CD147 Promotes Tumor Lymphangiogenesis in Melanoma via PROX-1
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Specimens
2.2. Immunohistochemistry
2.3. Immunofluorescence Staining
2.4. Cell Culture
2.5. In Vivo Monitoring Assays
2.6. Metastasis Foci Counting
2.7. In Vitro Migration, Proliferation, and Apoptosis Assays
2.8. Tube Formation Assay
2.9. Plasmids and siRNA Transfection
2.10. Western Blot Analyses
2.11. Real-Time Quantitative PCR (qRT-PCR)
2.12. Chromatin Immunoprecipitation (ChIP)
2.13. Human Phosphokinase Array
2.14. Statistical Analysis
3. Results
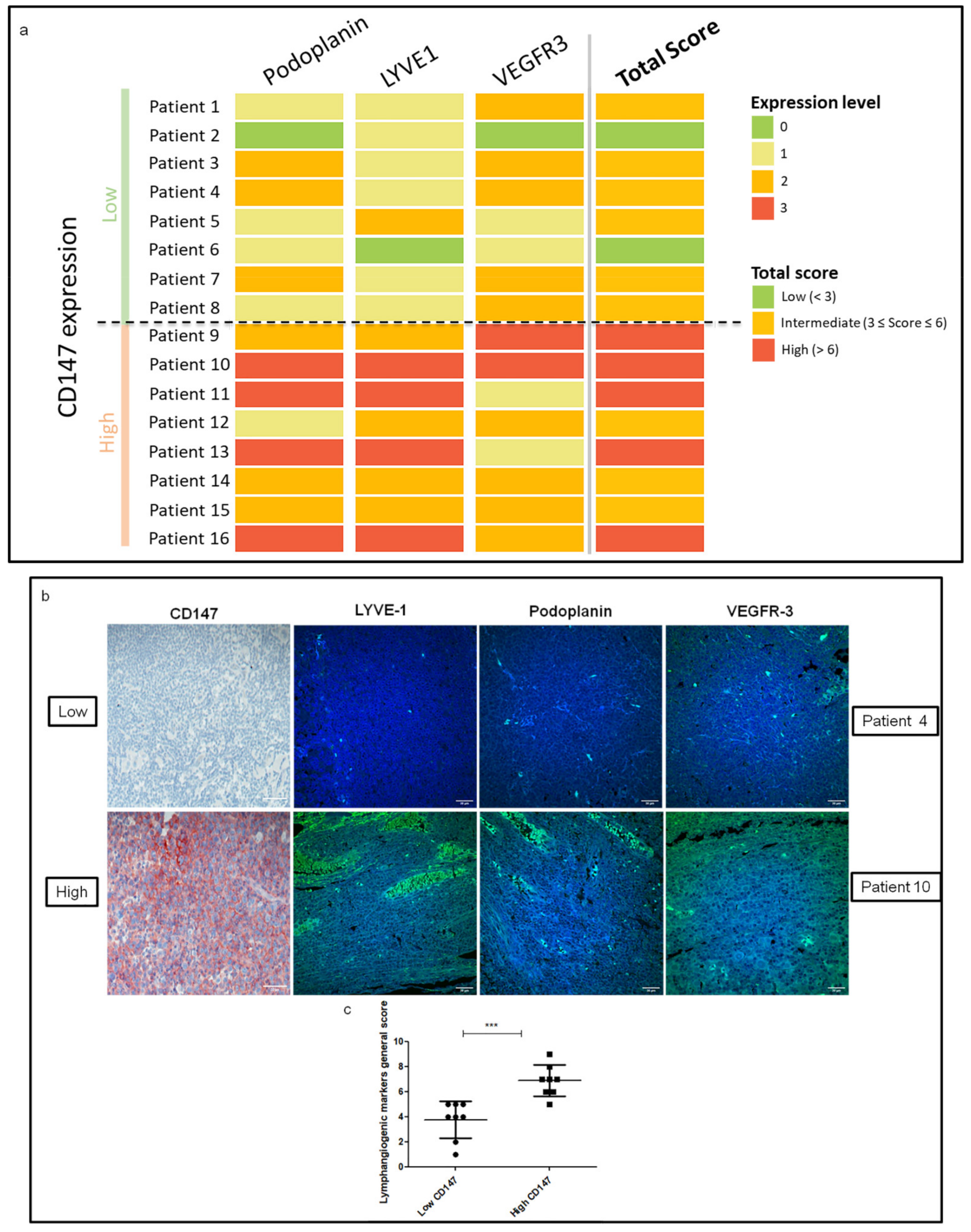
3.1. CD147 Expression Is Associated with Lymphangiogenesis Mediators in Human Melanoma Lymph Node Metastasis
3.2. CD147 Promotes In Vitro Lymphangiogenesis
3.3. CD147 Regulates Lymphangiogenesis Mediators through PROX-1 Transcription Factor
3.4. CD147 Downregulation Decreases Metastasis Formation and Lymphangiogenic Factors in a Human Melanoma Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christiansen, A.; Detmar, M. Lymphangiogenesis and Cancer. Genes Cancer 2011, 2, 1146–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albrecht, I.; Christofori, G. Molecular mechanisms of lymphangiogenesis in development and cancer. Int. J. Dev. Biol. 2011, 55, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Loges, S.; Clausen, H.; Reichelt, U.; Bubenheim, M.; Erbersdobler, A.; Schurr, P.; Yekebas, E.; Schuch, G.; Izbicki, J.; Pantel, K.; et al. Determination of Microvessel Density by Quantitative Real-time PCR in Esophageal Cancer: Correlation with Histologic Methods, Angiogenic Growth Factor Expression, and Lymph Node Metastasis. Clin. Cancer Res. 2007, 13, 76–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coso, S.; Zeng, Y.; Opeskin, K.; Williams, E.D. Vascular Endothelial Growth Factor Receptor-3 Directly Interacts with Phosphatidylinositol 3-Kinase to Regulate Lymphangiogenesis. PLoS ONE 2012, 7, e39558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirakawa, S.; Brown, L.F.; Kodama, S.; Paavonen, K.; Alitalo, K.; Detmar, M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2007, 109, 1010–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stacker, S.A.; Baldwin, M.E.; Achen, M.G. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002, 16, 922–934. [Google Scholar] [CrossRef] [PubMed]
- Prevo, R.; Banerji, S.; Ferguson, D.J.P.; Clasper, S.; Jackson, D.G. Mouse LYVE-1 Is an Endocytic Receptor for Hyaluronan in Lymphatic Endothelium. J. Biol. Chem. 2001, 276, 19420–19430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiteneder-Geleff, S.; Soleiman, A.; Horvat, R.; Amann, G.; Kowalski, H.; Kerjaschki, D. Podoplanin—A specific marker for lymphatic endothelium expressed in angiosarcoma. Verh. Dtsch. Ges. Pathol. 1999, 83, 270–275. [Google Scholar]
