Use of FOLFIRINOX or Nab-Paclitaxel Plus Gemcitabine for the Treatment of Locally Advanced Pancreatic Adenocarcinoma: A Single Institution Observational Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Inclusion Criteria
2.3. Efficacy and Survival Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Treatment
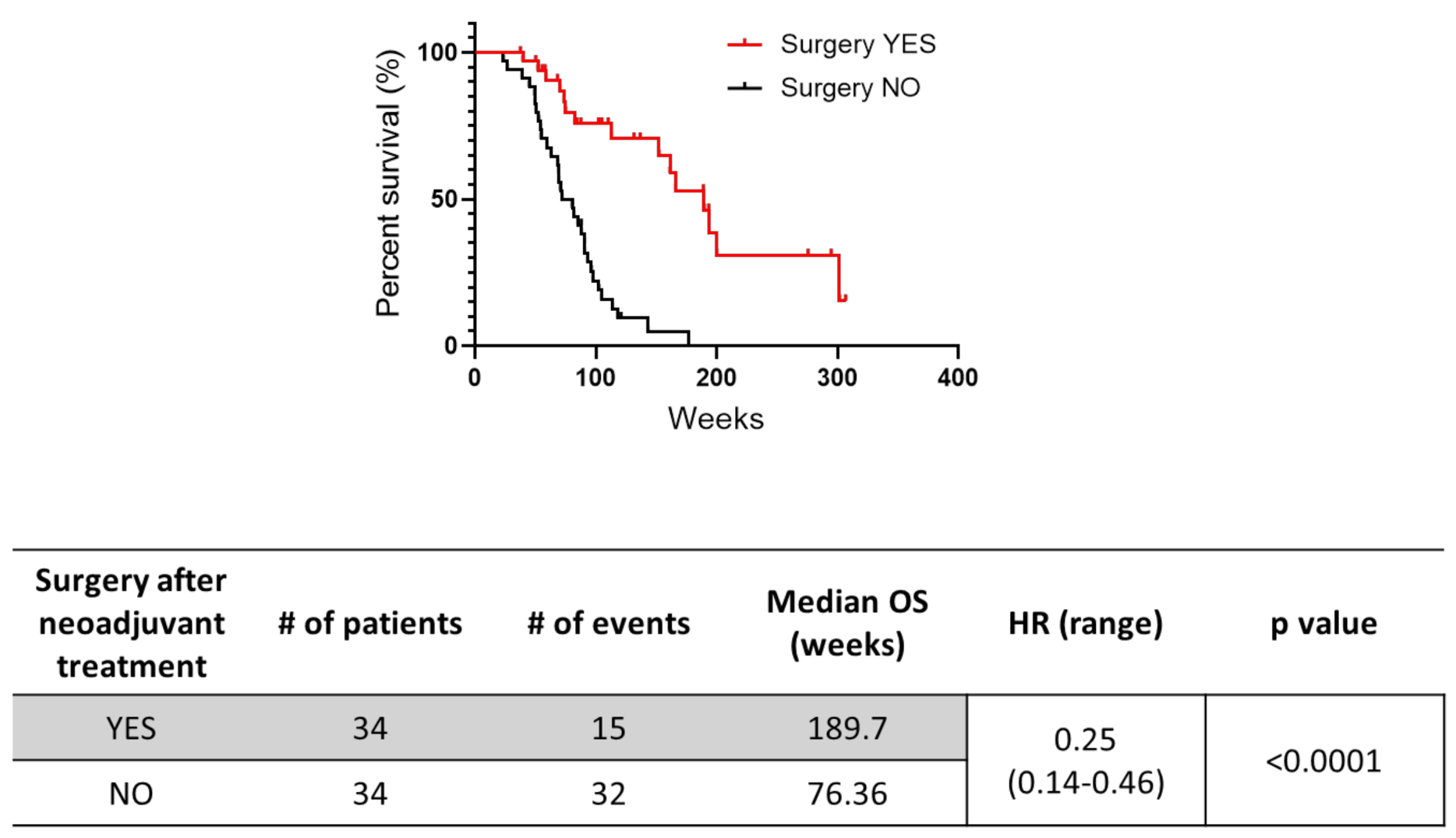
3.2. Efficacy of Neoadjuvant Treatment
3.3. Adverse Events
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Mocci, E.; Kundu, P.; Wheeler, W.; Arslan, A.A.; Beane Freeman, L.E.; Bracci, P.M.; Brennan, P.; Canzian, F.; Du, M.; Gallinger, S.; et al. Smoking modifies pancreatic cancer risk loci on 2q21.3. Cancer Res. 2021, 81, 3134–3143. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Smith, G.L.; Hurria, A.; Hortobagyi, G.N.; Buchholz, T.A. Future of Cancer Incidence in the United States: Burdens Upon an Aging, Changing Nation. J. Clin. Oncol. 2009, 27, 2758–2765. [Google Scholar] [CrossRef] [PubMed]
- Silvestris, N.; Brunetti, O.; Bittoni, A.; Cataldo, I.; Corsi, D.; Crippa, S.; D’Onofrio, M.; Fiore, M.; Giommoni, E.; Milella, M.; et al. Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up of Exocrine Pancreatic Ductal Adenocarcinoma: Evidence Evaluation and Recommendations by the Italian Association of Medical Oncology (AIOM). Cancers 2020, 12, 1681. [Google Scholar] [CrossRef]
- Hidalgo, M.; Cascinu, S.; Kleeff, J.; Labianca, R.; Löhr, J.-M.; Neoptolemos, J.; Real, F.X.; Van Laethem, J.-L.; Heinemann, V. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology 2015, 15, 8–18. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v56–v68. [Google Scholar] [CrossRef]
- Conroy, T.; Desseigne, F.; Ychou, M.; Bouché, O.; Guimbaud, R.; Bécouarn, Y.; Adenis, A.; Raoul, J.-L.; Gourgou-Bourgade, S.; de la Fouchardière, C.; et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. N. Engl. J. Med. 2011, 364, 1817–1825. [Google Scholar] [CrossRef] [Green Version]
- Von Hoff, D.D.; Ervin, T.; Arena, F.P.; Chiorean, E.G.; Infante, J.; Moore, M.; Seay, T.; Tjulandin, S.A.; Ma, W.W.; Saleh, M.N.; et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl. J. Med. 2013, 369, 1691–1703. [Google Scholar] [CrossRef] [Green Version]
- Huguet, F.; Girard, N.; Guerche, C.S.-E.; Hennequin, C.; Mornex, F.; Azria, D. Chemoradiotherapy in the Management of Locally Advanced Pancreatic Carcinoma: A Qualitative Systematic Review. J. Clin. Oncol. 2009, 27, 2269–2277. [Google Scholar] [CrossRef]
