Anus-Preserving Surgery in Advanced Low-Lying Rectal Cancer: A Perspective on Oncological Safety of Intersphincteric Resection
Abstract
:Simple Summary
Abstract
1. Introduction
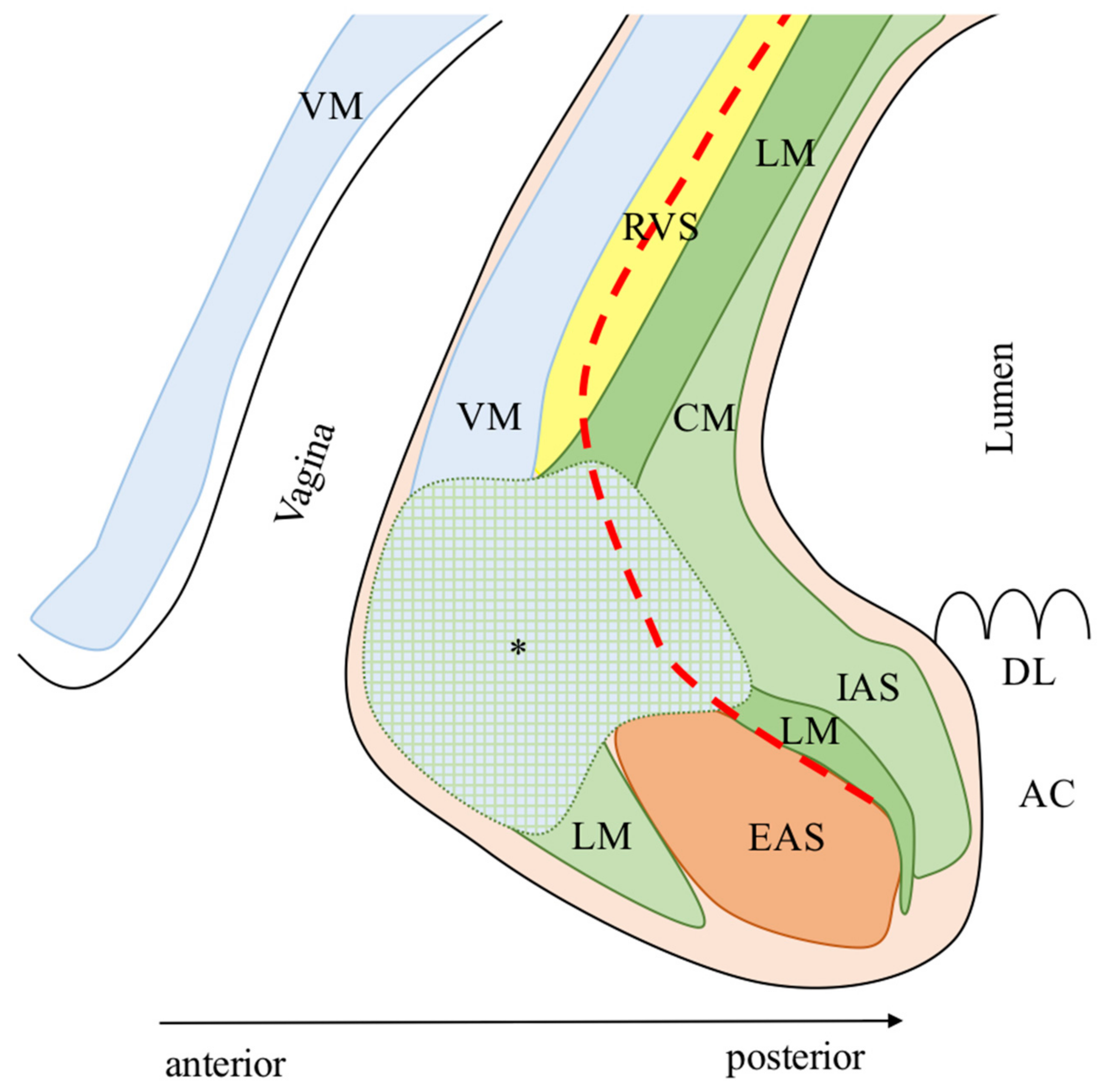
2. Anatomy of the Deep Pelvis
2.1. Posterior Anatomy
2.2. Anterior Anatomy
2.3. Lateral Anatomy
3. Definition of ISR
4. Indication of ISR
5. Distal Resection Margin and ISR
6. Circumferential Resection Margin and ISR
7. Neoadjuvant CRT and ISR
8. Surgical Approach: Open vs. Laparoscopic vs. Robotic
9. Risk Factors for Oncological Outcomes after ISR
10. ISR vs. APR
11. Patterns of LR after ISR
12. Considerations on Functional Outcomes after ISR
13. Learning Curve and Surgical Education on ISR
14. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AB | Anterior bundle of the longitudinal muscle |
| AC | Anal canal |
| ACL | Anococcygeal ligament |
| AM | Anal margin |
| APR | Abdominoperineal resection |
| AV | Anal verge |
| BM | Bulbospongiosus muscle |
| CAA | Coloanal anastomosis |
| CEA | Carcinoembryonic antigen |
| CI | Confidence interval |
| CM | Circumferential muscle |
| CRM | Circumferential margin |
| CSP | Corpus spongiosum of the penis |
| CT | Adjuvant chemotherapy |
| Cx | Coccyx |
| DFS | Disease free survival |
| DK | Dukes stage |
| DL | Dentate line |
| DM | Distant metastases |
| DRE | Digital rectal examination |
| DRM | Distal resection margin |
| EAS | External anal sphincter |
| ECOG PS | Eastern Cooperative Oncology Group scale of Performance Status |
| ESR | External sphincter resection |
| EUS | Endoscopic ultrasound |
| FU | Follow-up |
| HL | Hiatal ligament |
| HR | Hazard ratio |
| JSCCR | Japanese Society for Cancer of the Colon and Rectum |
| IAS | Internal anal sphincter |
| ISG | Intersphincteric groove |
| ISP | intersphincteric plane |
| ISR | Intersphincteric resection |
| LAM | Levator ani muscle |
| LISR | Laparoscopic intersphincteric resection |
| LM | Longitudinal muscle |
| LN | Lymph node |
| LR | Local recurrence |
| LRC | Low rectal cancer |
| LRFS | Local recurrence free survival |
| MRI | Magnetic resonance imaging |
| nCRT | Neoadjuvant chemoradiotherapy |
| OISR | Open intersphincteric resection |
| OR | Odds ratio |
| OS | Overall survival |
| PL | Parks’ ligament |
| PR | Prostate |
| RFS | Relapse free survival |
| RIP | Raphe of the iliococcygeus and pubococcygeus muscle |
| RISR | Robotic intersphincteric resection |
| RS | Urethral rhabdosphincter |
| RT | Radiotherapy |
| RU | Rectourethralis muscle |
| RVS | Rectovaginal septum |
| SP | Single port |
| TILME | Total intersphincteric longitudinal muscle excision |
| TME | Total mesorectal excision |
| UR | Urethra |
| VM | Vaginal smooth muscle layer |
| AB | Anterior bundle of the longitudinal muscle |
| AC | Anal canal |
| ACL | Anococcygeal ligament |
| AM | Anal margin |
| APR | Abdominoperineal resection |
References
- Miles, W.E. A method of performing abdomino-perineal excision for carcinoma of the rectum and of the terminal portion of the pelvic colon. Lancet 1908, 2, 1812–1813. [Google Scholar] [CrossRef] [Green Version]
- Ungley, H.G. The abdominoperineal excision (Miles’ operation). Proc. R. Soc. Med. 1959, 52, 36–40. [Google Scholar] [CrossRef] [Green Version]
- Hawkins, A.T.; Albutt, K.; Wise, P.E.; Alavi, K.; Sudan, R.; Kaiser, A.M.; Bordeianou, L. Abdominoperineal Resection for Rectal Cancer in the Twenty-First Century: Indications, Techniques, and Outcomes. J. Gastrointest. Surg. 2018, 22, 1477–1487. [Google Scholar] [CrossRef]
- Dixon, C.F. Anterior Resection for Malignant Lesions of the Upper Part of the Rectum and Lower Part of the Sigmoid. Ann. Surg. 1948, 128, 425–442. [Google Scholar] [CrossRef]
- Griffen, F.D.; Knight, C.D., Sr.; Whitaker, J.M.; Knight, C.D., Jr. The double stapling technique for low anterior resection. Results, modifications, and observations. Ann. Surg. 1990, 211, 745–752. [Google Scholar] [CrossRef]
- Toiyama, Y.; Kusunoki, M. Changes in surgical therapies for rectal cancer over the past 100 years: A review. Ann. Gastroenterol. Surg. 2020, 4, 331–342. [Google Scholar] [CrossRef]
- Heald, R.J.; Husband, E.M.; Ryall, R.D. The mesorectum in rectal cancer surgery—The clue to pelvic recurrence? Br. J. Surg. 1982, 69, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.J.; Moran, B.J.; Ryall, R.D.; Sexton, R.; MacFarlane, J.K. Rectal cancer: The Basingstoke experience of total mesorectal excision, 1978–1997. Arch. Surg. 1998, 133, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacFarlane, J.K.; Ryall, R.D.; Heald, R.J. Mesorectal excision for rectal cancer. Lancet 1993, 341, 457–460. [Google Scholar] [CrossRef]
- Minsky, B.D.; Cohen, A.M.; Kemeny, N.; Enker, W.E.; Kelsen, D.P.; Saltz, L.; Frankel, J. The efficacy of preoperative 5-fluorouracil, high-dose leucovorin, and sequential radiation therapy for unresectable rectal cancer. Cancer 1993, 71, 3486–3492. [Google Scholar] [CrossRef]
- Bujko, K.; Nowacki, M.P.; Oledzki, J.; Sopylo, R.; Skoczylas, J.; Chwalinski, M. Sphincter preservation after short-term preoperative radiotherapy for low rectal cancer—Presentation of own data and a literature review. Acta Oncol. 2001, 40, 593–601. [Google Scholar] [CrossRef]
- Theodoropoulos, G.; Wise, W.E.; Padmanabhan, A.; Kerner, B.A.; Taylor, C.W.; Aguilar, P.S.; Khanduja, K.S. T-level downstaging and complete pathologic response after preoperative chemoradiation for advanced rectal cancer result in decreased recurrence and improved disease-free survival. Dis. Colon Rectum 2002, 45, 895–903. [Google Scholar] [CrossRef] [PubMed]
- dePrisco, G. MRI Local Staging and Restaging in Rectal Cancer. Clin. Colon Rectal Surg. 2015, 28, 194–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.E.; Park, B.J.; Sung, D.J.; Kim, M.J.; Han, N.Y.; Sim, K.C.; Cho, S.B.; Kim, J.; Kim, S.H.; An, H. How to accurately measure the distance from the anal verge to rectal cancer on MRI: A prospective study using anal verge markers. Abdom. Radiol. 2020, 46, 449–458. [Google Scholar] [CrossRef]
- Kapiteijn, E.; Marijnen, C.A.; Nagtegaal, I.D.; Putter, H.; Steup, W.H.; Wiggers, T.; Rutten, H.J.; Pahlman, L.; Glimelius, B.; van Krieken, J.H.; et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N. Engl. J. Med. 2001, 345, 638–646. [Google Scholar] [CrossRef] [Green Version]
- Martling, A.L.; Holm, T.; Rutqvist, L.E.; Moran, B.J.; Heald, R.J.; Cedemark, B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet 2000, 356, 93–96. [Google Scholar] [CrossRef]
- Wibe, A.; Syse, A.; Andersen, E.; Tretli, S.; Myrvold, H.E.; Soreide, O.; on behalf of the Norwegian Rectal Cancer Group. Oncological outcomes after total mesorectal excision for cure for cancer of the lower rectum: Anterior vs. abdominoperineal resection. Dis. Colon Rectum 2004, 47, 48–58. [Google Scholar] [CrossRef]
- Schiessel, R.; Karner-Hanusch, J.; Herbst, F.; Teleky, B.; Wunderlich, M. Intersphincteric resection for low rectal tumours. Br. J. Surg. 1994, 81, 1376–1378. [Google Scholar] [CrossRef]
- Schiessel, R.; Novi, G.; Holzer, B.; Rosen, H.R.; Renner, K.; Holbling, N.; Feil, W.; Urban, M. Technique and long-term results of intersphincteric resection for low rectal cancer. Dis. Colon Rectum 2005, 48, 1858–1865; discussion 1865–1867. [Google Scholar] [CrossRef]
