Processing Speed and Time since Diagnosis Predict Adaptive Functioning Measured with WeeFIM in Pediatric Brain Tumor Survivors
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Demographic and Clinical Information
2.2.2. AF Assessment
- Complete dependence: 1 = total assistance (subject = 0%–24%); 2 = maximal assistance (subject = 25%–49%);
- Modified dependence: 3 = moderate assistance (subject = 50% or more); 4 = minimal contact assistance (subject = 75% or more); 5 = supervision;
- Independence: 6 = modified independence (with device(s)); 7 = complete independence (no device, completing the task promptly and safely) [37].
2.2.3. Cognitive Assessment
- The Verbal Comprehension Index (VCI) assesses verbal reasoning skills;
- The Perceptual Reasoning Index (PRI) measures visual-spatial reasoning skills;
- The Full Scale Intelligence Quotient (FSIQ) is the sum of the two previous indices and a measure of overall intellectual functioning;
- The Processing Speed Index (PSI) is a measure of the ability to respond promptly and to focus attention on a task; and
- The Working Memory Index (WMI) is a measure of auditory attention, concentration, and mental manipulation of information in short-term memory.
2.2.4. Selection of the Explanatory Variables
2.2.5. Data Diagnostics and Statistical Analysis
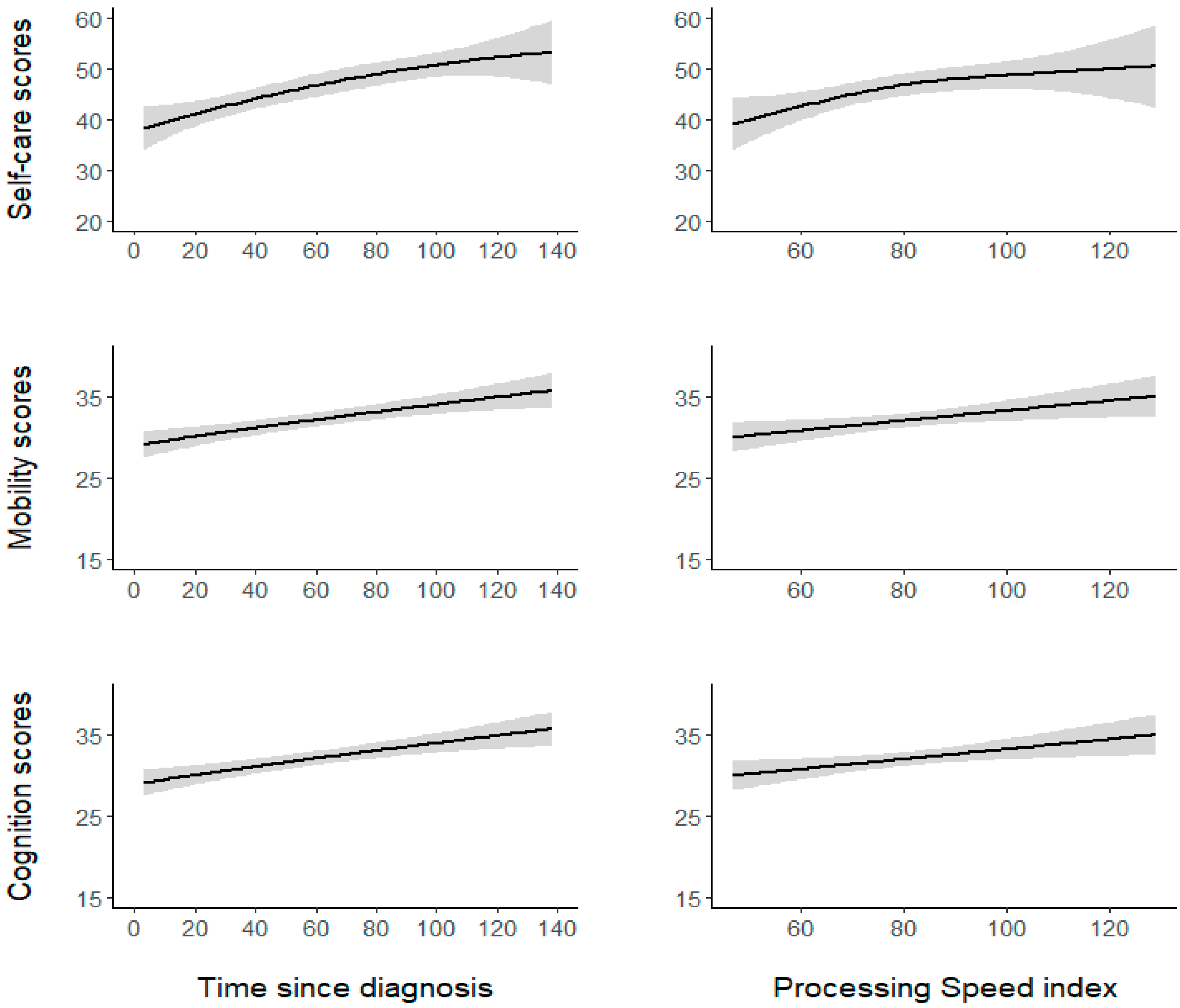
3. Results
4. Discussion
4.1. PS Effects on AF
4.2. Clinical Variable Effects on AF
4.2.1. Time since Diagnosis
4.2.2. Age at Diagnosis
4.2.3. History of Hydrocephalus
4.3. BT Survivors’ AF Characteristics
4.4. Parent Report Implications
4.5. Hypotheses of Intervention
4.6. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tassé, M.J.; Schalock, R.L.; Balboni, G.; Bersani, H., Jr.; Borthwick-Duffy, S.A.; Spreat, S.; Thissen, D.; Widaman, K.F.; Zhang, D. The construct of adaptive behavior: Its conceptualization, measurement, and use in the field of intellectual disability. Am. J. Intellect. Dev. Disabil. 2012, 117, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Tassé, M.J. The Oxford Handbook of Positive Psychology and Disability; Oxford University Press: Oxford, UK, 2013; pp. 105–106. [Google Scholar]
- Steinberg, L. Cognitive and affective development in adolescence. Trends Cogn. Sci. 2005, 9, 69–74. [Google Scholar] [CrossRef]
- Hoskinson, K.R.; Wolfe, K.R.; Yeats, K.O.; Mahone, E.M.; Cecil, K.M.; Ris, M.D. Predicting changes in adaptive functioning and behavioral adjustment following treatment for a pediatric brain tumor: A report from the Brain Radiation Investigative Study Consortium. Psycho-Oncology 2018, 27, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Panwala, T.F.; Fox, M.E.; Tucker, T.D.; King, T.Z. The Effects of Radiation and Sex Differences on Adaptive Functioning in Adult Survivors of Pediatric Posterior Fossa Brain Tumors. J. Int. Neuropsychol. Soc. 2019, 25, 729–739. [Google Scholar] [CrossRef]
- Taiwo, Z.; Na, S.; King, T.Z. The Neurological Predictor Scale: A Predictive Tool for Long-Term Core Cognitive Outcomes in Survivors of Childhood Brain Tumors. Pediatr. Blood Cancer 2017, 64, 172–179. [Google Scholar] [CrossRef] [Green Version]
- King, T.Z.; Ailion, A.S.; Fox, M.E.; Hufstetler, S.M. Neurodevelopmental model of long-term outcomes of adult survivors of childhood brain tumors. Child Neuropsychol. 2019, 25, 1–21. [Google Scholar] [CrossRef]
- Wolfe, K.R.; Madan-Swain, A.; Kana, R.K. Executive dysfunction in pediatric posterior fossa tumor survivors: A systematic literature review of neurocognitive deficits and interventions. Dev. Neuropsychol. 2012, 37, 153–175. [Google Scholar] [CrossRef] [Green Version]