- Varadhachary, G.R.; Tamm, E.P.; Abbruzzese, J.L.; Xiong, H.Q.; Crane, C.H.; Wang, H.; Lee, J.E.; Pisters, P.W.T.; Evans, D.B.; Wolff, R.A. Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy. Ann. Surg. Oncol. 2006, 13, 1035–1046. [Google Scholar] [CrossRef]
- Van Veldhuisen, E.; van den Oord, C.; Brada, L.J.; Walma, M.S.; Vogel, J.A.; Wilmink, J.W.; del Chiaro, M.; van Lienden, K.P.; Meijerink, M.R.; van Tienhoven, G.; et al. Locally Advanced Pancreatic Cancer: Work-Up, Staging, and Local Intervention Strategies. Cancers 2019, 11, 976. [Google Scholar] [CrossRef] [Green Version]
- Katz, M.H.G.; Marsh, R.; Herman, J.M.; Shi, Q.; Collison, E.; Venook, A.P.; Kindler, H.L.; Alberts, S.R.; Philip, P.; Lowy, A.M.; et al. Borderline Resectable Pancreatic Cancer: Need for Standardization and Methods for Optimal Clinical Trial Design. Ann. Surg. Oncol. 2013, 20, 2787–2795. [Google Scholar] [CrossRef]
- Massucco, P.; Capussotti, L.; Magnino, A.; Sperti, E.; Gatti, M.; Muratore, A.; Sgotto, E.; Gabriele, P.; Aglietta, M. Pancreatic Resections after Chemoradiotherapy for Locally Advanced Ductal Adenocarcinoma: Analysis of Perioperative Outcome and Survival. Ann. Surg. Oncol. 2006, 13, 1201–1208. [Google Scholar] [CrossRef]
- Gillen, S.; Schuster, T.; Meyer zum Büschenfelde, C.; Friess, H.; Kleeff, J. Preoperative/Neoadjuvant Therapy in Pancreatic Cancer: A Systematic Review and Meta-analysis of Response and Resection Percentages. PLoS Med. 2010, 7, e1000267. [Google Scholar] [CrossRef] [Green Version]
- Månsson, C.; Bergenfeldt, M.; Brahmstaedt, R.; Karlson, B.-M.; Nygren, P.; Nilsson, A. Safety and preliminary efficacy of ultrasound-guided percutaneous irreversible electroporation for treatment of localized pancreatic cancer. Anticancer Res. 2014, 34, 289–293. [Google Scholar]
- Sohal, D.P.S.; Duong, M.; Ahmad, S.A.; Gandhi, N.S.; Beg, M.S.; Wang-Gillam, A.; Wade, J.L.; Chiorean, E.G.; Guthrie, K.A.; Lowy, A.M.; et al. Efficacy of Perioperative Chemotherapy for Resectable Pancreatic Adenocarcinoma. JAMA Oncol. 2021, 7, 421. [Google Scholar] [CrossRef]
- Tempero, M.A.; Malafa, M.P.; Chiorean, E.G.; Czito, B.; Scaife, C.; Narang, A.K.; Fountzilas, C.; Wolpin, B.M.; Al-Hawary, M.; Asbun, H.; et al. Guidelines Insights: Pancreatic Adenocarcinoma, Version 1.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Edge, S.B.; Compton, C.C. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann. Surg. Oncol. 2010, 17, 1471–1474. [Google Scholar] [CrossRef]