- Cueni, L.N.; Hegyi, I.; Shin, J.W.; Albinger-Hegyi, A.; Gruber, S.; Kunstfeld, R.; Moch, H.; Detmar, M. Tumor Lymphangiogenesis and Metastasis to Lymph Nodes Induced by Cancer Cell Expression of Podoplanin. Am. J. Pathol. 2010, 177, 1004–1016. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J. BMP-9 balances endothelial cell fate. Proc. Natl. Acad. Sci. USA 2013, 110, 18746–18747. [Google Scholar] [CrossRef] [Green Version]
- Wigle, J.; Oliver, G. Prox1 Function Is Required for the Development of the Murine Lymphatic System. Cell 1999, 98, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Dadras, S.S.; Paul, T.; Bertoncini, J.; Brown, L.F.; Muzikansky, A.; Jackson, D.G.; Ellwanger, U.; Garbe, C.; Mihm, M.C.; Detmar, M. Tumor Lymphangiogenesis: A novel prognostic indicator for cutaneous melanoma metastasis and survival. Am. J. Pathol. 2003, 162, 1951–1960. [Google Scholar] [CrossRef]
- Cianfarani, F.; Mastroeni, S.; Odorisio, T.; Passarelli, F.; Cattani, C.; Mannooranparampil, T.J.; Fortes, C.; Failla, C.M. Expression of vascular endothelial growth factor-C in primary cutaneous melanoma predicts sentinel lymph node positivity. J. Cutan. Pathol. 2012, 39, 826–834. [Google Scholar] [CrossRef] [PubMed]
- Doeden, K.; Ma, Z.; Narasimhan, B.; Swetter, S.M.; Detmar, M.; Dadras, S.S. Lymphatic invasion in cutaneous melanoma is associated with sentinel lymph node metastasis. J. Cutan. Pathol. 2009, 36, 772–780. [Google Scholar] [CrossRef]
- Pastushenko, I.; Vermeulen, P.B.; Vicente-Arregui, S.; Van den Eynden, G.G.; Alvarez-Alegret, R.; Querol, I.; Rutten, A.; Carapeto, F.J.; Dirix, L.Y.; Van Laere, S. Peritumoral D2-40 Chalkley score independently predicts metastases and survival in patients with cutaneous malignant melanoma. J. Cutan. Pathol. 2015, 42, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, A.C.; Muenzner, J.K.; Andrade, F.; Rius, F.E.; Ostalecki, C.; Geppert, C.I.; Agaimy, A.; Hartmann, A.; Fujita, A.; Schneider-Stock, R.; et al. Gene expression and promoter methylation of angiogenic and lymphangiogenic factors as prognostic markers in melanoma. Mol. Oncol. 2019, 13, 1433–1449. [Google Scholar] [CrossRef] [Green Version]
- Mouawad, R.; Spano, J.-P.; Comperat, E.; Capron, F.; Khayat, D. Tumoural expression and circulating level of VEGFR-3 (Flt-4) in metastatic melanoma patients: Correlation with clinical parameters and outcome. Eur. J. Cancer 2009, 45, 1407–1414. [Google Scholar] [CrossRef]
- Suresh, R.; Ziemys, A.; Holder, A.M. Dissecting the Lymphatic System to Predict Melanoma Metastasis. Front. Oncol. 2020, 10. [Google Scholar] [CrossRef]
- Špirić, Z.; Vještica, M.; Erić, M. Survival prediction in patients with cutaneous melanoma by tumour lymphangiogenesis. Acta Clin. Belg. 2020, 75, 379–387. [Google Scholar] [CrossRef]