- Stargatt, R.; Rosenfeld, J.V.; Anderson, V.; Hassall, T.; Maixner, W.; Ashley, D. Intelligence and adaptive function in children diagnosed with brain tumour during infancy. J. Neuro-Oncol. 2006, 80, 295–303. [Google Scholar] [CrossRef]
- Kunin-Batson, A.; Kadan-Lottick, N.; Zhu, L.; Cox, C.; Bordes-Edgar, V.; Srivastava, D.K.; Zeltzer, L.; Robison, L.L.; Krull, K.R. Predictors of independent living status in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2011, 57, 1197–1203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, A.K.; Ris, M.D.; Orobio, J.; Xue, J.; Mahajan, A.; Paulino, A.C.; Grosshans, D.; Okcu, M.F.; Chintagumpala, M.; Kahalley, L.S. Cognitive mediators of adaptive functioning outcomes in survivors of pediatric brain tumors treated with proton radiotherapy. Pediatr. Blood Cancer 2020, 67, e28064. [Google Scholar] [CrossRef] [PubMed]
- Treble-Barna, A.; Zang, H.; Zhang, N.; Taylor, H.G.; Yeates, K.O.; Wade, S. Long-Term Neuropsychological Profiles and Their Role as Mediators of Adaptive Functioning after Traumatic Brain Injury in Early Childhood. J. Neurotrauma 2017, 34, 353–362. [Google Scholar] [CrossRef] [Green Version]
- Shultz, E.L.; Hoskinson, K.R.; Keim, M.C.; Dennis, M.; Taylor, H.G.; Bigler, E.D.; Rubin, K.H.; Vannatta, K.; Gerhardt, C.A.; Stancin, T.; et al. Adaptive functioning following pediatric traumatic brain injury: Relationship to executive function and processing speed. Neuropsychology 2016, 30, 830–840. [Google Scholar] [CrossRef]
- Papazoglou, A.; King, T.Z.; Morris, R.D.; Krawiecki, N.S. Cognitive predictors of adaptive functioning vary according to pediatric brain tumor location. Dev. Neuropsychol. 2008, 33, 505–520. [Google Scholar] [CrossRef]
- Semmel, E.S.; Quadri, T.R.; King, T.Z. Oral processing speed as a key mechanism in the relationship between neurological risk and adaptive functioning in survivors of pediatric brain tumors. Pediatr. Blood Cancer 2020, 67, e28575. [Google Scholar] [CrossRef] [PubMed]
- Scantlebury, N.; Bouffet, E.; Laughlin, S.; Strother, D.; McConnell, D.; Hukin, J.; Fryer, C.; Laperriere, N.; Montour-Proulx, I.; Keene, D.; et al. White matter and information processing speed following treatment with cranial-spinal radiation for pediatric brain tumor. Neuropsychology 2016, 30, 425–438. [Google Scholar] [CrossRef] [PubMed]
- Kahalley, L.S.; Conklin, H.M.; Tyc, V.L.; Hudson, M.M.; Wilson, S.J.; Wu, S.; Xiong, X.; Hinds, P.S. Slower processing speed after treatment for pediatric brain tumor and acute lymphoblastic leukemia. Psychooncology 2013, 22, 1979–1986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, M.; Karunamuni, R.; McDonald, C.; Seibert, T.; White, N.; Moiseenko, V.; Bartsch, H.; Farid, N.; Kuperman, J.; Krishnan, A.; et al. Regional susceptibility to dose-dependent white matter damage after brain radiotherapy. Radiother. Oncol. 2017, 123, 209–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connor, M.; Karunamuni, R.; McDonald, C.; White, N.; Pettersson, N.; Moiseenko, V.; Seibert, T.; Marshall, D.; Cervino, L.; Bartsch, H.; et al. Dose-dependent white matter damage after brain radiotherapy. Radiother. Oncol. 2016, 121, 209–216. [Google Scholar] [CrossRef] [Green Version]
- de Ruiter, M.A.; van Mourik, R.; Schouten-van Meeteren, A.Y.; Grootenhuis, M.A.; Oosterlaan, J. Neurocognitive consequences of a paediatric brain tumour and its treatment: A meta-analysis. Dev. Med. Child Neurol. 2013, 55, 408–417. [Google Scholar] [CrossRef]
- Brinkman, T.M.; Palmer, S.L.; Chen, S.; Zhang, H.; Evankovich, K.; Swain, M.A.; Bonner, M.J.; Janzen, L.; Knight, S.; Armstrong, C.L.; et al. Parent-reported social outcomes after treatment for pediatric embryonal tumors: A prospective longitudinal study. J. Clin. Oncol. 2012, 30, 4134–4140. [Google Scholar] [CrossRef] [Green Version]