- Suker, M.; Beumer, B.R.; Sadot, E.; Marthey, L.; Faris, J.E.; Mellon, E.A.; El-Rayes, B.F.; Wang-Gillam, A.; Lacy, J.; Hosein, P.J.; et al. FOLFIRINOX for locally advanced pancreatic cancer: A systematic review and patient-level meta-analysis. Lancet Oncol. 2016, 17, 801–810. [Google Scholar] [CrossRef] [Green Version]
- Philip, P.A.; Lacy, J.; Portales, F.; Sobrero, A.; Pazo-Cid, R.; Manzano Mozo, J.L.; Kim, E.J.; Dowden, S.; Zakari, A.; Borg, C.; et al. Nab-paclitaxel plus gemcitabine in patients with locally advanced pancreatic cancer (LAPACT): A multicentre, open-label phase 2 study. Lancet Gastroenterol. Hepatol. 2020, 5, 285–294. [Google Scholar] [CrossRef]
- Auclin, E.; Marthey, L.; Abdallah, R.; Mas, L.; Francois, E.; Saint, A.; Cunha, A.S.; Vienot, A.; Lecomte, T.; Hautefeuille, V.; et al. Role of FOLFIRINOX and chemoradiotherapy in locally advanced and borderline resectable pancreatic adenocarcinoma: Update of the AGEO cohort. Br. J. Cancer 2021. [Google Scholar] [CrossRef]
- Abrams, R.A.; Lowy, A.M.; O’Reilly, E.M.; Wolff, R.A.; Picozzi, V.J.; Pisters, P.W.T. Combined Modality Treatment of Resectable and Borderline Resectable Pancreas Cancer: Expert Consensus Statement. Ann. Surg. Oncol. 2009, 16, 1751–1756. [Google Scholar] [CrossRef]
- Guion-Dusserre, J.-F.; Bertaut, A.; Ghiringhelli, F.; Vincent, J.; Quipourt, V.; Marilier, S.; Tharin, Z.; Bengrine-Lefevre, L. Folfirinox in elderly patients with pancreatic or colorectal cancer-tolerance and efficacy. World J. Gastroenterol. 2016, 22, 9378. [Google Scholar] [CrossRef]
- Mellon, E.A.; Hoffe, S.E.; Springett, G.M.; Frakes, J.M.; Strom, T.J.; Hodul, P.J.; Malafa, M.P.; Chuong, M.D.; Shridhar, R. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol. 2015, 54, 979–985. [Google Scholar] [CrossRef]
- Chen, Y. Combined radiochemotherapy in patients with locally advanced pancreatic cancer: A meta-analysis. World J. Gastroenterol. 2013, 19, 7461. [Google Scholar] [CrossRef]
- Tong, H.; Fan, Z.; Liu, B.; Lu, T. The benefits of modified FOLFIRINOX for advanced pancreatic cancer and its induced adverse events: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 8666. [Google Scholar] [CrossRef] [Green Version]
