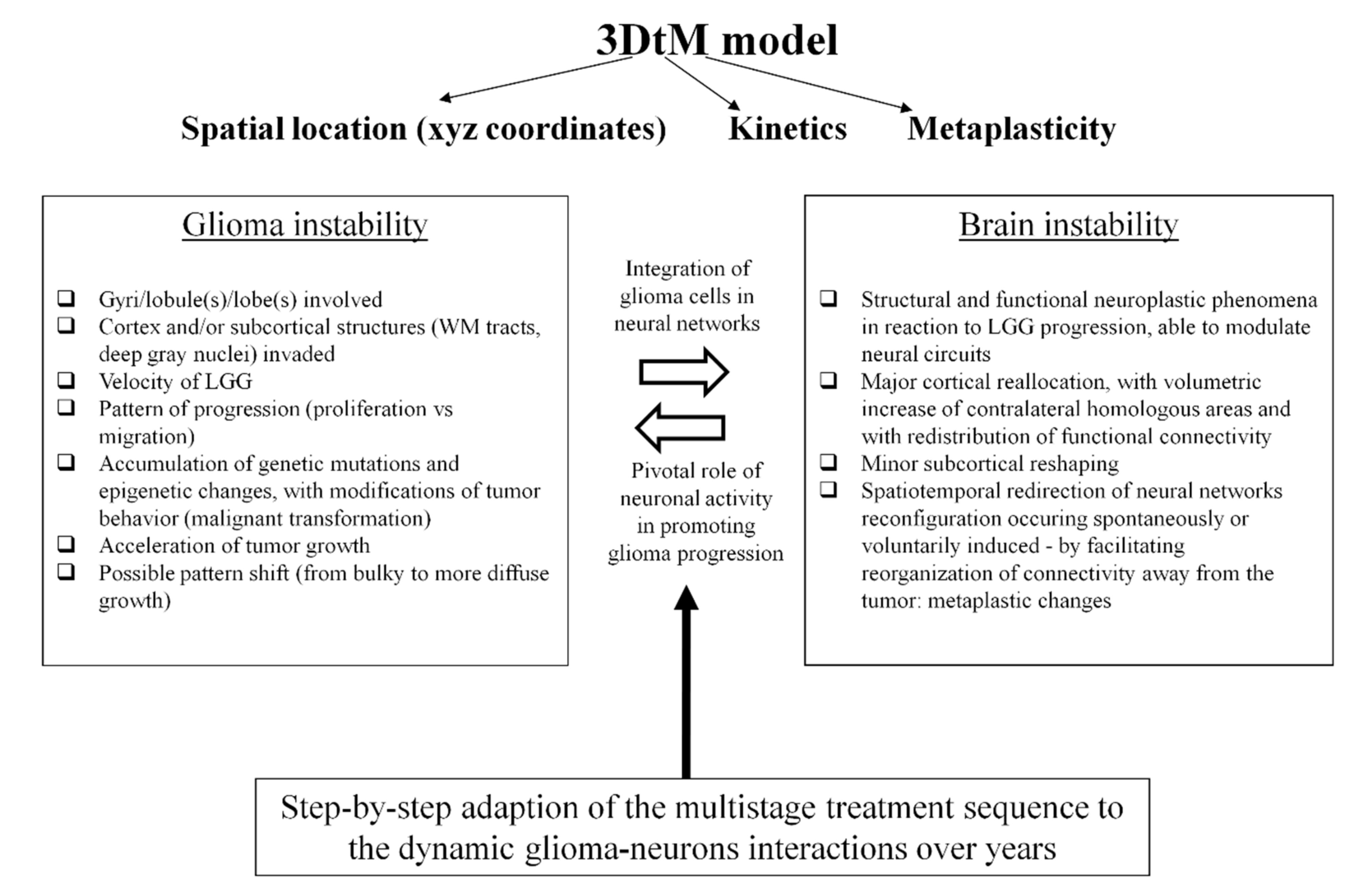
2. Proposal of an Original Model Based on Dynamic LGG–Connectome Interplay
3. Clinical Implications of the Model: Towards New Insights into the Oncofunctional Balance
4. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duffau, H. Diffuse Low-Grade Gliomas in Adults, 2nd ed.; Duffau, H., Ed.; Springer: London, UK, 2017. [Google Scholar]
- Pallud, J.; Blonski, M.; Mandonnet, E.; Audureau, E.; Fontaine, D.; Sanai, N.; Bauchet, L.; Peruzzi, P.; Frénay, M.; Colin, P.; et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol. 2013, 15, 595–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brat, D.J.; Verhaak, R.G.; Aldape, K.D.; Yung, W.K.; Salama, S.R.; Cooper, L.A.; Rheinbay, E.; Miller, C.R.; Vitucci, M.; et al.; Cancer Genome Atlas Research Network Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 2015, 372, 2481–2498. [Google Scholar] [PubMed] [Green Version]
- Suzuki, H.; Aoki, K.; Chiba, K.; Sato, Y.; Shiozawa, Y.; Shiraishi, Y.; Shimamura, T.; Niida, A.; Motomura, K.; Ohka, F.; et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 2015, 47, 458–468. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Barthel, F.; Malta, T.; Sabedot, T.S.; Salama, S.; Murray, B.A.; Morozova, O.; Newton, Y.; Radenbaugh, A.; Pagnotta, S.M.; et al. Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma. Cell 2016, 164, 550–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazor, T.; Chesnelong, C.; Pankov, A.; Jalbert, L.E.; Hong, C.; Hayes, J.; Smirnov, I.V.; Marshall, R.; Souza, C.F.; Shen, Y.; et al. Clonal expansion and epigenetic reprogramming following deletion or amplification of mutant IDH1. Proc. Natl. Acad. Sci. USA 2017, 114, 10743–10748. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Villanueva-Meyer, J.; Grimmer, M.R.; Hilz, S.; Solomon, D.A.; Choi, S.; Wahl, M.; Mazor, T.; Hong, C.; Shai, A.; et al. Temozolomide-induced hypermutation is associated with distant recurrence and reduced survival after high-grade transformation of low-grade IDH-mutant gliomas. Neuro Oncol. 2021. [Google Scholar] [CrossRef]
- Touat, M.; Li, Y.Y.; Boynton, A.N.; Spurr, L.F.; Iorgulescu, J.B.; Bohrson, C.L.; Cortes-Ciriano, I.; Birzu, C.; Geduldig, J.E.; Pelton, K.; et al. Mechanisms and therapeutic implications of hypermutation in gliomas. Nature 2020, 580, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Robbins, M.E. Radiation-induced cognitive impairment-from bench to bedside. Neuro Oncol. 2012, 14, iv37–iv44. [Google Scholar] [CrossRef] [Green Version]
- Heeran, A.B.; Berrigan, H.P.; O’Sullivan, J.N. The Radiation-Induced Bystander Effect (RIBE) and its Connections with the Hallmarks of Cancer. Radiat. Res. 2019, 192, 668. [Google Scholar] [CrossRef]
- Ferracci, F.-X.; Michaud, K.; Duffau, H. The landscape of postsurgical recurrence patterns in diffuse low-grade gliomas. Crit. Rev. Oncol. Hematol. 2019, 138, 148–155. [Google Scholar] [CrossRef]
- De Eulate-Beramendi, S.A.; Rigau, V.; Taillandier, L.; Duffau, H. Delayed leptomeningeal and subependymal seeding after multiple surgeries for supratentorial diffuse low-grade gliomas in adults. J. Neurosurg. 2014, 120, 833–839. [Google Scholar] [CrossRef]