- Khayati, F.; Pérez-Cano, L.; Maouche, K.; Sadoux, A.; Boutalbi, Z.; Podgorniak, M.-P.; Maskos, U.; Setterblad, N.; Janin, A.; Calvo, F.; et al. EMMPRIN/CD147 is a novel coreceptor of VEGFR-2 mediating its activation by VEGF. Oncotarget 2015, 6, 9766–9780. [Google Scholar] [CrossRef] [Green Version]
- Bougatef, F.; Quemener, C.; Kellouche, S.; Naimi, B.; Podgorniak, M.-P.; Millot, G.; Gabison, E.E.; Calvo, F.; Dosquet, C.; Lebbé, C.; et al. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2α–mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood 2009, 114, 5547–5556. [Google Scholar] [CrossRef] [Green Version]
- Landras, A.; de Moura, C.R.; Jouenne, F.; Lebbe, C.; Menashi, S.; Mourah, S. CD147 is a promising target of tumor progres-sion and a prognostic biomarker. Cancers 2019, 11, 1803. [Google Scholar] [CrossRef] [Green Version]
- Caudron, A.; Battistella, M.; Feugeas, J.-P.; Pagès, C.; Basset-Séguin, N.; Mazouz Dorval, S.; Brentano, E.F.; Sadoux, A.; Podgorniak, M.-P.; Menashi, S.; et al. EMMPRIN/CD147 is an independent prognostic biomarker in cutaneous melanoma. Exp. Dermatol. 2016, 25, 618–622. [Google Scholar] [CrossRef]
- Liu, B.; Wan, Z.; Sheng, B.; Lin, Y.; Fu, T.; Zeng, Q.; Qi, C. Overexpression of EMMPRIN is associated with lymph node metastasis and advanced stage of non-small cell lung cancer: A retrospective study. BMC Pulm. Med. 2017, 17, 214. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Abudumijiti, H.; Xu, L.; Hasim, A. CD147 promotes cervical cancer migration and invasion by up-regulating fatty acid synthase expression. Int J Clin Exp Pathol 2019, 12, 4280–4288. [Google Scholar] [PubMed]
- Van Muijen, G.N.P.; Jansen, K.F.J.; Cornelissen, I.M.H.A.; Smeets, D.F.C.M.; Beck, J.L.M.; Ruiter, D.J. Establishment and characterization of a human melanoma cell line (MV3) which is highly metastatic in nude mice. Int. J. Cancer 2007, 48, 85–91. [Google Scholar] [CrossRef]
- Van Muijen, G.N.P.; Cornelissen, L.M.H.A.; Jansen, C.F.J.; Figdor, C.G.; Johnson, J.P.; Brockerr, E.-B.; Ruitert, D.J. Antigen expression of metastasizing and non-metastasizing human melanoma cells xenografted into nude mice. Clin. Exp. Metastasis 1991, 9, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Gabison, E.; Khayati, F.; Mourah, S.; Menashi, S. Cell Membrane Vesicles as a Tool for the Study of Direct Epithelial–Stromal Interaction: Lessons from CD147. Adv. Struct. Saf. Stud. 2013, 1066, 103–111. [Google Scholar] [CrossRef]
- Gabison, E.E.; Mourah, S.; Steinfels, E.; Yan, L.; Hoang-Xuan, T.; Watsky, M.A.; De Wever, B.; Calvo, F.; Mauviel, A.; Menashi, S. Differential Expression of Extracellular Matrix Metalloproteinase Inducer (CD147) in Normal and Ulcerated Corneas: Role in Epithelio-Stromal Interactions and Matrix Metalloproteinase Induction. Am. J. Pathol. 2005, 166, 209–219. [Google Scholar] [CrossRef]