- Blomstrand, H.; Scheibling, U.; Bratthäll, C.; Green, H.; Elander, N.O. Real world evidence on gemcitabine and nab-paclitaxel combination chemotherapy in advanced pancreatic cancer. BMC Cancer 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Yamao, K.; Takenaka, M.; Yoshikawa, T.; Ishikawa, R.; Okamoto, A.; Yamazaki, T.; Nakai, A.; Omoto, S.; Kamata, K.; Minaga, K.; et al. Clinical Safety and Efficacy of Secondary Prophylactic Pegylated G-CSF in Advanced Pancreatic Cancer Patients Treated with mFOLFIRINOX: A Single-center Retrospective Study. Intern. Med. 2019, 58, 1993–2002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canova, S.; Cicchiello, F.; Agustoni, F.; Bianchini, G.; Abbate, M.I.; Bidoli, P.; Cortinovis, D.L. Gemcitabine-induced Thrombocytosis as a Potential Predictive Factor in Non-small Cell Lung Cancer: Analysis of 318 Patients. Tumori J. 2017, 103, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Mulder, F.I.; Candeloro, M.; Kamphuisen, P.W.; Di Nisio, M.; Bossuyt, P.M.; Guman, N.; Smit, K.; Büller, H.R.; van Es, N. The Khorana score for prediction of venous thromboembolism in cancer patients: A systematic review and meta-analysis. Haematologica 2019, 104, 1277–1287. [Google Scholar] [CrossRef] [Green Version]


| Characteristics of Patients | FOLFIRINOX (n = 67) | Nab-Paclitaxel + Gemcitabine (n = 27) | p |
|---|---|---|---|
| Age at inclusion, Median (range) | 59.2 (35.4–74.9) | 67.8 (43.8–77.4) | 0.0003 |
| Sex Male Female | 34 33 | 14 13 | >0.99 |
| Site (n): Head Body/Tail | 42 25 | 21 6 | 0.23 |
| PS ECOG: 0 1 | 58 9 | 23 4 | >0.99 |
| Basal CA19.9, UI/mL ≤37 >37 N/A | 17 47 3 | 3 21 3 | 0.19 |
| Staging IIA IIB III | 3 14 50 | 4 5 18 | 0.22 |
| T classification: T2 T3 T4 | 4 13 50 | 0 9 18 | 0.18 |
| N classification: N0 N+ N/A | 14 45 8 | 7 15 5 | 0.54 |
| Outcome after Neoadjuvant Treatment | FOLFIRINOX (%) (n = 67) | Nab-Paclitaxel + Gemcitabine (%) (n = 27) | p |
|---|---|---|---|
| Partial Response (PR) | 31 (46.3) | 10 (37.1) | 0.19 |
| Stable Disease (SD) | 21 (34.3) | 6 (22.2) | |
| Progressive Disease (PD) | 15 (22.4) | 11 (40.7) |
| Surgery | FOLFIRINOX (%) (n = 67) | Nab-Paclitaxel + Gemcitabine (%) (n = 27) | p |
|---|---|---|---|
| YES | 28 (41.8) | 6 (22.2) | 0.097 |
| NO | 39 (58.2) | 21 (77.8) |
| Stage | FOLFIRINOX | Nab-Paclitaxel + Gemcitabine |
|---|---|---|
| IIA Resected Total | 0 3 | 2 4 |
| IIB Resected Total | 9 14 | 1 5 |
| III Resected Total | 19 50 | 3 18 |
| Variables | Hazard Ratio | p |
|---|---|---|
| Treatment Group Reference: FFN GemNab | ||
| 1.78 [1.03; 3.07] | 0.04 | |
| Age at Inclusion (years) Risk for each 1-unit increase | ||
| 1.02 [0.987; 1.05] | 0.274 | |
| Adverse Event | FOLFIRINOX n = 67 (%) | Nab-Paclitaxel + Gemcitabine N = 27 (%) | ||