- Duffau, H.; Taillandier, L. New concepts in the management of diffuse low-grade glioma: Proposal of a multistage and individualized therapeutic approach. Neuro Oncol. 2015, 17, 332–342. [Google Scholar] [CrossRef] [Green Version]
- Mandonnet, E.; Duffau, H. An attempt to conceptualize the individual onco-functional balance: Why a standardized treatment is an illusion for diffuse low-grade glioma patients. Crit. Rev. Oncol. 2018, 122, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H. The huge plastic potential of adult brain and the role of connectomics: New insights provided by serial mappings in glioma surgery. Cortex 2014, 58, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Duffau, H. Revisiting the Functional Anatomy of the Human Brain: Toward a Meta-Networking Theory of Cerebral Functions. Physiol. Rev. 2020, 100, 1181–1228. [Google Scholar] [CrossRef]
- Duffau, H. Functional Mapping before and after Low-Grade Glioma Surgery: A New Way to Decipher Various Spatiotemporal Patterns of Individual Neuroplastic Potential in Brain Tumor Patients. Cancers 2020, 12, 2611. [Google Scholar] [CrossRef] [PubMed]
- Almairac, F.; Deverdun, J.; Cochereau, J.; Coget, A.; Lemaitre, A.-L.; Moritz-Gasser, S.; Duffau, H.; Herbet, G. Homotopic redistribution of functional connectivity in insula-centered diffuse low-grade glioma. NeuroImage Clin. 2021, 29, 102571. [Google Scholar] [CrossRef]
- Duffau, H. Lessons from brain mapping in surgery for low-grade glioma: Insights into associations between tumour and brain plasticity. Lancet Neurol. 2005, 4, 476–486. [Google Scholar] [CrossRef]
- Desmurget, M.; Bonnetblanc, F.; Duffau, H. Contrasting acute and slow-growing lesions: A new door to brain plasticity. Brain 2007, 130, 898–914. [Google Scholar] [CrossRef] [Green Version]
- Duffau, H. Stimulation mapping of white matter tracts to study brain functional connectivity. Nat. Rev. Neurol. 2015, 11, 255–265. [Google Scholar] [CrossRef]
- Sarubbo, S.; Tate, M.; De Benedictis, A.; Merler, S.; Moritz-Gasser, S.; Herbet, G.; Duffau, H. Mapping critical cortical hubs and white matter pathways by direct electrical stimulation: An original functional atlas of the human brain. NeuroImage 2020, 205, 116237. [Google Scholar] [CrossRef]
- Monje, M. Synaptic Communication in Brain Cancer. Cancer Res. 2020, 80, 2979–2982. [Google Scholar] [CrossRef] [PubMed]
- Venkataramani, V.; Tanev, D.I.; Strahle, C.; Studier-Fischer, A.; Fankhauser, L.; Kessler, T.; Körber, C.; Kardorff, M.; Ratliff, M.; Xie, R.; et al. Glutamatergic synaptic input to glioma cells drives brain tumour progression. Nature 2019, 573, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Alfonso, J.; Osswald, M.; Monyer, H.; Wick, W.; Winkler, F. Emerging intersections between neuroscience and glioma biology. Nat. Neurosci. 2019, 22, 1951–1960. [Google Scholar] [CrossRef]
- Venkatesh, H.S.; Morishita, W.; Geraghty, A.C.; Silverbush, D.; Gillespie, S.M.; Arzt, M.; Tam, L.T.; Espenel, C.; Ponnuswami, A.; Ni, L.; et al. Electrical and synaptic integration of glioma into neural circuits. Nature 2019, 573, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Szalisznyo, K.; Silverstein, D.N.; Duffau, H.; Smits, A. Pathological Neural Attractor Dynamics in Slowly Growing Gliomas Supports an Optimal Time Frame for White Matter Plasticity. PLoS ONE 2013, 8, e69798. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; Maheu, M.; Costi, E.; LaFargue, G.; Duffau, H. Mapping neuroplastic potential in brain-damaged patients. Brain 2016, 139, 829–844. [Google Scholar] [CrossRef]