- Jussila, L.; Alitalo, K. Vascular Growth Factors and Lymphangiogenesis. Physiol. Rev. 2002, 82, 673–700. [Google Scholar] [CrossRef] [Green Version]
- Breiteneder-Geleff, S.; Soleiman, A.; Kowalski, H.; Horvat, R.; Amann, G.; Kriehuber, E.; Diem, K.; Weninger, W.; Tschachler, E.; Alitalo, K.; et al. Angiosarcomas Express Mixed Endothelial Phenotypes of Blood and Lymphatic Capillaries: Podoplanin as a Specific Marker for Lymphatic Endothelium. Am. J. Pathol. 1999, 154, 385–394. [Google Scholar] [CrossRef]
- Stump, B.; Shrestha, S.; LaMattina, A.M.; Louis, P.H.; Cho, W.; Perrella, M.A.; Ai, X.; Rosas, I.O.; Wagner, F.F.; Priolo, C.; et al. Glycogen synthase kinase 3-β inhibition induces lymphangiogenesis through β-catenin-dependent and mTOR-independent pathways. PLoS ONE 2019, 14, e0213831. [Google Scholar] [CrossRef] [Green Version]
- Shi, F.; Wang, Y.-C.; Zhao, T.-Z.; Zhang, S.; Du, T.-Y.; Yang, C.-B.; Li, Y.-H.; Sun, X.-Q. Effects of Simulated Microgravity on Human Umbilical Vein Endothelial Cell Angiogenesis and Role of the PI3K-Akt-eNOS Signal Pathway. PLoS ONE 2012, 7, e40365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turner, C.E. Paxillin and focal adhesion signalling. Nat. Cell Biol. 2000, 2, E231–E236. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Yang, H.-Y.; Hsu, Y.-F.; Chiu, P.-T.; Ou, G.; Hsu, M.-J. Src contributes to IL6-induced vascular endothelial growth factor-C expression in lymphatic endothelial cells. Angiogenesis 2014, 17, 407–418. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Yang, H.-Y.; Huang, S.-W.; Ou, G.; Hsu, Y.-F.; Hsu, M.-J. Interleukin-6 Induces Vascular Endothelial Growth Factor-C Expression via Src-FAK-STAT3 Signaling in Lymphatic Endothelial Cells. PLoS ONE 2016, 11, e0158839. [Google Scholar] [CrossRef]
- Davis, R.J. Signal Transduction by the JNK Group of MAP Kinases. Cell 2000, 103, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Lutze, G.; Haarmann, A.; Demanou Toukam, J.A.; Buttler, K.; Wilting, J.; Becker, J. Non-canonical WNT-signaling controls differentiation of lymphatics and extension lymphangiogenesis via RAC and JNK signaling. Sci. Rep. 2019, 9, 4739. [Google Scholar] [CrossRef] [PubMed]
- Lockshin, A.; Giovanella, B.C.; De Ipolyi, P.D.; Williams, L.J.; Mendoza, J.T.; O Yim, S.; Stehlin, J.S. Exceptional lethality for nude mice of cells derived from a primary human melanoma. Cancer Res. 1985, 45. [Google Scholar]