- Piozzi, G.N.; Park, H.; Choi, T.S.; Kim, S.H. Intersphincteric Resection for Low Rectal Cancer: A Review on Anatomy and Surgical Technique, Oncologic and Functional Outcomes and the Role of Robotics. Turk. J. Colorectal Dis. 2020, 30, 76–85. [Google Scholar] [CrossRef]
- Piozzi, G.N.; Park, H.; Kim, J.S.; Choi, H.B.; Lee, T.H.; Baek, S.J.; Kwak, J.M.; Kim, J.; Kim, S.H. Anatomical Landmarks for Transabdominal Robotic-Assisted Intersphincteric Dissection for Ultralow Anterior Resection. Dis. Colon Rectum 2021, 64, e87–e88. [Google Scholar] [CrossRef]
- Muro, S.; Yamaguchi, K.; Nakajima, Y.; Watanabe, K.; Harada, M.; Nimura, A.; Akita, K. Dynamic intersection of the longitudinal muscle and external anal sphincter in the layered structure of the anal canal posterior wall. Surg. Radiol. Anat. 2014, 36, 551–559. [Google Scholar] [CrossRef]
- Kraima, A.C.; West, N.P.; Roberts, N.; Magee, D.R.; Smit, N.N.; van de Velde, C.J.H.; DeRuiter, M.C.; Rutten, H.J.; Quirke, P. The role of the longitudinal muscle in the anal sphincter complex: Implications for the Intersphincteric Plane in Low Rectal Cancer Surgery? Clin. Anat. 2020, 33, 567–577. [Google Scholar] [CrossRef]
- Muro, S.; Kagawa, R.; Habu, M.; Ka, H.; Harada, M.; Akita, K. Coexistence of Dense and Sparse Areas in the Longitudinal Smooth Muscle of the Anal Canal: Anatomical and Histological Analyses Inspired by Magnetic Resonance Images. Clin. Anat. 2020, 33, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parks, A.G. A note on the anatomy of the anal canal. Proc. R. Soc. Med. 1954, 47, 997–998. [Google Scholar] [CrossRef] [Green Version]
- Toldt, B. Anatomischer Atlas für Studierende und Arzte; Berlin; Urban & Schwarzenberg: Munich, Germany, 1903; pp. 526–538. [Google Scholar]
- Jorge, J.M.N.; Habr-Gama, A. Anatomy and Embriology. In The ASCRS Textbook of Colon and Rectum Surgery, 2nd ed.; Beck, D.E., Ed.; Springer Science and Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Milligan, E.; Morgan, C. Surgical anatomy of the anal canal with special reference to anorectal fistule. Lancet 1934, 224, 1150–1156. [Google Scholar] [CrossRef]
- Suriyut, J.; Muro, S.; Baramee, P.; Harada, M.; Akita, K. Various significant connections of the male pelvic floor muscles with special reference to the anal and urethral sphincter muscles. Anat. Sci. Int. 2020, 95, 305–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsukada, Y.; Ito, M.; Watanabe, K.; Yamaguchi, K.; Kojima, M.; Hayashi, R.; Akita, K.; Saito, N. Topographic Anatomy of the Anal Sphincter Complex and Levator Ani Muscle as It Relates to Intersphincteric Resection for Very Low Rectal Disease. Dis. Colon Rectum 2016, 59, 426–433. [Google Scholar] [CrossRef]
- Shafik, A. New concept of the anatomy of the anal sphincter mechanism and the physiology of defecation. II. Anatomy of the levator ani muscle with special reference to puborectalis. Investig. Urol. 1975, 13, 175–182. [Google Scholar]
- Nakajima, Y.; Muro, S.; Nasu, H.; Harada, M.; Yamaguchi, K.; Akita, K. Morphology of the region anterior to the anal canal in males: Visualization of the anterior bundle of the longitudinal muscle by transanal ultrasonography. Surg. Radiol. Anat. 2017, 39, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Muro, S.; Tsukada, Y.; Harada, M.; Ito, M.; Akita, K. Anatomy of the smooth muscle structure in the female anorectal anterior wall: Convergence and anterior extension of the internal anal sphincter and longitudinal muscle. Colorectal Dis. 2019, 21, 472–480. [Google Scholar] [CrossRef]
- Muro, S.; Tsukada, Y.; Harada, M.; Ito, M.; Akita, K. Spatial distribution of smooth muscle tissue in the male pelvic floor with special reference to the lateral extent of the rectourethralis muscle: Application to prostatectomy and proctectomy. Clin. Anat. 2018, 31, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Uchimoto, K.; Murakami, G.; Kinugasa, Y.; Arakawa, T.; Matsubara, A.; Nakajima, Y. Rectourethralis muscle and pitfalls of anterior perineal dissection in abdominoperineal resection and intersphincteric resection for rectal cancer. Anat. Sci. Int. 2007, 82, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Nyangoh Timoh, K.; Deffon, J.; Moszkowicz, D.; Lebacle, C.; Creze, M.; Martinovic, J.; Zaitouna, M.; Diallo, D.; Lavoue, V.; Fautrel, A.; et al. Smooth muscle of the male pelvic floor: An anatomic study. Clin. Anat. 2020, 33, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Lyttle, J.A.; Parks, A.G. Intersphincteric excision of the rectum. Br. J. Surg. 1977, 64, 413–416. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.G. Endoanal technique of low colonic anastomosis. Surg. Tech. 1977, 2, 63–70. [Google Scholar]
- Schiessel, R. Surgical technique of intersphincteric resection. In Intersphincteric Resection for Low Rectal Tumors; Schiessel, R., Metzger, P., Eds.; Springer: Vienna, Austria, 2012; pp. 73–84. [Google Scholar]
- Kang, D.W.; Kwak, H.D.; Sung, N.S.; Yang, I.S.; Baek, S.J.; Kwak, J.M.; Kim, J.; Kim, S.H. Oncologic outcomes in rectal cancer patients with a ≤1-cm distal resection margin. Int. J. Colorectal Dis. 2017, 32, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Akagi, Y.; Kinugasa, T.; Shirouzu, K. Intersphincteric resection for very low rectal cancer: A systematic review. Surg. Today 2013, 43, 838–847. [Google Scholar] [CrossRef]
- Saito, N.; Moriya, Y.; Shirouzu, K.; Maeda, K.; Mochizuki, H.; Koda, K.; Hirai, T.; Sugito, M.; Ito, M.; Kobayashi, A. Intersphincteric resection in patients with very low rectal cancer: A review of the Japanese experience. Dis. Colon Rectum 2006, 49, S13–S22. [Google Scholar] [CrossRef]
- Kohler, A.; Athanasiadis, S.; Ommer, A.; Psarakis, E. Long-term results of low anterior resection with intersphincteric anastomosis in carcinoma of the lower one-third of the rectum: Analysis of 31 patients. Dis. Colon Rectum 2000, 43, 843–850. [Google Scholar] [CrossRef]
- Tiret, E.; Poupardin, B.; McNamara, D.; Dehni, N.; Parc, R. Ultralow anterior resection with intersphincteric dissection--what is the limit of safe sphincter preservation? Colorectal Dis. 2003, 5, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Rullier, E.; Laurent, C.; Bretagnol, F.; Rullier, A.; Vendrely, V.; Zerbib, F. Sphincter-saving resection for all rectal carcinomas: The end of the 2-cm distal rule. Ann. Surg. 2005, 241, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Hohenberger, W.; Merkel, S.; Matzel, K.; Bittorf, B.; Papadopoulos, T.; Gohl, J. The influence of abdomino-peranal (intersphincteric) resection of lower third rectal carcinoma on the rates of sphincter preservation and locoregional recurrence. Colorectal Dis. 2006, 8, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Chin, C.C.; Yeh, C.Y.; Huang, W.S.; Wang, J.Y. Clinical outcome of intersphincteric resection for ultra-low rectal cancer. World J. Gastroenterol. 2006, 12, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Chamlou, R.; Parc, Y.; Simon, T.; Bennis, M.; Dehni, N.; Parc, R.; Tiret, E. Long-term results of intersphincteric resection for low rectal cancer. Ann. Surg. 2007, 246, 916–921; discussion 921–922. [Google Scholar] [CrossRef]