- Bonner, M.J.; Hardy, K.K.; Willard, V.W.; Anthony, K.K.; Hood, M.; Gururangan, S. Social functioning and facial expression recognition in survivors of pediatric brain tumors. J. Pediatr. Psychol. 2008, 33, 1142–1152. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.E.; Wolfe, K.R.; Yeates, K.O.; Mahone, E.M.; Cecil, K.M.; Ris, M.D. Predictors of adaptive functioning and psychosocial adjustment in children with pediatric brain tumor: A report from the Brain Radiation Investigative Study Consortium. Pediatr. Blood Cancer 2015, 62, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Beebe, D.W.; Ris, M.D.; Armstrong, F.D.; Fontanesi, J.; Mulhern, R.; Holmes, E.; Wisoff, J.H. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: Evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130). J. Clin. Oncol. 2005, 23, 5198–5204. [Google Scholar] [CrossRef] [PubMed]
- Kautiainen, R.J.; Fox, M.E.; King, T.Z. The Neurological Predictor Scale Predicts Adaptive Functioning via Executive Dysfunction in Young Adult Survivors of Childhood Brain Tumor. J. Int. Neuropsychol. Soc. 2021, 27, 1–11. [Google Scholar] [CrossRef]
- Netson, K.L.; Conklin, H.M.; Wu, S.; Xiong, X.; Merchant, T.E. A 5-year investigation of children’s adaptive functioning following conformal radiation therapy for localized ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, 217–223.e1. [Google Scholar] [CrossRef] [Green Version]
- Zeltzer, L.K.; Recklitis, C.; Buchbinder, D.; Zebrack, B.; Casillas, J.; Tsao, J.C.; Lu, Q.; Krull, K. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2009, 27, 2396–2404. [Google Scholar] [CrossRef]
- Ness, K.K.; Mertens, A.C.; Hudson, M.M.; Wall, M.M.; Leisenring, W.M.; Oeffinger, K.C.; Sklar, C.A.; Robison, L.L.; Gurney, J.G. Limitations on physical performance and daily activities among long-term survivors of childhood Cancer. Ann. Intern. Med. 2005, 143, 639–647. [Google Scholar] [CrossRef]
- Netson, K.L.; Conklin, H.M.; Wu, S.; Xiong, X.; Merchant, T.E. Longitudinal investigation of adaptive functioning following conformal irradiation for pediatric craniopharyngioma and low-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 2013, 85, 1301–1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willard, V.W.; Conklin, H.M.; Wu, S.; Merchant, T.E. Prospective longitudinal evaluation of emotional and behavioral functioning in pediatric patients with low-grade glioma treated with conformal radiation therapy. J. Neuro-Oncol. 2015, 122, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Willard, V.W.; Conklin, H.M.; Boop, F.A.; Wu, S.; Merchant, T.E. Emotional and behavioral functioning after conformal radiation therapy for pediatric ependymoma. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 814–821. [Google Scholar] [CrossRef] [Green Version]
- Sands, S.A.; Milner, J.S.; Goldberg, J.; Mukhi, V.; Moliterno, J.A.; Maxfield, C.; Wisoff, J.H. Quality of life and behavioral follow-up study of pediatric survivors of craniopharyngioma. J. Neurosurg. 2005, 103, 302–311. [Google Scholar] [CrossRef]
- Ashford, J.M.; Netson, K.L.; Clark, K.N.; Merchant, T.E.; Santana, V.M.; Wu, S.; Conklin, H.M. Adaptive functioning of childhood brain tumor survivors following conformal radiation therapy. J. Neuro-Oncol. 2014, 118, 193–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beretta, E.; Molteni, E.; Galbiati, S.; Stefanoni, G.; Strazzer, S. Five-year motor functional outcome in children with acquired brain injury. Yet to the end of the story? Dev. Neurorehabilit. 2018, 21, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.; Wong, S.; Chan, K.; Wong, W. Functional Independence Measure (WeeFIM) for Chinese children: Hong Kong Cohort. Pediatrics 2002, 109, e36. [Google Scholar] [CrossRef] [Green Version]