|---|---|---|---|---|
| G1/2 | G3/4 | G1/2 | G3/4 | |
| Anemia | 45 (67.2) | 4 (6.0) | 23 (85.2) | 3 (11.1) |
| Neutropenia | 23 (34.3) | 25 (37.3) | 12 (44.4) | 6 (22.2) |
| Thrombocytopenia | 41 (61.2) | 2 (1.5) | 13 (48.1) | 5 (18.5) |
| Increased AST and/or ALT levels | 34 (50.8) | 3 (4.5) | 16 (59.3) | 3 (11.1) |
| Oral Mucositis | 13 (19.4) | 0 (0) | 5 (18.5) | 0 (0) |
| Diarrhea | 33 (49.3) | 4 (6.0) | 12 (44.4) | 2 (7.4) |
| Nausea | 43 (64.2) | 1 (1.5) | 11 (40.8) | 0 (0) |
| Vomiting | 24 (35.8) | 1 (1.5) | 5 (18.5) | 0 (0) |
| Fatigue | 51 (76.1) | 0 (0) | 20 (74.1) | 1 (3.7) |
| Periferal neuropathy | 40 (59.7) | 1 (1.5) | 6 (22.2) | 3 (11.1) |
| Thrombocytosis (platelet count > 500 × 103) | 1 (1.5) | 11 (40.7) | ||
| Peripheral edema | 1 (1.5) | 8 (29.6) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servetto, A.; Santaniello, A.; Napolitano, F.; Foschini, F.; Marciano, R.; Mozzillo, E.; Cascetta, P.; Amato, A.R.; Augurio, M.R.; Maresca, L.; et al. Use of FOLFIRINOX or Nab-Paclitaxel Plus Gemcitabine for the Treatment of Locally Advanced Pancreatic Adenocarcinoma: A Single Institution Observational Study. Cancers 2021, 13, 4939. https://doi.org/10.3390/cancers13194939
Servetto A, Santaniello A, Napolitano F, Foschini F, Marciano R, Mozzillo E, Cascetta P, Amato AR, Augurio MR, Maresca L, et al. Use of FOLFIRINOX or Nab-Paclitaxel Plus Gemcitabine for the Treatment of Locally Advanced Pancreatic Adenocarcinoma: A Single Institution Observational Study. Cancers. 2021; 13(19):4939. https://doi.org/10.3390/cancers13194939
Chicago/Turabian StyleServetto, Alberto, Antonio Santaniello, Fabiana Napolitano, Francesca Foschini, Roberta Marciano, Eleonora Mozzillo, Priscilla Cascetta, Anna Rita Amato, Maria Rosaria Augurio, Lucia Maresca, and et al. 2021. "Use of FOLFIRINOX or Nab-Paclitaxel Plus Gemcitabine for the Treatment of Locally Advanced Pancreatic Adenocarcinoma: A Single Institution Observational Study" Cancers 13, no. 19: 4939. https://doi.org/10.3390/cancers13194939
APA StyleServetto, A., Santaniello, A., Napolitano, F., Foschini, F., Marciano, R., Mozzillo, E., Cascetta, P., Amato, A. R., Augurio, M. R., Maresca, L., De Placido, P., De Placido, S., Formisano, L., & Bianco, R. (2021). Use of FOLFIRINOX or Nab-Paclitaxel Plus Gemcitabine for the Treatment of Locally Advanced Pancreatic Adenocarcinoma: A Single Institution Observational Study. Cancers, 13(19), 4939. https://doi.org/10.3390/cancers13194939