- Portier, G.; Ghouti, L.; Kirzin, S.; Guimbaud, R.; Rives, M.; Lazorthes, F. Oncological outcome of ultra-low coloanal anastomosis with and without intersphincteric resection for low rectal adenocarcinoma. Br. J. Surg. 2007, 94, 341–345. [Google Scholar] [CrossRef]
- Akasu, T.; Takawa, M.; Yamamoto, S.; Fujita, S.; Moriya, Y. Incidence and patterns of recurrence after intersphincteric resection for very low rectal adenocarcinoma. J. Am. Coll. Surg. 2007, 205, 642–647. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yin, L.; Huang, L.; Zhang, H.B.; Han, Y.; Lin, M.B. Long-term results of intersphincteric resection for low rectal cancer. J. Investig. Surg. 2013, 26, 217–222. [Google Scholar] [CrossRef]
- Tokoro, T.; Okuno, K.; Hida, J.; Ueda, K.; Yoshifuji, T.; Daito, K.; Takemoto, M.; Sugiura, F. Analysis of the clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. World J. Surg. Oncol. 2013, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Akagi, Y.; Shirouzu, K.; Ogata, Y.; Kinugasa, T. Oncologic outcomes of intersphincteric resection without preoperative chemoradiotherapy for very low rectal cancer. Surg. Oncol. 2013, 22, 144–149. [Google Scholar] [CrossRef]
- Mahalingam, S.; Seshadri, R.A.; Veeraiah, S. Long-Term Functional and Oncological Outcomes Following Intersphincteric Resection for Low Rectal Cancers. Indian J. Surg. Oncol. 2017, 8, 457–461. [Google Scholar] [CrossRef]
- Matsunaga, R.; Kojima, M.; Nishizawa, Y.; Yokota, M.; Hasegawa, H.; Saito, N.; Ito, M.; Ochiai, A. The utility of longitudinal slicing method for rectal specimen: Pathological analysis of circumferential resection margin of intersphincteric resection for low-lying rectal cancer. Pathol. Int. 2019, 69, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Park, S.Y.; Kim, H.J.; Cho, S.H.; Kwak, S.G.; Choi, G.S. Long-term Oncologic Outcomes After Neoadjuvant Chemoradiation Followed by Intersphincteric Resection With Coloanal Anastomosis for Locally Advanced Low Rectal Cancer. Dis. Colon Rectum 2019, 62, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Piozzi, G.N.; Park, H.; Lee, T.H.; Kim, J.S.; Choi, H.B.; Baek, S.J.; Kwak, J.M.; Kim, J.; Kim, S.H. Risk factors for local recurrence and long term survival after minimally invasive intersphincteric resection for very low rectal cancer: Multivariate analysis in 161 patients. Eur. J. Surg. Oncol. 2021, 47, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, G.I.; Odaryuk, T.S.; Tsarkov, P.V.; Talalakin, A.I.; Rybakov, E.G. Resection of the rectum and total excision of the internal anal sphincter with smooth muscle plasty and colonic pouch for treatment of ultralow rectal carcinoma. Br. J. Surg. 2004, 91, 1506–1512. [Google Scholar] [CrossRef] [PubMed]
- Krand, O.; Yalti, T.; Tellioglu, G.; Kara, M.; Berber, I.; Titiz, M.I. Use of smooth muscle plasty after intersphincteric rectal resection to replace a partially resected internal anal sphincter: Long-term follow-up. Dis. Colon Rectum 2009, 52, 1895–1901. [Google Scholar] [CrossRef]
- Han, J.G.; Wei, G.H.; Gao, Z.G.; Zheng, Y.; Wang, Z.J. Intersphincteric resection with direct coloanal anastomosis for ultralow rectal cancer: The experience of People’s Republic of China. Dis. Colon Rectum 2009, 52, 950–957. [Google Scholar] [CrossRef]
- Weiser, M.R.; Quah, H.M.; Shia, J.; Guillem, J.G.; Paty, P.B.; Temple, L.K.; Goodman, K.A.; Minsky, B.D.; Wong, W.D. Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection. Ann. Surg. 2009, 249, 236–242. [Google Scholar] [CrossRef]
- Kuo, L.J.; Hung, C.S.; Wu, C.H.; Wang, W.; Tam, K.W.; Liang, H.H.; Chang, Y.J.; Wei, P.L. Oncological and functional outcomes of intersphincteric resection for low rectal cancer. J. Surg. Res. 2011, 170, e93–e98. [Google Scholar] [CrossRef]
- Gong, X.; Jin, Z.; Zheng, Q. Anorectal function after partial intersphincteric resection in ultra-low rectal cancer. Colorectal Dis. 2012, 14, e802–e806. [Google Scholar] [CrossRef]
- Saito, N.; Ito, M.; Kobayashi, A.; Nishizawa, Y.; Kojima, M.; Nishizawa, Y.; Sugito, M. Long-term outcomes after intersphincteric resection for low-lying rectal cancer. Ann. Surg. Oncol. 2014, 21, 3608–3615. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Gawad, W.; Zaghloul, A.; Fakhr, I.; Sakr, M.; Shabana, A.; Lotayef, M.; Mansour, O. Evaluation of the frequency and pattern of local recurrence following intersphincteric resection for ultra-low rectal cancer. J. Egypt. Natl. Cancer Inst. 2014, 26, 87–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koide, Y.; Maeda, K.; Katsuno, H.; Hanai, T.; Masumori, K.; Matsuoka, H.; Endo, T.; Cheong, Y.C.; Uyama, I. Exfoliated cancer cells during intersphincteric resection for very low rectal cancer. Surg. Today 2020, 50, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Choi, G.S.; Park, J.S.; Kim, H.J.; Choi, W.H.; Ryuk, J.P. Robotic-assisted transabdominal intersphincteric resection: A technique involving a completely abdominal approach and coloanal anastomosis. Surg. Laparosc. Endosc. Percutaneous Tech. 2013, 23, e5–e10. [Google Scholar] [CrossRef]
- Kim, J.C.; Kim, C.W.; Lee, J.L.; Yoon, Y.S.; Park, I.J.; Kim, J.R.; Kim, J.; Park, S.H. Complete intersphincteric longitudinal muscle excision May Be key to reducing local recurrence during intersphincteric resection. Eur. J. Surg. Oncol. 2021, 47, 1629–1636. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.T.; Heneghan, H.M.; Winter, D.C. Systematic review of outcomes after intersphincteric resection for low rectal cancer. Br. J. Surg. 2012, 99, 603–612. [Google Scholar] [CrossRef]
- Shirouzu, K.; Murakami, N.; Akagi, Y. Intersphincteric resection for very low rectal cancer: A review of the updated literature. Ann. Gastroenterol. Surg. 2017, 1, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.; Saiki, Y.; Takano, S.; Iwamoto, K.; Tanaka, M.; Fukunaga, M.; Noguchi, T.; Nakamura, Y.; Hisano, S.; Fukami, K.; et al. Long-term results of intersphincteric resection for low rectal cancer in Japan. Surg. Today 2019, 49, 275–285. [Google Scholar] [CrossRef]
- Rullier, E.; Denost, Q.; Vendrely, V.; Rullier, A.; Laurent, C. Low rectal cancer: Classification and standardization of surgery. Dis. Colon Rectum 2013, 56, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Shirouzu, K.; Ogata, Y.; Araki, Y.; Kishimoto, Y.; Sato, Y. A new ultimate anus-preserving operation for extremely low rectal cancer and for anal canal cancer. Tech. Coloproctol. 2003, 7, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Shelygin, Y.A.; Vorobiev, G.I.; Pikunov, D.Y.; Markova, E.V.; Djhanaev, Y.A.; Fomenko, O.Y. Intersphincteric resection with partial removal of external anal sphincter for low rectal cancer. Acta Chir. Iugosl. 2008, 55, 45–53. [Google Scholar] [CrossRef]
- Kang, B.M.; Park, Y.K.; Park, S.J.; Lee, K.Y.; Kim, C.W.; Lee, S.H. Does circumferential tumor location affect the circumferential resection margin status in mid and low rectal cancer? Asian J. Surg. 2018, 41, 257–263. [Google Scholar] [CrossRef]
- Goligher, J.C.; Dukes, C.E.; Bussey, H.J.R. Local recurrences after sphincter saving excisions for carcinoma of the rectum and rectosigmoid. Br. J. Surg. 1951, 39, 199–211. [Google Scholar] [CrossRef]