- Wong, V.; Chung, B.; Hui, S.; Fong, A.; Lau, C.; Law, B.; Lo, K.; Shum, T.; Wong, R. Cerebral palsy: Correlation of risk factors and functional performance using the Functional Independence Measure for Children (WeeFIM). J. Child Neurol. 2004, 19, 887–893. [Google Scholar] [CrossRef]
- World Health Organization. International Classification of Impairments, Disabilities, and Handicaps: A Manual of Classification Relating to the Consequences of Disease; WHO: Geneva, Switzerland, 1980; p. 11. Available online: https://apps.who.int/iris/bitstream/handle/10665/41003/9241541261_eng.pdf;jsessionid=A32A3302C76B209A51EF9B75434652F5?sequence=1 (accessed on 7 September 2021).
- Williams, K.S.; Young, D.K.; Burke, G.A.A.; Fountain, D.M. Comparing the WeeFIM and PEDI in neurorehabilitation for children with acquired brain injury: A systematic review. Dev. Neurorehabilit. 2017, 20, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Maddux, A.B.; Cox-Martin, M.; Dichiaro, M.; Bennett, T.D. The Association Between the Functional Status Scale and the Pediatric Functional Independence Measure in Children Who Survive Traumatic Brain Injury. Pediatr. Crit. Care Med. 2018, 19, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Kahn, D.A.; Asbell, S.J.; Donders, J. Clinical utility of the LANSE-A in adolescents with traumatic brain injury. Rehabil. Psychol. 2015, 60, 187–192. [Google Scholar] [CrossRef] [PubMed]
- McBride, T. Neuropsychological scores and WeeFIM cognitive ratings of children with traumatic brain injury: A brief report. Brain Inj. 2015, 29, 951–954. [Google Scholar] [CrossRef]
- Austin, C.A.; Slomine, B.S.; Dematt, E.J.; Salorio, C.F.; Suskauer, S.J. Time to follow commands remains the most useful injury severity variable for predicting WeeFIM scores 1 year after paediatric TBI. Brain Inj. 2013, 27, 1056–1062. [Google Scholar] [CrossRef] [Green Version]
- Slomine, B.; Eikenberg, J.; Salorio, C.; Suskauer, S.; Trovato, M.; Christensen, J. Preliminary evaluation of the Cognitive and Linguistic Scale: A measure to assess recovery in inpatient rehabilitation following pediatric brain injury. J. Head Trauma Rehabil. 2008, 23, 286–293. [Google Scholar] [CrossRef]
- Kerr, E.R.; Fayed, N. Cognitive predictors of adaptive functioning in children with symptomatic epilepsy. Epilepsy Res. 2017, 136, 67–76. [Google Scholar] [CrossRef]
- Howarth, R.A.; Vova, J.; Blackwell, L.S. Early Functional Outcomes for Pediatric Patients Diagnosed with Anti-N-Methyl-D-Aspartate Receptor Encephalitis during Inpatient Rehabilitation. Am. J. Phys. Med. Rehabil. 2019, 98, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.L.; Moura, M.C.D.S.; Becker, K.K.; Teixeira, R.A.A.; Voos, M.C.; Hasue, R.H. The relationship between motor function, cognition, independence and quality of life in myelomeningocele patients. Arq. Neuropsiquiatr. 2017, 75, 509–514. [Google Scholar] [CrossRef] [Green Version]
- Bart, O.; Sarah, A.; Tzafrir, D. Validation of the Israeli version of the Rivermead Behavioral Memory Test for children following acquired brain injury. J. Head Trauma Rehabil. 2013, 28, 419–425. [Google Scholar] [CrossRef]
- Long, C.E.; Blackman, J.A.; Farrell, W.J.; Smolkin, M.E.; Conaway, M.R. A comparison of developmental versus functional assessment in the rehabilitation of young children. Pediatr. Rehabil. 2005, 8, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Uniform Data System for Medical Rehabilitation (UDSMR). WeeFIM System SM. Clinical Guide, Version 5.01; University at Buffalo: Buffalo, NY, USA, 2000. [Google Scholar]