- Van Kempen, L.C.L.T.; Meier, F.; Egeblad, M.; Kersten-Niessen, M.J.F.; Garbe, C.; Weidle, U.H.; van Muijen, G.N.P.; Herlyn, M.; Bloemers, H.P.J.; Swart, G.W.M. Truncation of Activated Leukocyte Cell Adhesion Molecule: A Gateway to Melanoma Metastasis. J. Investig. Dermatol. 2004, 122, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Küsters, B.; Westphal, J.R.; Smits, D.; Ruiter, D.J.; Wesseling, P.; Keilholz, U.; de Waal, R.M.W. The pattern of metastasis of human melanoma to the central nervous system is not influenced by integrin αvβ3 expression. Int. J. Cancer 2001, 92, 176–180. [Google Scholar] [CrossRef]
- Lee, J.Y.; Hong, S.-H.; Shin, M.; Heo, H.-R.; Jang, I.H. Blockade of FLT4 suppresses metastasis of melanoma cells by impaired lymphatic vessels. Biochem. Biophys. Res. Commun. 2016, 478, 733–738. [Google Scholar] [CrossRef]
- Shields, J.D.; Emmett, M.S.; Dunn, D.B.A.; Joory, K.D.; Sage, L.M.; Rigby, H.; Mortimer, P.S.; Orlando, A.; Levick, J.R.; Bates, D.O. Chemokine-mediated migration of melanoma cells towards lymphatics—A mechanism contributing to metastasis. Oncogene 2006, 26, 2997–3005. [Google Scholar] [CrossRef] [Green Version]
- Ubellacker, J.M.; Tasdogan, A.; Ramesh, V.; Shen, B.; Mitchell, E.C.; Martin-Sandoval, M.S.; Gu, Z.; McCormick, M.L.; Durham, A.B.; Spitz, D.R.; et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature 2020, 585, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Riethdorf, S.; Reimers, N.; Assmann, V.; Kornfeld, J.-W.; Terracciano, L.; Sauter, G.; Pantel, K. High incidence of EMMPRIN expression in human tumors. Int. J. Cancer 2006, 119, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Biswas, C.; Zhang, Y.; DeCastro, R.; Guo, H.; Nakamura, T.; Kataoka, H.; Nabeshima, K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res. 1995, 55, 55. [Google Scholar]
- Knutti, N.; Kuepper, M.; Friedrich, K. Soluble extracellular matrix metalloproteinase inducer (EMMPRIN, EMN) regulates cancer-related cellular functions by homotypic interactions with surface CD147. FEBS J. 2015, 282, 4187–4200. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Nakada, M.T.; Kesavan, P.; McCabe, F.; Millar, H.; Rafferty, P.; Bugelski, P.; Yan, L. Extracellular Matrix Metalloproteinase Inducer Stimulates Tumor Angiogenesis by Elevating Vascular Endothelial Cell Growth Factor and Matrix Metalloproteinases. Cancer Res. 2005, 65, 3193–3199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egawa, N.; Koshikawa, N.; Tomari, T.; Nabeshima, K.; Isobe, T.; Seiki, M. Membrane Type 1 Matrix Metalloproteinase (MT1-MMP/MMP-14) Cleaves and Releases a 22-kDa Extracellular Matrix Metalloproteinase Inducer (EMMPRIN) Fragment from Tumor Cells. J. Biol. Chem. 2006, 281, 37576–37585. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.; Rode, A.; Nicoll, A.; E Maczurek, A.; Lim, L.; Lim, S.; Angus, P.; Kronborg, I.; Arachchi, N.; Gorelik, A.; et al. Circulating CD147 predicts mortality in advanced hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2016, 31, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Menck, K.; Scharf, C.; Bleckmann, A.; Dyck, L.; Rost, U.; Wenzel, D.; Dhople, V.M.; Siam, L.; Pukrop, T.; Binder, C.; et al. Tumor-derived microvesicles mediate human breast cancer invasion through differentially glycosylated EMMPRIN. J. Mol. Cell Biol. 2015, 7, 143–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sidhu, S.S.; Mengistab, A.T.; Tauscher, A.N.; Lavail, J.H.; Basbaum, C. The microvesicle as a vehicle for EMMPRIN in tumor–stromal interactions. Oncogene 2004, 23, 956–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.; Zhang, Y.; Wu, K.; Wang, L.; Jiang, Y.; Chen, W.; Yan, M. CD147 promotes progression of head and neck squamous cell carcinoma via NF-kappa B signaling. J. Cell. Mol. Med. 2019, 23, 954–966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Honda, K.; Nanjo, H.; Iikawa, N.; Tsuji, T.; Kawasaki, Y.; Yamazaki, K.; Sato, T.; Saito, H.; Shiina, K.; et al. CD147 expression correlates with lymph node metastasis in T1-T2 squamous cell carcinoma of the tongue. Oncol. Lett. 2017, 14, 4670–4676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrova, T.V.; Makinen, T.; Makela, T.; Saarela, J.; Virtanen, I.; Ferrell, R.E.; Finegold, D.; Kerjaschki, D.; Ylä-Herttuala, S.; Alitalo, K. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J. 2002, 21, 4593–4599. [Google Scholar] [CrossRef] [Green Version]
- Ueta, K.; Otowa, Y.; Kakeji, Y.; Hirashima, M. PROX1 Is Associated with Cancer Progression and Prognosis in Gastric Cancer. Anticancer Res. 2018, 38, 6139–6145. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.-H.; Huang, C.-C.; Pan, M.-R.; Chen, H.-H.; Hung, W.-C. Prospero Homeobox 1 Promotes Epithelial–Mesenchymal Transition in Colon Cancer Cells by Inhibiting E-cadherin via miR-9. Clin. Cancer Res. 2012, 18, 6416–6425. [Google Scholar] [CrossRef] [Green Version]
- Yokobori, T.; Bao, P.; Fukuchi, M.; Altan, B.; Ozawa, D.; Rokudai, S.; Bai, T.; Kumakura, Y.; Honjo, H.; Hara, K.; et al. Nuclear PROX1 is Associated with Hypoxia-Inducible Factor 1α Expression and Cancer Progression in Esophageal Squamous Cell Carcinoma. Ann. Surg. Oncol. 2015, 22, 1566–1573. [Google Scholar] [CrossRef]
- Elsir, T.; Smits, A.; Lindström, M.S.; Nistér, M. Transcription factor PROX1: Its role in development and cancer. Cancer Metastasis Rev. 2012, 31, 793–805. [Google Scholar] [CrossRef]
- Huber, R.; Meier-Schiesser, B.; Otsuka, A.; Fenini, G.; Satoh, T.; Gehrke, S.; Widmer, D.; Levesque, M.P.; Mangana, J.; Kerl, K.; et al. Tumour hypoxia promotes melanoma growth and metastasis via High Mobility Group Box-1 and M2-like macrophages. Sci. Rep. 2016, 6, 29914. [Google Scholar] [CrossRef] [Green Version]