- Williams, N.S.; Dixon, M.F.; Johnston, D. Reappraisal of the 5 centimetre rule of distal excision for carcinoma of the rectum: A study of distal intramural spread and of patients’ survival. Br. J. Surg. 1983, 70, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Pollett, W.G.; Nicholls, R.J. The relationship between the extent of distal clearance and survival and local recurrence rates after curative anterior resection for carcinoma of the rectum. Ann. Surg. 1983, 198, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Tsarkov, P. Abdominoperineal resection. In Rectal Cancer: New Frontiers in Diagnosis, Treatment and Rehabilitation; Springer: Milan, Italy, 2006; Volume 157Y65. [Google Scholar]
- Kuvshinoff, B.; Maghfoor, I.; Miedema, B.; Bryer, M.; Westgate, S.; Wilkes, J.; Ota, D. Distal margin requirements after preoperative chemoradiotherapy for distal rectal carcinomas: Are <or = 1 cm distal margins sufficient? Ann. Surg. Oncol. 2001, 8, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Moore, H.G.; Riedel, E.; Minsky, B.D.; Saltz, L.; Paty, P.; Wong, D.; Cohen, A.M.; Guillem, J.G. Adequacy of 1-cm distal margin after restorative rectal cancer resection with sharp mesorectal excision and preoperative combined-modality therapy. Ann. Surg. Oncol. 2003, 10, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mochizuki, H.; Hashiguchi, Y.; Ishikawa, K.; Fujimoto, H.; Shinto, E.; Hase, K. Preoperative parameters expanding the indication of sphincter preserving surgery in patients with advanced low rectal cancer. Ann. Surg. 2004, 239, 34–42. [Google Scholar] [CrossRef]
- Bernstein, T.E.; Endreseth, B.H.; Romundstad, P.; Wibe, A.; Norwegian Colorectal Cancer, R. What is a safe distal resection margin in rectal cancer patients treated by low anterior resection without preoperative radiotherapy? Colorectal Dis. 2012, 14, e48–e55. [Google Scholar] [CrossRef]
- Mezhir, J.J.; Shia, J.; Riedel, E.; Temple, L.K.; Nash, G.M.; Weiser, M.R.; Paty, P.B.; Wong, W.D.; Guillem, J.G. Whole-mount pathologic analysis of rectal cancer following neoadjuvant therapy: Implications of margin status on long-term oncologic outcome. Ann. Surg. 2012, 256, 274–279. [Google Scholar] [CrossRef]
- Rutkowski, A.; Nowacki, M.P.; Chwalinski, M.; Oledzki, J.; Bednarczyk, M.; Liszka-Dalecki, P.; Gornicki, A.; Bujko, K. Acceptance of a 5-mm distal bowel resection margin for rectal cancer: Is it safe? Colorectal Dis. 2012, 14, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Karanjia, N.D.; Schache, D.J.; North, W.R.; Heald, R.J. ‘Close shave’ in anterior resection. Br. J. Surg. 1990, 77, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.S.; Soman, A.; Sacksner, J. Disparate surgical margin lengths of colorectal resection specimens between in vivo and in vitro measurements. The effects of surgical resection and formalin fixation on organ shrinkage. Am. J. Clin. Pathol. 1999, 111, 349–351. [Google Scholar] [CrossRef] [Green Version]
- Koyama, M.; Murata, A.; Sakamoto, Y.; Morohashi, H.; Takahashi, S.; Yoshida, E.; Hakamada, K. Long-term clinical and functional results of intersphincteric resection for lower rectal cancer. Ann. Surg. Oncol. 2014, 21 (Suppl. S3), S422–S428. [Google Scholar] [CrossRef]
- Klose, J.; Tarantino, I.; Kulu, Y.; Bruckner, T.; Trefz, S.; Schmidt, T.; Schneider, M.; Hackert, T.; Buchler, M.W.; Ulrich, A. Sphincter-Preserving Surgery for Low Rectal Cancer: Do We Overshoot the Mark? J. Gastrointest. Surg. 2017, 21, 885–891. [Google Scholar] [CrossRef]
- Molnar, C.; Nicolescu, C.; Grigorescu, B.L.; Botoncea, M.; Butiurca, V.O.; Petrisor, M.D.; Gurzu, S. Comparative oncological outcomes and survival following surgery for low rectal cancer—A single center experience. Rom. J. Morphol. Embryol. 2019, 60, 847–852. [Google Scholar]
- Quirke, P.; Durdey, P.; Dixon, M.F.; Williams, N.S. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet 1986, 2, 996–999. [Google Scholar] [CrossRef]
- Birbeck, K.F.; Macklin, C.P.; Tiffin, N.J.; Parsons, W.; Dixon, M.F.; Mapstone, N.P.; Abbott, C.R.; Scott, N.; Finan, P.J.; Johnston, D.; et al. Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery. Ann. Surg. 2002, 235, 449–457. [Google Scholar] [CrossRef]
- Marijnen, C.A.; Nagtegaal, I.D.; Kapiteijn, E.; Kranenbarg, E.K.; Noordijk, E.M.; van Krieken, J.H.; van de Velde, C.J.; Leer, J.W.; Cooperative Investigators of the Dutch Colorectal Cancer Group. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: Report of a multicenter randomized trial. Int. J. Radiat. Oncol. Biol. Phys. 2003, 55, 1311–1320. [Google Scholar] [CrossRef]
- Adam, I.J.; Mohamdee, M.O.; Martin, I.G.; Scott, N.; Finan, P.J.; Johnston, D.; Dixon, M.F.; Quirke, P. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet 1994, 344, 707–711. [Google Scholar] [CrossRef]
- Tilney, H.S.; Rasheed, S.; Northover, J.M.; Tekkis, P.P. The influence of circumferential resection margins on long-term outcomes following rectal cancer surgery. Dis. Colon Rectum 2009, 52, 1723–1729. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Quirke, P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J. Clin. Oncol. 2008, 26, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Kelly, S.B.; Mills, S.J.; Bradburn, D.M.; Ratcliffe, A.A.; Borowski, D.W.; Northern Region Colorectal Cancer Audit Group. Effect of the circumferential resection margin on survival following rectal cancer surgery. Br. J. Surg. 2011, 98, 573–581. [Google Scholar] [CrossRef]
- Nikberg, M.; Kindler, C.; Chabok, A.; Letocha, H.; Shetye, J.; Smedh, K. Circumferential resection margin as a prognostic marker in the modern multidisciplinary management of rectal cancer. Dis. Colon Rectum 2015, 58, 275–282. [Google Scholar] [CrossRef]
- Park, J.S.; Huh, J.W.; Park, Y.A.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y.; Chun, H.K. A circumferential resection margin of 1 mm is a negative prognostic factor in rectal cancer patients with and without neoadjuvant chemoradiotherapy. Dis. Colon Rectum 2014, 57, 933–940. [Google Scholar] [CrossRef]
- Tilly, C.; Lefevre, J.H.; Svrcek, M.; Shields, C.; Flejou, J.F.; Tiret, E.; Parc, Y. R1 rectal resection: Look up and don’t look down. Ann. Surg. 2014, 260, 794–799; discussion 799–800. [Google Scholar] [CrossRef] [PubMed]
- Campa-Thompson, M.; Weir, R.; Calcetera, N.; Quirke, P.; Carmack, S. Pathologic processing of the total mesorectal excision. Clin. Colon Rectal Surg. 2015, 28, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Lefevre, J.H.; Mineur, L.; Kotti, S.; Rullier, E.; Rouanet, P.; de Chaisemartin, C.; Meunier, B.; Mehrdad, J.; Cotte, E.; Desrame, J.; et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J. Clin. Oncol. 2016, 34, 3773–3780. [Google Scholar] [CrossRef]
- Kinoshita, H.; Watanabe, T.; Yanagisawa, A.; Nagawa, H.; Kato, Y.; Muto, T. Pathological changes of advanced lower-rectal cancer by preoperative radiotherapy. Hepatogastroenterology 2004, 51, 1362–1366. [Google Scholar]
- Peeters, K.C.; Marijnen, C.A.; Nagtegaal, I.D.; Kranenbarg, E.K.; Putter, H.; Wiggers, T.; Rutten, H.; Pahlman, L.; Glimelius, B.; Leer, J.W.; et al. The TME trial after a median follow-up of 6 years: Increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann. Surg. 2007, 246, 693–701. [Google Scholar] [CrossRef] [PubMed]
- National Comprehensive Cancer Network. Rectal Cancer (Version 1.2021). Available online: https://www.nccn.org/professionals/physician_gls/PDF/rectal.pdf (accessed on 29 January 2021).