- Sanders, J.O.; McConnell, S.L.; King, R.; Lanford, A.; Montpetit, K.; Gates, P.; Rich, M.M.; Shepherd, K.; Cupp, T.; Haynes, R.; et al. A prospective evaluation of the WeeFIM in patients with cerebral palsy undergoing orthopaedic surgery. J. Pediatr. Orthop. 2006, 26, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Suskauer, S.J.; Slomine, B.S.; Inscore, A.B.; Lewelt, A.J.; Kirk, J.W.; Salorio, C.F. Injury severity variables as predictors of WeeFIM scores in pediatric TBI: Time to follow commands is best. J. Pediatr. Rehabil. Med. 2009, 2, 297–307. [Google Scholar] [CrossRef]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; Organizzazioni Speciali: Firenze, Italy, 2012; pp. 28–34. (In Italian) [Google Scholar]
- Thornton, C.P.; Ruble, K.; Jacobson, L.A. Beyond Risk-Based Stratification: Impacts of Processing Speed and Executive Function on Adaptive Skills in Adolescent and Young Adult Cancer Survivors. J. Adolesc. Young Adult Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.A.; Mahone, E.M.; Yeates, K.O.; Ris, M.D. Processing speed in children treated for brain tumors: Effects of radiation therapy and age. Child Neuropsychol. 2019, 25, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.G.; Quintana-Ascencio, P.F. A solution to minimum sample size for regressions. PLoS ONE 2020, 15, e0229345. [Google Scholar] [CrossRef] [Green Version]
- Peña, E.A.; Slate, E.H. Global Validation of Linear Model Assumptions. J. Am. Stat. Assoc. 2006, 101, 341–354. [Google Scholar] [CrossRef] [PubMed]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. Ser. B 2011, 73, 3–36. [Google Scholar] [CrossRef] [Green Version]
- Wood, S.N. Stable and Efficient Multiple Smoothing Parameter Estimation for Generalized Additive Models. J. Am. Stat. Assoc. 2004, 99, 673–686. Available online: http://www.jstor.org/stable/27590439 (accessed on 7 September 2021). [CrossRef] [Green Version]
- Wood, S. Generalized Additive Models: An Introduction with R, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Tingley, D.; Yamamoto, T.; Hirose, K.; Imai, K.K. Mediation: R Package for Causal Mediation Analysis. J. Softw. 2014, 59, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Hedvall, Å.; Fernell, E.; Holm, A.; Åsberg Johnels, J.; Gillberg, C.; Billstedt, E. Autism, processing speed, and adaptive functioning in preschool children. Sci. World J. 2013, 158263. [Google Scholar] [CrossRef] [PubMed]
- Oliveras-Rentas, R.E.; Kenworthy, L.; Roberson, R.B., 3rd; Martin, A.; Wallace, G.L. WISC-IV profile in high-functioning autism spectrum disorders: Impaired processing speed is associated with increased autism communication symptoms and decreased adaptive communication abilities. J. Autism Dev. Disord. 2012, 42, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoza, B.; Shoulberg, E.K.; Tompkins, C.L.; Martin, C.P.; Krasner, A.; Dennis, M.; Meyer, L.E.; Cook, H. Moderate-to-vigorous physical activity and processing speed: Predicting adaptive change in ADHD levels and related impairments in preschoolers. J. Child Psychol. Psychiatry 2020, 61, 1380–1387. [Google Scholar] [CrossRef]
- Adalio, C.J.; Owens, E.B.; McBurnett, K.; Hinshaw, S.P.; Pfiffner, L.J. Processing Speed Predicts Behavioral Treatment Outcomes in Children with Attention-Deficit/Hyperactivity Disorder Predominantly Inattentive Type. J. Abnorm. Child Psychol. 2018, 46, 701–711. [Google Scholar] [CrossRef]
- Palmer, S. Neurodevelopmental impacts on children treated for medulloblastoma: A review and proposed conceptual model. Dev. Disabil. Res. Rev. 2008, 14, 203–210. [Google Scholar] [CrossRef] [Green Version]
- Test, D.W.; Mazzotti, V.L.; Mustian, A.L.; Fowler, C.H.; Kortering, L.; Kohler, P. Evidence-Based Secondary Transition Predictors for Improving Postschool Outcomes for Students with Disabilities. Career Dev. Except. Individ. 2009, 32, 160–181. [Google Scholar] [CrossRef]