- Ribatti, D. Antiangiogenic therapy accelerates tumor metastasis. Leuk. Res. 2011, 35, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Pàez-Ribes, M.; Allen, E.; Hudock, J.; Takeda, T.; Okuyama, H.; Viñals, F.; Inoue, M.; Bergers, G.; Hanahan, D.; Casanovas, O. Antiangiogenic Therapy Elicits Malignant Progression of Tumors to Increased Local Invasion and Distant Metastasis. Cancer Cell 2009, 15, 220–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saif, M.W.; Knost, J.A.; Chiorean, E.G.; Kambhampati, S.R.P.; Yu, D.; Pytowski, B.; Qin, A.; Kauh, J.S.; O’Neil, B.H. Phase 1 study of the anti-vascular endothelial growth factor receptor 3 monoclonal antibody LY3022856/IMC-3C5 in patients with advanced and refractory solid tumors and advanced colorectal cancer. Cancer Chemother. Pharmacol. 2016, 78, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Jones, D. Parallels of Resistance between Angiogenesis and Lymphangiogenesis Inhibition in Cancer Therapy. Cells 2020, 9, 762. [Google Scholar] [CrossRef] [Green Version]
- Yamakawa, M.; Doh, S.J.; Santosa, S.M.; Montana, M.; Qin, E.C.; Kong, H.; Han, K.-Y.; Yu, C.; Rosenblatt, M.I.; Kazlauskas, A.; et al. Potential lymphangiogenesis therapies: Learning from current antiangiogenesis therapies-A review. Med. Res. Rev. 2018, 38, 1769–1798. [Google Scholar] [CrossRef]
- Han, B.; Li, K.; Wang, Q.; Zhang, L.; Shi, J.; Wang, Z.; Cheng, Y.; He, J.; Shi, Y.; Zhao, Y.; et al. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients with Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1569–1575. [Google Scholar] [CrossRef]
- Qin, E.A.T.; Liu, Z.; Wang, J.; Xia, J.; Liu, S.; Jia, Y.; Liu, H.; Li, K. Anlotinib suppresses lymphangiogenesis and lymphatic metastasis in lung adenocarcinoma through a process potentially involving VEGFR-3 signaling. Cancer Biol. Med. 2020, 17, 753–767. [Google Scholar] [CrossRef]
- Dieterich, L.C.; Detmar, M. Tumor lymphangiogenesis and new drug development. Adv. Drug Deliv. Rev. 2016, 99, 148–160. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reger de Moura, C.; Landras, A.; Khayati, F.; Maskos, U.; Maouche, K.; Battistella, M.; Menashi, S.; Lebbé, C.; Mourah, S. CD147 Promotes Tumor Lymphangiogenesis in Melanoma via PROX-1. Cancers 2021, 13, 4859. https://doi.org/10.3390/cancers13194859
Reger de Moura C, Landras A, Khayati F, Maskos U, Maouche K, Battistella M, Menashi S, Lebbé C, Mourah S. CD147 Promotes Tumor Lymphangiogenesis in Melanoma via PROX-1. Cancers. 2021; 13(19):4859. https://doi.org/10.3390/cancers13194859
Chicago/Turabian StyleReger de Moura, Coralie, Alexandra Landras, Farah Khayati, Uwe Maskos, Kamel Maouche, Maxime Battistella, Suzanne Menashi, Céleste Lebbé, and Samia Mourah. 2021. "CD147 Promotes Tumor Lymphangiogenesis in Melanoma via PROX-1" Cancers 13, no. 19: 4859. https://doi.org/10.3390/cancers13194859