- Ito, M.; Saito, N.; Sugito, M.; Kobayashi, A.; Nishizawa, Y.; Tsunoda, Y. Analysis of clinical factors associated with anal function after intersphincteric resection for very low rectal cancer. Dis. Colon Rectum 2009, 52, 64–70. [Google Scholar] [CrossRef] [Green Version]
- Hassan, I.; Larson, D.W.; Wolff, B.G.; Cima, R.R.; Chua, H.K.; Hahnloser, D.; O’Byrne, M.M.; Larson, D.R.; Pemberton, J.H. Impact of pelvic radiotherapy on morbidity and durability of sphincter preservation after coloanal anastomosis for rectal cancers. Dis. Colon Rectum 2008, 51, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Gervaz, P.; Rotholtz, N.; Wexner, S.D.; You, S.Y.; Saigusa, N.; Kaplan, E.; Secic, M.; Weiss, E.G.; Nogueras, J.J.; Belin, B. Colonic J-pouch function in rectal cancer patients: Impact of adjuvant chemoradiotherapy. Dis. Colon Rectum 2001, 44, 1667–1675. [Google Scholar] [CrossRef]
- Bonnel, C.; Parc, Y.R.; Pocard, M.; Dehni, N.; Caplin, S.; Parc, R.; Tiret, E. Effects of preoperative radiotherapy for primary resectable rectal adenocarcinoma on male sexual and urinary function. Dis. Colon Rectum 2002, 45, 934–939. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Park, I.J.; Joh, Y.G.; Hahn, K.Y. Laparoscopic resection of rectal cancer: A comparison of surgical and oncologic outcomes between extraperitoneal and intraperitoneal disease locations. Dis. Colon Rectum 2008, 51, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.J.; Kim, S.H.; Kwak, J.M.; Cho, J.S.; Shin, J.W.; Amar, A.H.; Kim, J. Selective use of preoperative chemoradiotherapy for T3 rectal cancer can be justified: Analysis of local recurrence. World J. Surg. 2013, 37, 220–226. [Google Scholar] [CrossRef]
- Feeney, G.; Sehgal, R.; Sheehan, M.; Hogan, A.; Regan, M.; Joyce, M.; Kerin, M. Neoadjuvant radiotherapy for rectal cancer management. World J. Gastroenterol. 2019, 25, 4850–4869. [Google Scholar] [CrossRef]
- Guillou, P.J.; Quirke, P.; Thorpe, H.; Walker, J.; Jayne, D.G.; Smith, A.M.; Heath, R.M.; Brown, J.M.; for the MRC CLASICC Trial Group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet 2005, 365, 1718–1726. [Google Scholar] [CrossRef]
- Kang, S.B.; Park, J.W.; Jeong, S.Y.; Nam, B.H.; Choi, H.S.; Kim, D.W.; Lim, S.B.; Lee, T.G.; Kim, D.Y.; Kim, J.S.; et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): Short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010, 11, 637–645. [Google Scholar] [CrossRef]
- Leung, K.L.; Kwok, S.P.; Lam, S.C.; Lee, J.F.; Yiu, R.Y.; Ng, S.S.; Lai, P.B.; Lau, W.Y. Laparoscopic resection of rectosigmoid carcinoma: Prospective randomised trial. Lancet 2004, 363, 1187–1192. [Google Scholar] [CrossRef]
- Milsom, J.W.; Bohm, B.; Hammerhofer, K.A.; Fazio, V.; Steiger, E.; Elson, P. A prospective, randomized trial comparing laparoscopic versus conventional techniques in colorectal cancer surgery: A preliminary report. J. Am. Coll. Surg. 1998, 187, 46–54; discussion 54–55. [Google Scholar] [CrossRef]
- Van der Pas, M.H.; Haglind, E.; Cuesta, M.A.; Furst, A.; Lacy, A.M.; Hop, W.C.; Bonjer, H.J.; COlorectal Cancer Laparoscopic or Open Resection II (COLOR II) Study Group. Laparoscopic versus open surgery for rectal cancer (COLOR II): Short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013, 14, 210–218. [Google Scholar] [CrossRef]
- Kim, N.K.; Kim, M.S.; Al-Asari, S.F. Update and debate issues in surgical treatment of middle and low rectal cancer. J. Korean Soc. Coloproctol. 2012, 28, 230–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clinical Outcomes of Surgical Therapy Study Group; Nelson, H.; Sargent, D.J.; Wieand, H.S.; Fleshman, J.; Anvari, M.; Stryker, S.J.; Beart, R.W., Jr.; Hellinger, M.; Flanagan, R., Jr.; et al. A comparison of laparoscopically assisted and open colectomy for colon cancer. N. Engl. J. Med. 2004, 350, 2050–2059. [Google Scholar] [CrossRef] [Green Version]
- Chouhan, H.; Shin, J.; Kim, S.H. Is robotic rectal resection the preferred option for resectable cancer? Mini-Invasive Surg. 2018, 2, 18. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.J.; Kim, C.H.; Cho, M.S.; Bae, S.U.; Hur, H.; Min, B.S.; Baik, S.H.; Lee, K.Y.; Kim, N.K. Robotic surgery for rectal cancer can overcome difficulties associated with pelvic anatomy. Surg. Endosc. 2015, 29, 1419–1424. [Google Scholar] [CrossRef]
- Park, S.Y.; Choi, G.S.; Park, J.S.; Kim, H.J.; Ryuk, J.P. Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: A retrospective comparison with conventional laparoscopy. Surg. Endosc. 2013, 27, 48–55. [Google Scholar] [CrossRef]
- Leong, Q.M.; Son, D.N.; Cho, J.S.; Baek, S.J.; Kwak, J.M.; Amar, A.H.; Kim, S.H. Robot-assisted intersphincteric resection for low rectal cancer: Technique and short-term outcome for 29 consecutive patients. Surg. Endosc. 2011, 25, 2987–2992. [Google Scholar] [CrossRef]
- Hellan, M.; Stein, H.; Pigazzi, A. Totally robotic low anterior resection with total mesorectal excision and splenic flexure mobilization. Surg. Endosc. 2009, 23, 447–451. [Google Scholar] [CrossRef]
- Kim, S.H.; Kwak, J.M. Robotic total mesorectal excision: Operative technique and review of the literature. Tech. Coloproctol. 2013, 17 (Suppl. S1), S47–S53. [Google Scholar] [CrossRef]
- Kim, J.C.; Lim, S.B.; Yoon, Y.S.; Park, I.J.; Kim, C.W.; Kim, C.N. Completely abdominal intersphincteric resection for lower rectal cancer: Feasibility and comparison of robot-assisted and open surgery. Surg. Endosc. 2014, 28, 2734–2744. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, J.L.; Bong, J.W.; Seo, J.H.; Kim, C.W.; Park, S.H.; Kim, J. Oncological and anorectal functional outcomes of robot-assisted intersphincteric resection in lower rectal cancer, particularly the extent of sphincter resection and sphincter saving. Surg. Endosc. 2019, 34, 2082–2094. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Lee, J.L.; Alotaibi, A.M.; Yoon, Y.S.; Kim, C.W.; Park, I.J. Robot-assisted intersphincteric resection facilitates an efficient sphincter-saving in patients with low rectal cancer. Int. J. Colorectal Dis. 2017, 32, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Yamamoto, S.; Ito, M.; Yamaguchi, S.; Sakamoto, K.; Kinugasa, Y.; Kokuba, Y.; Okuda, J.; Yoshimura, K.; Watanabe, M. Short-term outcomes of laparoscopic intersphincteric resection from a phase II trial to evaluate laparoscopic surgery for stage 0/I rectal cancer: Japan Society of Laparoscopic Colorectal Surgery Lap RC. Surg. Endosc. 2012, 26, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Choi, G.S.; Jun, S.H.; Hasegawa, S.; Sakai, Y. Laparoscopic versus open intersphincteric resection and coloanal anastomosis for low rectal cancer: Intermediate-term oncologic outcomes. Ann. Surg. 2011, 254, 941–946. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Q.; Hu, T.; Gu, C.; Bi, L.; Wang, Z. Laparoscopic Versus Conventional Open Surgery in Intersphincteric Resection for Low Rectal Cancer: A Systematic Review and Meta-Analysis. J. Laparoendosc Adv. Surg. Tech. A 2018, 28, 189–200. [Google Scholar] [CrossRef]