- Carter, E.W.; Austin, D.; Trainor, A.A. Predictors of Postschool Employment Outcomes for Young Adults with Severe Disabilities. J. Disabil. Policy Stud. 2012, 23, 50–63. [Google Scholar] [CrossRef] [Green Version]
- Dockstader, C.; Gaetz, W.; Bouffet, E.; Tabori, U.; Wang, F.; Bostan, S.R.; Laughlin, S.; Mabbott, D.J. Neural correlates of delayed visual-motor performance in children treated for brain tumours. Cortex 2013, 49, 2140–2150. [Google Scholar] [CrossRef]
- van der Holst, H.M.; Tuladhar, A.M.; Zerbi, V.; van Uden, I.W.M.; de Laat, K.F.; van Leijsen, E.M.C.; Ghafoorian, M.; Platel, B.; Bergkamp, M.I.; van Norden, A.G.W.; et al. White matter changes and gait decline in cerebral small vessel disease. Neuroimage Clin. 2017, 17, 731–738. [Google Scholar] [CrossRef]
- Aukema, E.J.; Caan, M.W.; Oudhuis, N.; Majoie, C.B.; Vos, F.M.; Reneman, L.; Last, B.F.; Grootenhuis, M.A.; Schouten-van Meeteren, A.Y. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Urgesi, C.; Massimino, M.; Gandola, L.; Bardoni, A.; Poggi, G. Effects of supratentorial and infratentorial tumor location on cognitive functioning of children with brain tumor. Childs Nerv. Syst. 2020, 36, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Spiegler, B.J.; Bouffet, E.; Greenberg, M.L.; Rutka, J.T.; Mabbott, D.J. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J. Clin. Oncol. 2004, 22, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, E.; Gómez, C.M.; Quintero, E.A.; González-Rosa, J.J.; Márquez, J. Differential prefrontal-like deficit in children after cerebellar astrocytoma and medulloblastoma tumor. Behav. Brain Funct. 2008, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Palmer, S.L.; Lesh, S.; Wallace, D.; Bonner, M.J.; Swain, M.; Chapieski, L.; Janzen, L.; Mabbott, D.; Knight, S.; Boyle, R.; et al. How parents cope with their child’s diagnosis and treatment of an embryonal tumor: Results of a prospective and longitudinal study. J. Neuro-Oncol. 2011, 105, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Biassoni, V.; Massimino, M.; Oprandi, M.C.; Clerici, C.A.; Veneroni, L.; Corti, C.; Schiavello, E.; Spreafico, F.; Poggi, G. Rehabilitation for children and young people surviving a brain tumor, and their transition to adult services: The main challenges. Expert Rev. Qual. Life Cancer Care 2017, 2, 137–152. [Google Scholar] [CrossRef]
- Pruitt, D.W.; Ayyangar, R.; Craig, K.; White, A.; Neufeld, J.A. Pediatric brain tumor rehabilitation. J. Pediatr. Rehabil. Med. 2011, 4, 59–70. [Google Scholar] [CrossRef]
- Inhestern, L.; Peikert, M.L.; Krauth, K.A.; Escherich, G.; Rutkowski, S.; Kandels, D.; Bergelt, C. Parents’ perception of their children’s process of reintegration after childhood cancer treatment. PLoS ONE 2020, 15, e0239967. [Google Scholar] [CrossRef] [PubMed]
- Cederberg, T.J.; Weineland, S.; Dahl, J.; Ljungman, G. Validation of the Swedish Acceptance and Action Questionnaire (SAAQ) for parents of children with Cancer. J. Context. Behav. Sci. 2018, 10, 50–54. [Google Scholar] [CrossRef]
- Cernvall, M.; Skogseid, E.; Carlbring, P.; Ljungman, L.; Ljungman, G.; von Essen, L. Experiential Avoidance and Rumination in Parents of Children on Cancer Treatment: Relationships with Posttraumatic Stress Symptoms and Symptoms of Depression. J. Clin. Psychol. Med. Settings 2016, 23, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, M.; Antoniou, G.; Toogood, I.; Rice, M.; Baghurst, P. Childhood cancer: A 4-year prospective study of the psychological adjustment of children and parents. J. Pediatr. Hematol. Oncol. 2000, 22, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Boman, K.; Lindahl, A.; Björk, O. Disease-related distress in parents of children with cancer at various stages after the time of diagnosis. Acta Oncol. 2003, 42, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, M.A.; Szumowski, E.; Blondis, T.A.; Roizen, N.J. Adaptive skills dysfunction in ADD and ADHD children. J. Child Psychol. Psychiatry 1995, 36, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Tonning Olsson, I.; Perrin, S.; Lundgren, J.; Hjorth, L.; Johanson, A. Long-term cognitive sequelae after pediatric brain tumor related to medical risk factors, age, and sex. Pediatr. Neurol. 2014, 51, 515–521. [Google Scholar] [CrossRef]