- Liu, D.; Liu, Y.; Hu, X.; Hu, G.; Yang, K.; Xiao, C.; Hu, J.; Li, Z.; Zou, Y.; Chen, J.; et al. Alterations of white matter integrity associated with cognitive deficits in patients with glioma. Brain Behav. 2020, 10, e01639. [Google Scholar] [CrossRef]
- Almairac, F.; Herbet, G.; Moritz-Gasser, S.; De Champfleur, N.M.; Duffau, H. The left inferior fronto-occipital fasciculus subserves language semantics: A multilevel lesion study. Brain Struct. Funct. 2015, 220, 1983–1995. [Google Scholar] [CrossRef]
- Incekara, F.; Satoer, D.; Visch-Brink, E.; Vincent, A.; Smits, M. Changes in language white matter tract microarchitecture associated with cognitive deficits in patients with presumed low-grade glioma. J. Neurosurg. 2019, 130, 1538–1546. [Google Scholar] [CrossRef] [Green Version]
- Duffau, H. Introducing the concept of brain meta-plasticity in glioma: How to re-orientate the pattern of neural reconfiguration to optimize the therapeutic strategy? J. Neurosurg. in press. 2021. [Google Scholar]
- Abraham, W.C.; Bear, M.F. Metaplasticity: The plasticity of synaptic plasticity. Trends Neurosci. 1996, 19, 126–130. [Google Scholar] [CrossRef]
- Hebb, D.O. The Organization of Behavior: A Neuropsychological Theory; Science Education: New York, NY, USA, 1949; Volume 44. [Google Scholar]
- Lee, H.-K.; Kirkwood, A. Mechanisms of Homeostatic Synaptic Plasticity in vivo. Front. Cell. Neurosci. 2019, 13, 520. [Google Scholar] [CrossRef] [Green Version]
- Yger, P.; Gilson, M. Models of Metaplasticity: A Review of Concepts. Front. Comput. Neurosci. 2015, 9, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehgal, M.; Song, C.; Ehlers, V.L.; Moyer, J.R. Learning to learn—intrinsic plasticity as a metaplasticity mechanism for memory formation. Neurobiol. Learn. Mem. 2013, 105, 186–199. [Google Scholar] [CrossRef] [Green Version]
- Duffau, H. Can non-invasive brain stimulation be considered to facilitate reoperation for low-grade glioma relapse by eliciting neuroplasticity? Front. Neurol. 2020, 11, 582489. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Angelini, E.; de Schotten, M.T.; Mandonnet, E.; Duffau, H. Evidence for potentials and limitations of brain plasticity using an atlas of functional resectability of WHO grade II gliomas: Towards a “minimal common brain”. NeuroImage 2011, 56, 992–1000. [Google Scholar] [CrossRef]
- Duffau, H. Why brain radiation therapy should take account of the individual structural and functional connectivity: Toward an irradiation “à la carte”. Crit. Rev. Oncol. Hematol. 2020, 154, 103073. [Google Scholar] [CrossRef] [PubMed]