- Chen, H.; Ma, B.; Gao, P.; Wang, H.; Song, Y.; Tong, L.; Li, P.; Wang, Z. Laparoscopic intersphincteric resection versus an open approach for low rectal cancer: A meta-analysis. World J. Surg. Oncol. 2017, 15, 229. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.K.; Kim, H.C.; Lee, W.Y.; Yun, S.H.; Cho, Y.B.; Huh, J.W.; Park, Y.A. Minimally invasive versus open intersphincteric resection of low rectal cancer regardless of neoadjuvant chemoradiotherapy: Long-term oncologic outcomes. Sci. Rep. 2021, 11, 11001. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, D.H.; Lim, S.W. Robotic versus laparoscopic intersphincteric resection for low rectal cancer: A systematic review and meta-analysis. Int. J. Colorectal Dis. 2018, 33, 1741–1753. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, N.K.; Kim, S.H.; Lee, K.Y.; Lee, K.Y.; Shin, J.Y.; Kim, C.N.; Choi, G.S.; Korean Laparoscopic Colorectal Surgery Study Group. Multicentre study of robotic intersphincteric resection for low rectal cancer. Br. J. Surg. 2015, 102, 1567–1573. [Google Scholar] [CrossRef]
- Kuo, L.J.; Lin, Y.K.; Chang, C.C.; Tai, C.J.; Chiou, J.F.; Chang, Y.J. Clinical outcomes of robot-assisted intersphincteric resection for low rectal cancer: Comparison with conventional laparoscopy and multifactorial analysis of the learning curve for robotic surgery. Int. J. Colorectal Dis. 2014, 29, 555–562. [Google Scholar] [CrossRef]
- Baek, S.J.; Al-Asari, S.; Jeong, D.H.; Hur, H.; Min, B.S.; Baik, S.H.; Kim, N.K. Robotic versus laparoscopic coloanal anastomosis with or without intersphincteric resection for rectal cancer. Surg. Endosc. 2013, 27, 4157–4163. [Google Scholar] [CrossRef]
- Yoo, B.E.; Cho, J.S.; Shin, J.W.; Lee, D.W.; Kwak, J.M.; Kim, J.; Kim, S.H. Robotic versus laparoscopic intersphincteric resection for low rectal cancer: Comparison of the operative, oncological, and functional outcomes. Ann. Surg. Oncol. 2015, 22, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Rullier, E.; Sa Cunha, A.; Couderc, P.; Rullier, A.; Gontier, R.; Saric, J. Laparoscopic intersphincteric resection with coloplasty and coloanal anastomosis for mid and low rectal cancer. Br. J. Surg. 2003, 90, 445–451. [Google Scholar] [CrossRef]
- Laurent, C.; Paumet, T.; Leblanc, F.; Denost, Q.; Rullier, E. Intersphincteric resection for low rectal cancer: Laparoscopic vs open surgery approach. Colorectal Dis. 2012, 14, 35–41; discussion 42–43. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, G.S.; Song, S.H.; Park, J.S.; Park, S.Y.; Lee, S.M.; Choi, J.A. An initial experience with a novel technique of single-port robotic resection for rectal cancer. Tech. Coloproctol. 2021, 25, 857–864. [Google Scholar] [CrossRef]
- Cheong, J.Y.; Choo, J.M.; Kim, J.S.; Rusli, S.M.; Kim, J.; Kim, S.H. Da Vinci SP system optimized for intersphincteric resection (ISR) of very low rectal cancer. Dis. Colon Rectum 2021. accepted for publication. [Google Scholar]
- Lee, S.Y.; Jo, J.S.; Kim, H.J.; Kim, C.H.; Kim, Y.J.; Kim, H.R. Prognostic factors for low rectal cancer patients undergoing intersphincteric resection after neoadjuvant chemoradiation. J. Surg. Oncol. 2015, 111, 1054–1058. [Google Scholar] [CrossRef]
- Piozzi, G.N.; Lee, T.H.; Kwak, J.M.; Kim, J.; Kim, S.H. Robotic-assisted resection for beyond TME rectal cancer: A novel classification and analysis from a specialized center. Updates Surg. 2021, 73, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Lu, J.; Wu, Z.; Li, G.; Wei, F.; Cao, J.; Li, W. Intersphincteric Resection Versus Abdominoperineal Resection for Low Rectal Cancer: A Meta-Analysis. Surg. Innov. 2020, 27, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Holm, T. Abdominoperineal Excision: Technical Challenges in Optimal Surgical and Oncological Outcomes after Abdominoperineal Excision for Rectal Cancer. Clin. Colon Rectal Surg. 2017, 30, 357–367. [Google Scholar] [CrossRef]
- Shen, Z.; Bu, Z.; Li, A.; Lu, J.; Zhu, L.; Chong, C.S.; Gao, Z.; Jiang, K.; Wang, S.; Li, F.; et al. Multicenter study of surgical and oncologic outcomes of extra-levator versus conventional abdominoperineal excision for lower rectal cancer. Eur. J. Surg. Oncol. 2020, 46, 115–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, N.; Sugito, M.; Ito, M.; Kobayashi, A.; Nishizawa, Y.; Yoneyama, Y.; Nishizawa, Y.; Minagawa, N. Oncologic outcome of intersphincteric resection for very low rectal cancer. World J. Surg. 2009, 33, 1750–1756. [Google Scholar] [CrossRef]
- Okamura, R.; Hida, K.; Yamaguchi, T.; Akagi, T.; Konishi, T.; Yamamoto, M.; Ota, M.; Matoba, S.; Bando, H.; Goto, S.; et al. Local control of sphincter-preserving procedures and abdominoperineal resection for locally advanced low rectal cancer: Propensity score matched analysis. Ann. Gastroenterol. Surg. 2017, 1, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Miyake, M.; Shida, D.; Ochiai, H.; Yamada, K.; Kanemitsu, Y. Intersphincteric Resection Has Similar Long-term Oncologic Outcomes Compared With Abdominoperineal Resection for Low Rectal Cancer Without Preoperative Therapy: Results of Propensity Score Analyses. Dis. Colon Rectum 2018, 61, 1035–1042. [Google Scholar] [CrossRef]
- Keller, D.S.; Reif de Paula, T.; Kiran, R.P. Ready for the National Accreditation Programs for Rectal Cancer? Auditing rectal cancer outcomes in the United States. Colorectal Dis. 2019, 21, 1213–1215. [Google Scholar] [CrossRef]
- Ghadban, T.; Reeh, M.; Bockhorn, M.; Heumann, A.; Grotelueschen, R.; Bachmann, K.; Izbicki, J.R.; Perez, D.R. Minimally invasive surgery for colorectal cancer remains underutilized in Germany despite its nationwide application over the last decade. Sci. Rep. 2018, 8, 15146. [Google Scholar] [CrossRef] [Green Version]