- Eaton, B.R.; Goldberg, S.; Tarbell, N.J.; Lawell, M.P.; Gallotto, S.L.; Weyman, E.A.; Kuhlthau, K.A.; Ebb, D.H.; MacDonald, S.M.; Yock, T.I. Long-term health-related quality of life in pediatric brain tumor survivors receiving proton radiotherapy at <4 years of age. Neuro Oncol. 2020, 29, 1379–1387. [Google Scholar] [CrossRef]
- Marusak, H.A.; Iadipaolo, A.S.; Harper, F.W.; Elrahal, F.; Taub, J.W.; Goldberg, E.; Rabinak, C.A. Neurodevelopmental consequences of pediatric cancer and its treatment: Applying an early adversity framework to understanding cognitive, behavioral, and emotional outcomes. Neuropsychol. Rev. 2018, 28, 123–175. [Google Scholar] [CrossRef]
- Duffner, P.K. Risk factors for cognitive decline in children treated for brain tumors. Eur. J. Paediatr. Neurol. 2010, 14, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.; Baron, I.S.; Fantie, B.D. Neuropsychological functioning in early hydrocephalus: Review from a developmental perspective. Child Neuropsychol. 2001, 7, 199–229. [Google Scholar] [CrossRef] [PubMed]
- Aarsen, F.K.; Van Dongen, H.R.; Paquier, P.F.; Van Mourik, M.; Catsman-Berrevoets, C.E. Long term sequelae in children after cerebellar astrocytoma surgery. Neurology 2002, 62, 1311–1316. [Google Scholar] [CrossRef]
- Cámara, S.; Fournier, M.C.; Cordero, P.; Melero, J.; Robles, F.; Esteso, B.; Vara, M.T.; Rodríguez, S.; Lassaletta, Á.; Budke, M. Neuropsychological Profile in Children with Posterior Fossa Tumors with or Without Postoperative Cerebellar Mutism Syndrome (CMS). Cerebellum 2020, 19, 78–88. [Google Scholar] [CrossRef]
- Rourke, B.P. Nonverbal Learning Disability: The Syndrome and the Model; Guilford Press: New York, NY, USA, 1989. [Google Scholar]
- Ness, K.K.; Hudson, M.M.; Ginsberg, J.P.; Nagarajan, R.; Kaste, S.C.; Marina, N.; Whitton, J.; Robison, L.L.; Gurney, J.G. Physical performance limitations in the Childhood Cancer Survivor Study Cohort. J. Clin. Oncol. 2009, 27, 2382–2389. [Google Scholar] [CrossRef] [Green Version]
- Tassé, M.J.; Luckasson, R.; Schalock, R.L. The Relation Between Intellectual Functioning and Adaptive Behavior in the Diagnosis of Intellectual Disability. Intellect Dev. Disabil. 2016, 54, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.M.; Holm, K.E.; Gurney, J.G. The impact of childhood cancer on the family: A qualitative analysis of strains, resources, and coping behaviors. Psychooncology 2004, 13, 390–407. [Google Scholar] [CrossRef]
- Lazarus, R.S. Coping theory and research: Past, present, and future. Psychosom. Med. 1993, 55, 234–247. [Google Scholar] [CrossRef]
- Papay, C.K.; Bambara, L.M. Best practices in transition to adult life for youth with intellectual disabilities. Career Dev. Except. Individ. 2014, 37, 136–148. [Google Scholar] [CrossRef]
- Morris, J. Cognitive rehabilitation: Where we are and what is on the horizon. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, D.M.; Ledbetter, C.; Moore, A.L. LearningRx Cognitive Training Effects in Children Ages 8–14: A Randomized Controlled Trial. Appl. Cogn. Psychol. 2016, 30, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Doger de Spéville, E.; Kieffer, V.; Dufour, C.; Grill, J.; Noulhiane, M.; Hertz-Pannier, L.; Chevignard, M. Neuropsychological consequences of childhood medulloblastoma and possible interventions: A review. Neurochirurgie 2021, 67, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.W.; Copeland, D.R. Attentional processes and their remediation in children treated for cancer: A literature review and the development of a therapeutic approach. J. Int. Neuropsychol. Soc. 2002, 8, 115–124. [Google Scholar] [CrossRef]