- Castellano, A.; Donativi, M.; Rudà, R.; De Nunzio, G.; Riva, M.; Iadanza, A.; Bertero, L.; Rucco, M.; Bello, L.; Soffietti, R.; et al. Evaluation of low-grade glioma structural changes after chemotherapy using DTI-based histogram analysis and functional diffusion maps. Eur. Radiol. 2016, 26, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Mandonnet, E.; Delattre, J.Y.; Tanguy, M.L.; Swanson, K.R.; Carpentier, A.F.; Duffau, H.; Cornu, P.; Van Effenterre, R.; Alvord, E.C., Jr.; Capelle, L. Continuous growth of mean tumor diameter in a subset of grade II gliomas. Ann. Neurol. 2003, 53, 524–528. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Ng, S.; Herbet, G.; Lemaitre, A.L.; Cochereau, J.; Moritz-Gasser, S.; Duffau, H. Neuropsychological assessments before and after awake surgery for incidental low-grade gliomas. J. Neurosurg. 2021, 163, 1257–1267. [Google Scholar]
- Ng, S.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Return to Work Following Surgery for Incidental Diffuse Low-Grade Glioma: A Prospective Series With 74 Patients. Neurosurgery 2020, 87, 720–729. [Google Scholar] [CrossRef]
- Mandonnet, E.; Jbabdi, S.; Taillandier, L.; Galanaud, D.; Benali, H.; Capelle, L.; Duffau, H. Preoperative estimation of residual volume for WHO grade II glioma resected with intraoperative functional mapping. Neuro Oncol. 2007, 9, 63–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capelle, L.; Fontaine, D.; Mandonnet, E.; Taillandier, L.; Golmard, J.L.; Bauchet, L.; Pallud, J.; Peruzzi, P.; Baron, M.H.; Kujas, M.; et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: A series of 1097 cases: Clinical article. J. Neurosurg. 2013, 118, 1157–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roelz, R.; Strohmaier, D.; Jabbarli, R.; Kraeutle, R.; Egger, K.; Coenen, V.A.; Weyerbrock, A.; Reinacher, P.C. Residual Tumor Volume as Best Outcome Predictor in Low Grade Glioma—A Nine-Years Near-Randomized Survey of Surgery vs. Biopsy. Sci. Rep. 2016, 6, srep32286. [Google Scholar] [CrossRef] [PubMed]
- Herbet, G.; LaFargue, G.; Bonnetblanc, F.; Moritz-Gasser, S.; De Champfleur, N.M.; Duffau, H. Inferring a dual-stream model of mentalizing from associative white matter fibres disconnection. Brain 2014, 137, 944–959. [Google Scholar] [CrossRef]
- Herbet, G.; Moritz-Gasser, S.; Boiseau, M.; Duvaux, S.; Cochereau, J.; Duffau, H. Converging evidence for a cortico-subcortical network mediating lexical retrieval. Brain 2016, 139, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- Obara, T.; Blonski, M.; Brzenczek, C.; Mézières, S.; Gaudeau, Y.; Pouget, C.; Gauchotte, G.; Verger, A.; Vogin, G.; Moureaux, J.-M.; et al. Adult Diffuse Low-Grade Gliomas: 35-Year Experience at the Nancy France Neurooncology Unit. Front. Oncol. 2020, 10, 574679. [Google Scholar] [CrossRef]
- Picart, T.; Herbet, G.; Moritz-Gasser, S.; Duffau, H. Iterative Surgical Resections of Diffuse Glioma with Awake Mapping: How to Deal with Cortical Plasticity and Connectomal Constraints? Neurosurgery 2019, 85, 105–116. [Google Scholar] [CrossRef]
- Hamdan, N.; Duffau, H. Extending the multistage surgical strategy for recurrent initially low-grade gliomas: Functional and oncological outcomes in 31 consecutive patients who underwent a third resection under awake mapping. J. Neurosurg. 2021, 1. [Google Scholar] [CrossRef]