- Marwan, K.; Staples, M.P.; Thursfield, V.; Bell, S.W. The rate of abdominoperineal resections for rectal cancer in the state of Victoria, Australia: A population-based study. Dis. Colon Rectum 2010, 53, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Rouanet, P.; Rivoire, M.; Gourgou, S.; Lelong, B.; Rullier, E.; Jafari, M.; Mineur, L.; Pocard, M.; Faucheron, J.L.; Dravet, F.; et al. Sphincter-saving surgery for ultra-low rectal carcinoma initially indicated for abdominoperineal resection: Is it safe on a long-term follow-up? J. Surg. Oncol. 2021, 123, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Beppu, N.; Kimura, F.; Aihara, T.; Doi, H.; Tomita, N.; Yanagi, H.; Yamanaka, N. Patterns of Local Recurrence and Oncologic Outcomes in T3 Low Rectal Cancer (≤5 cm from the Anal Verge) Treated With Short-Course Radiotherapy With Delayed Surgery: Outcomes in T3 Low Rectal Cancer Treated With Short-Course Radiotherapy With Delayed Surgery. Ann. Surg. Oncol. 2017, 24, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Kusters, M.; Marijnen, C.A.; van de Velde, C.J.; Rutten, H.J.; Lahaye, M.J.; Kim, J.H.; Beets-Tan, R.G.; Beets, G.L. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur. J. Surg. Oncol. 2010, 36, 470–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, K.; Shimoda, H.; Miura, T.; Sakamoto, Y.; Morohashi, H.; Watanabe, S.; Narita, H.; Mitsuhashi, Y.; Umemura, K.; Hakamada, K. Widespread anorectal lymphovascular networks and tissue drainage: Analyses from submucosal India ink injection and indocyanine green fluorescence imaging. Colorectal Dis. 2021, 23, 1334–1345. [Google Scholar] [CrossRef] [PubMed]
- Bittorf, B.; Stadelmaier, U.; Gohl, J.; Hohenberger, W.; Matzel, K.E. Functional outcome after intersphincteric resection of the rectum with coloanal anastomosis in low rectal cancer. Eur. J. Surg. Oncol. 2004, 30, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Denost, Q.; Moreau, J.B.; Vendrely, V.; Celerier, B.; Rullier, A.; Assenat, V.; Rullier, E. Intersphincteric resection for low rectal cancer: The risk is functional rather than oncological. A 25-year experience from Bordeaux. Colorectal Dis. 2020, 22, 1603–1613. [Google Scholar] [CrossRef]
- Celerier, B.; Denost, Q.; Van Geluwe, B.; Pontallier, A.; Rullier, E. The risk of definitive stoma formation at 10 years after low and ultralow anterior resection for rectal cancer. Colorectal Dis. 2016, 18, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kuo, L.J.; Hung, C.S.; Wang, W.; Tam, K.W.; Lee, H.C.; Liang, H.H.; Chang, Y.J.; Huang, M.T.; Wei, P.L. Intersphincteric resection for very low rectal cancer: Clinical outcomes of open versus laparoscopic approach and multidimensional analysis of the learning curve for laparoscopic surgery. J. Surg. Res. 2013, 183, 524–530. [Google Scholar] [CrossRef] [PubMed]



| Authors, Year | Indications | Contraindications |
|---|---|---|
| Schiessel, 1994–2012 [18,19,39] | -T1–T3 LRC -Tumor diameter >1 cm -Big villous adenomas -Mucosectomy/RT residual tumors -Low carcinoids/hemangiomas | -Undifferentiated tumors -EAS infiltration -T4 stage -Preoperative insufficient sphincter function -Distant metastases |
| Vorobiev, 2004 [58] | T2–3 (EUS) Well/moderately diff. adenoca. Fecal continence | -EAS/LAM infiltration -N+ (EUS) -M+ |
| Rullier, 2005 [45] | -≤4.5 cm AV -Distant metastases | -EAS/LAM infiltration -Fixed tumors (except partial vaginal fixity) -Fecal incontinence > 6 months before diagnosis |
| Hohenberger, 2005 [46] | -≥0.5 cm from DL (rectoscopy) -T1–2 (EUS) -T3 (above puborectal sling) -G1–2 -Patients with possibly distinct invasion of the pelvic floor musculature underwent prior nCRT | -EAS infiltration -Fecal incontinence |
| Chin, 2006 [47] | -T2 -T3–4 (after nCRT) -≤5 cm (maximal diameter) -1–3 cm from DL | -Distant metastases |
| Chamlou, 2007 [48] | -T1–3 -T4 if invasion is distant from the tumor’s lowest part/sphincter, and is resectable -Resectable distant metastases -uT1 with adverse pathologic features after transanal local excision | -EAS/LAM infiltration -Fecal incontinence |
| Krand, 2009 [59] | -(Study on ISR with partial IAS) -Distal excision at the DL or 1–2 mm distal to it -T2–3 -Well/moderately diff. adenoca. | -Total IAS for achieving acceptable DRM -Fecal incontinence -EAS/LAM infiltration -Poorly diff. adenoca. -Distant metastases (except resectable liver metastases) |
| Han, 2009 [60] | -T1–2 (IAS) -T1-T2 after nCRT -Tumor diameter > 1 cm but <5 cm -Well/moderately diff. adenoca. -Sufficient anal function (DRE, manometry) | -Infiltration of pelvic floor -Tumor diameter > 5 cm -Poorly diff. adenoca. -Insufficient anal function (DRE, manometry) -Distant metastases -Intestinal obstruction |
| Kuo, 2011 [62] | -T1–3 | -Infiltration EAS/LAR (even if submitted to nCRT with radiological clearance) |
| Martin, 2012 [69] (Review) | -≤1 cm from anorectal ring | -T4 tumors -EAS/LAM infiltration -Fixed tumors at DRE -Poorly diff. adenoca. -Fecal incontinence -Distant metastases |
| Tokoro, 2013 [52] | -T1–3 -Resectable metastases | -T4 tumors -Poorly diff. adenoca. -Infiltrating gross appearance -Fecal incontinence |
| Akagi, 2013 [53] | -T1–3 (mobile tumors) -≤4 cm from AV -Well/moderately diff. adenoca. -ECOG PS 0–2 -Good anal function | -T4 tumor -Fixed tumors -Untreatable distant metastases -Poorly diff. adenoca. -Psychiatric disease -Poor anal function (no discernable tone at DRE or the maximum squeeze pressure < 50 mmHg before operation) -Liver cirrhosis, renal dysfunction, cardiac failure, and respiratory dysfunction |
| Akagi, 2013 [41] (Review) | -T1–3 tumors -30–35 mm from AV -Independently to IAS invasion | -As for Schiessel et al. |
| Saito, 2014 [64] | -T1–4 -≤5 cm from AV | -EAS/LAM infiltration -Fecal incontinence |
| Shirouzu, 2017 [70] (Review) | -T1–3 -1–5 cm AV -Well-moderately diff. adenoca. | -T4 -Fixed tumors -EAS/LAM infiltration -Untreatable distant metastases -Poorly diff. adenoca. -Poor anal function -Severe preoperative pathologies (cardiac failure, liver cirrhosis, renal dysfunction, respiratory dysfunction) -Psychiatric disease |
| Park, 2019 [56] | -Tumor’s response to nCRT on restaging MRI -Evaluation of ymrT stage and ymrCRM status | -Poor nCRT responders |