- Butler, R.W.; Sahler, O.J.Z.; Askins, M.A.; Alderfer, M.A.; Katz, E.R.; Phipps, S.; Noll, R.B. Interventions to improve neuropsychological functioning in childhood cancer survivors. Dev. Disabil. Res. Rev. 2008, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Hardy, K.K.; Willard, V.W.; Bonner, M.J. Computerized cognitive training in survivors of childhood cancer: A pilot study. J. Pediatr. Oncol. Nurs. 2011, 28, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Lacayo, N.J.; Jo, B. A pilot study of an online cognitive rehabilitation program for executive function skills in children with cancer-related brain injury. Brain Inj. 2011, 25, 101–112. [Google Scholar] [CrossRef] [Green Version]
- Gorman, S.; Barnes, M.A.; Swank, P.R.; Prasad, M.; Cox, C.S., Jr.; Ewing-Cobbs, L. Does processing speed mediate the effect of pediatric traumatic brain injury on working memory? Neuropsychology 2016, 30, 263–273. [Google Scholar] [CrossRef] [PubMed]

| Categorical Clinical Variables | n (%) |
| Sex | |
| Male | 42 (57.5) |
| Female | 31 (42.5) |
| Histopathological tumor type | |
| Astrocytoma | 16 (21.9) |
| Ependymoma | 14 (19.2) |
| Medulloblastoma | 28 (38.4) |
| Others | 15 (20.5) |
| History of hydrocephalus | |
| Present | 13 (17.8) |
| Absent | 60 (82.2) |
| Tumor location | |
| Supratentorial | 31 (57.5) |
| Infratentorial | 42 (42.5) |
| Treatment | |
| Neurosurgery without adjuvant treatments | 17 (23.29) |
| Neurosurgery and chemotherapy | 7 (9.59) |
| Neurosurgery and radiotherapy with or without chemotherapy | 49 (67.12) |
| Continuous Clinical Variables | M (SE) |
| Time since diagnosis (months) | 59.5 (4.5) |
| Age at diagnosis (in months) | 71.1 (4.6) |
| Cognitive Variable | M (SD) |
|---|---|
| VCI | 88.41 (18.47) |
| PRI | 87.88 (19.47) |
| FSIQ | 85.88 (18.85) |
| WMI | 88.19 (19.33) |
| PSI | 80.27 (18.02) |
| WeeFIM Subscales | Intercept | SE | p-Value | R2 (adj.) |
|---|---|---|---|---|
| Self-care model | 46.34 | 2.26 | <0.0001 | 0.66 |
| Mobility model | 33.78 | 1.49 | <0.0001 | 0.33 |
| Cognition model | 30.87 | 1.42 | <0.0001 | 0.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oprandi, M.C.; Oldrati, V.; delle Fave, M.; Panzeri, D.; Gandola, L.; Massimino, M.; Bardoni, A.; Poggi, G. Processing Speed and Time since Diagnosis Predict Adaptive Functioning Measured with WeeFIM in Pediatric Brain Tumor Survivors. Cancers 2021, 13, 4776. https://doi.org/10.3390/cancers13194776
Oprandi MC, Oldrati V, delle Fave M, Panzeri D, Gandola L, Massimino M, Bardoni A, Poggi G. Processing Speed and Time since Diagnosis Predict Adaptive Functioning Measured with WeeFIM in Pediatric Brain Tumor Survivors. Cancers. 2021; 13(19):4776. https://doi.org/10.3390/cancers13194776
Chicago/Turabian StyleOprandi, Maria Chiara, Viola Oldrati, Morena delle Fave, Daniele Panzeri, Lorenza Gandola, Maura Massimino, Alessandra Bardoni, and Geraldina Poggi. 2021. "Processing Speed and Time since Diagnosis Predict Adaptive Functioning Measured with WeeFIM in Pediatric Brain Tumor Survivors" Cancers 13, no. 19: 4776. https://doi.org/10.3390/cancers13194776
APA StyleOprandi, M. C., Oldrati, V., delle Fave, M., Panzeri, D., Gandola, L., Massimino, M., Bardoni, A., & Poggi, G. (2021). Processing Speed and Time since Diagnosis Predict Adaptive Functioning Measured with WeeFIM in Pediatric Brain Tumor Survivors. Cancers, 13(19), 4776. https://doi.org/10.3390/cancers13194776