- Chapman, C.H.; Zhu, T.; Nazem-Zadeh, M.; Tao, Y.; Buchtel, H.A.; Tsien, C.I.; Lawrence, T.S.; Cao, Y. Diffusion tensor imaging predicts cognitive function change following partial brain radiotherapy for low-grade and benign tumors. Radiother. Oncol. 2016, 120, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bady, P.; Kurscheid, S.; Delorenzi, M.; Gorlia, T.; van den Bent, M.; Hoang-Xuan, K.; Vauléon, É.; Gijtenbeek, A.; Enting, R.; Thiessen, B.; et al. The DNA methylome of DDR genes and benefit from RT or TMZ in IDH mutant low-grade glioma treated in EORTC 22033. Acta Neuropathol. 2018, 135, 601–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blonski, M.; Taillandier, L.; Herbet, G.; Maldonado, I.; Beauchesne, P.; Fabbro, M.; Campello, C.; Gozé, C.; Rigau, V.; Moritz-Gasser, S.; et al. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: A study of cognitive status and quality of life. J. Neuro Oncol. 2012, 106, 353–366. [Google Scholar] [CrossRef] [PubMed]
| Stage of the Disease | 3DtM Model | Management | ||
|---|---|---|---|---|
| Variable-3D | Variable-t | Variable-M | ||
| Incidental discovery | + | + | + | Early maximal surgery |
| First seizure with normal cognitive assessment, limited invasion of WM, and slow LGG growth | + | + | + | Maximal surgery |
| Epilepsy with mild cognitive disorders related to moderate invasion of WM, and slow LGG growth | ± | + | ± | Surgery ± medical adjuvant treatment by privilegiating upfront chemotherapy |
| Epilepsy with significant cognitive disorders related to wide invasion of WM, and slow LGG growth | − | + | − | Neoadjuvant chemotherapy ± adjuvant surgery |
| Recurrent LGG with no or slight cognitive disorders, limited invasion of WM, and slow tumor growth | + | + | + | Reoperation |
| Recurrent glioma with no or slight cognitive disorders, limted invasion of WM, and acceleration of tumor growth rate | + | − | + | Reoperation followed by medical adjuvant treatments combining chemotherapy and radiotherapy |
| Recurrent LGG with moderate cognitive disorders despite wide invasion of WM but still slow tumor growth | − | + | ± | Medical adjuvant treatment by privilegiating upfront chemotherapy ± reoperation |
| Recurrent LGG with significant cognitive/neurological deficits related to wide invasion of WM and/or acceleration of growth rate | − | − | − | Medical adjuvant treatments combining chemotherapy and radiotherapy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duffau, H. Dynamic Interplay between Lower-Grade Glioma Instability and Brain Metaplasticity: Proposal of an Original Model to Guide the Therapeutic Strategy. Cancers 2021, 13, 4759. https://doi.org/10.3390/cancers13194759
Duffau H. Dynamic Interplay between Lower-Grade Glioma Instability and Brain Metaplasticity: Proposal of an Original Model to Guide the Therapeutic Strategy. Cancers. 2021; 13(19):4759. https://doi.org/10.3390/cancers13194759
Chicago/Turabian StyleDuffau, Hugues. 2021. "Dynamic Interplay between Lower-Grade Glioma Instability and Brain Metaplasticity: Proposal of an Original Model to Guide the Therapeutic Strategy" Cancers 13, no. 19: 4759. https://doi.org/10.3390/cancers13194759
APA StyleDuffau, H. (2021). Dynamic Interplay between Lower-Grade Glioma Instability and Brain Metaplasticity: Proposal of an Original Model to Guide the Therapeutic Strategy. Cancers, 13(19), 4759. https://doi.org/10.3390/cancers13194759