| Piozzi, 2021 [57] | -≤4 cm from AV -After nCRT for cT3-T4 -(y)cT4 if curative resection is technically feasible at the pre-operative MRI -Conversion from an ultra-low AR in case of involvement/threatening of the distal gross margin in the resected specimen or in case of stapler failure for any reason | -EAS/LAM infiltration (at restaging MRI after nCRT) -Abundant mucinous component -Anal canal involvement below DL (requiring total ISR) -Fecal incontinence -Patient’s refusal |
| First Author, Year | Country | n | Age | Sex, M | Distance-AV, cm | nCRT | Approach | ISR | Type (Par, Subt, Tot, ESR) | cT Stage | Stage 0/I/II/III/IV | DRM, cm | CRM, mm | R0 | FU, mo | LR Rate | DM | CT | OS-5 Years | DFS-5 Years | LRFS-5 Years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kohler [43], 2000 | Germany | 31 | 60 | 55% | 1.3 ± 0.9 (DL) | 0% | O | T-P | 0/31/0/0 | T1–3 | 0/18/4/9/0 | 1.6 ± 0.8 | nr | 100% | 6.8 ± 3.7 (y) | 9.7% | 19.4% | 35% | 79% | nr | nr |
| Vorobiev [58], 2004 | Russia | 27 | 55 (26–75) | 59% | 1.0 (0.5–1.5) (DL) | 7% | O | P | 0/0/27/0 | T2–3 | 0/8/18/1/0 | 1.9 (1.5–2.6) | 0.8 (0.6–1.5) | 100% | 38 (14–66) | 0 | 11.1% | 3.7% | 92.5% (3-y) | 88.9% (3-y) | 0% |
| Schiessel [19], 2005 | Austria | 121 | 65.2 | 68% | 3 (1–5) (AM) | nr | O | T-P | nr | T1–3 | DK. A49, B33, C37 | nr | nr | nr | 94 (24–185) | 5.3% | nr | nr | nr | nr | nr |
| Rullier [45], 2005 | France | 92 | 65 (25–86) | 62% | 3 (1.5–4.5) | 88% | O/L | T-P | nr | T1–4 | nr | 2 (0.5–3) | 5 (0–15) | 89% | 40 (63%) | 2% (63%) | 19% (63%) | nr | 81% | 70% | nr |
| Hohenberger [46], 2006 | Germany | 65 | nr | nr | <2 (DL) | 54% | O | T-P | 60/0/0/0 | nr | nr | 1.5–2.0 | nr | nr | nr | 22.7% | nr | nr | 85.1% | nr | nr |
| Chamlou [48], 2007 | France | 90 | 59 (27–82) | 65% | 3.5 (2.2–5.2) | 41% | O | T-P | 63/27/0/0 | T1–4 | 0/37/16/25/5 | 1.2 (0.5–3.5) | nr | 94.4% | 56.2 (13.3–168.4) | 8.8% | 8.8% | nr | 82% | 75% | nr |
| Portier [49], 2007 | France | 173 | 64 ± 11 | 33% | 4.1 ± 1.4 | 53% | O | T-P | 173/0/0/0 | T1–4 | 0/74/46/53/0 | 2.6 ± 1.2 | nr | 96% | 66.8 ± 52.1 | 10.6% | nr | nr | 86.1% | 83.9% | nr |
| Akasu [50], 2007 | Japan | 106 | 55 (26–75) | 78% | 3 (1–5) | 0% | O/L | T-P | 90/0/16/6 | T1–3 | 0/45/20/38/3 | 1.2 (0.3–4) | nr | 97% | 3.5 (0.9–11.7) (y) | 5.7% | 10% | 18% | 91% | 82% | 88% |
| Krand [59], 2009 | Turkey | 47 | 57 (27–72) | 66% | 3.3 (1.5–5) | 100% | O | P | 47/0/0/0 | T2–3 | 0/nr/nr/25/0 | 1.2 ± 0.3 | 5 ± 2.3 | 98% | 67.5 (9–132) | 2.1% | 15.2% | 53.2% | 85% | 82% | nr |
| Han [60], 2009 | China | 40 | 62 (34–73) | 60% | 1.5 (0.5–5.0) (DL) | 2.5% | O | P | 23/0/5/0 | T1–2 | 0/18/6/16/0 | 2.1 (2–5) | nr | 100% | 43 (12–94) | 5% | 2.5% | 7.5% | 97% | 86% | nr |
| Weiser [61], 2009 | U.S.A. | 44 | 54 (28–78) | 57% | 5 (3–6) | 100% | O | P | nr | T1–3 | 11/16/12/5/0 | nr | nr | 92% | 47 (33–59) | 0% | 16% | nr | nr | 96% | 83% |
| Kuo [62], 2011 | Taiwan | 26 | 51 (26–71) | 61% | 3.5 (2.5–5) | 88% | O | P | 26/0/0/0 | T1–3 | 0/14/2/9/0 | 1.4 (0.1–4.5) | 11 (1–31) | 87% | 55 (8–93) | 7.7% | 15% | nr | 83% | 76% | nr |
| Gong [63], 2012 | China | 43 | 53 | 63% | <5 | 0% | O | P | nr | T1–2 | nr | nr | nr | 100% | 20 (12–42) | 0% | 0% | nr | nr | nr | nr |
| Zhang [51], 2013 | China | 60 | 55 (30–77) | 65% | 4.2 (3–5) | 30% | O | T-P | 34/0/26/0 | T1–4 | 0/28/21/11/0 | 1.9 (1.0–3.2) | ≥1 mm | 100% | 49 (18–90) | 10% | 6.6% | nr | 90% | 83.3% | nr |
| Tokoro [52], 2013 | Japan | 30 | 60 ± 10 | 40% | 0.9 ± 0.8 (DL) | 0% | O | T-P | 12/4/14/4 | Tis–T3 | 1/16/5/7/1 | 0.7 (0.3–2.2) | 3 (0.5–9) | 93% (CRM) | 56.2 (13.3–168.4) | 20% | 16% | nr | 76.5% | 68.4% | nr |
| Akagi [53], 2013 | Japan | 124 | 65 (32–81) | 62% | 3 (1–4) | 0% | O | T-P | nr | T1–3 | 0/43/41/40/0 | nr | nr | 97.6% (CRM) | 85 (14–122) | 4.8% | 10.5% | 46.8% | 84.2–78.6% | 90.5–83.6% | 81.7% |
| Saito [64], 2014 | Japan | 199 | 59 (27–80) | 72% | 3.8 | 25% | O | P | 64/80/55/41 | T1–4 | 9/69/46/75/0 | nr | 19.6% (≤1 mm) | 80.4% | 78 (12–164) | 13.6% | nr | 48% | 78.3% (7-y) | 66.7% (7-y) | 80.3% (7-y) |
| Abdel-Gawad [65], 2014 | Egypt | 55 | nr | nr | 2.3 (0–5) | 45% | O/L | T-P | 35/0/20/20 | T1–3 | nr | nr | nr | 94.5% (CRM) | 1.5 (1–4.6) (y) | 5.4% | 12.7% | nr | 88.7% (3-y) | 82.6% (3-y) | 85.2% (3-y) |
| Koyama [88], 2014 | Japan | 77 | 63 (24–86) | 73% | nr | 9% | nr | nr | nr | T1–4 | 0/20/25/32/0 | nr | nr | nr | 69 (56–87) | 7.8% | nr | nr | 76.4% | nr | 93.5% |
| Mahalingam [54], 2017 | India | 33 | 50 (26–69) | 64% | 3 (1.5–5) | 91% | nr | T-P | nr | nr | nr | 2 (0.4–4) | nr | 100% | 48 (18–83) | 0% | 5% | nr | 95% (3-y) | nr | nr |
| Klose [89], 2017 | Germany | 60 | 67 (41–86) | 72% | 3.4 (1–5) | 73% | nr | nr | nr | T1–4 | 0/36/12/9/3 | nr | nr | 95% | 58 (11–210) | nr | nr | 23% | 80% | 69% | nr |
| Matsunaga [55], 2019 | Japan | 197 | 61 (33–80) | 70% | 4 (0.6–6.5) | 0% | nr | T-P | 88/62/47/0 | T1–3 | nr | nr | 0.3 (0.01–2) | 88% (CRM) | 68 (9–182) | nr | nr | nr | 88.3% | 76.9% | nr |
| Molnar [90], 2019 | Romania | 37 | 66 ± 11 | 65% | 10–40 | nr | nr | nr | nr | nr | 0/7/13/16/1 | nr | nr | 87% (CRM) | 62 (55–80) | 5.4% | 5.4% | nr | 71% | nr | nr |
| Park [56], 2019 | Korea | 147 | 61 ± 11 | 72% | 2.8 ± 1.0 | 100% | L/R | T-P | 31/95/21/0 | T2–4 | 33/36/42/36/0 | nr | nr | 95% | 34 (8–94) | 11.6% | 22.4% | nr | nr | 64.9% (3-y) | nr |
| Kim [68], 2021 | Korea | 590 | 58 ± 11 | 59% | 3.3 ± 1.9 | 47% | R | TA | 155/93/42/70 | Tis–T3 | 41/103/59/77 | 1.5 ± 1.3 | 8 ± 6 | DRM ≤ 10 mm (45.4%), CRM ≤ 1 mm (7.8%) | 43 (21–59) | 2.4% (PS) | 15.1% (PS) | nr | 90.8% (PS) | 81.6% (PS) | nr |
| Piozzi [57], 2021 | Korea | 161 | 59 (51–68) | 75% | 3 (2.5–3.5) | 71% | L/R | T-P | nr | T1-4 | 15/51/34/44/17 | 0.8 (0.5–1.5) | 0.3 (0.2–0.5) | 91.3% (CRM) | 55 (34.5–77.5) | 11.1% | 26.1% | 55.9% | 80% | 64% | 87% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piozzi, G.N.; Baek, S.-J.; Kwak, J.-M.; Kim, J.; Kim, S.H. Anus-Preserving Surgery in Advanced Low-Lying Rectal Cancer: A Perspective on Oncological Safety of Intersphincteric Resection. Cancers 2021, 13, 4793. https://doi.org/10.3390/cancers13194793
Piozzi GN, Baek S-J, Kwak J-M, Kim J, Kim SH. Anus-Preserving Surgery in Advanced Low-Lying Rectal Cancer: A Perspective on Oncological Safety of Intersphincteric Resection. Cancers. 2021; 13(19):4793. https://doi.org/10.3390/cancers13194793
Chicago/Turabian StylePiozzi, Guglielmo Niccolò, Se-Jin Baek, Jung-Myun Kwak, Jin Kim, and Seon Hahn Kim. 2021. "Anus-Preserving Surgery in Advanced Low-Lying Rectal Cancer: A Perspective on Oncological Safety of Intersphincteric Resection" Cancers 13, no. 19: 4793. https://doi.org/10.3390/cancers13194793






