The Microbiome in Childhood Acute Lymphoblastic Leukemia
Abstract
:Simple Summary
Abstract
1. Current Status of Microbiome Analysis in Pediatric ALL
2. Microbiome in ALL
2.1. Microbiome at Time of Diagnosis
2.1.1. Oral Microbiome
2.1.2. Gut Microbiome
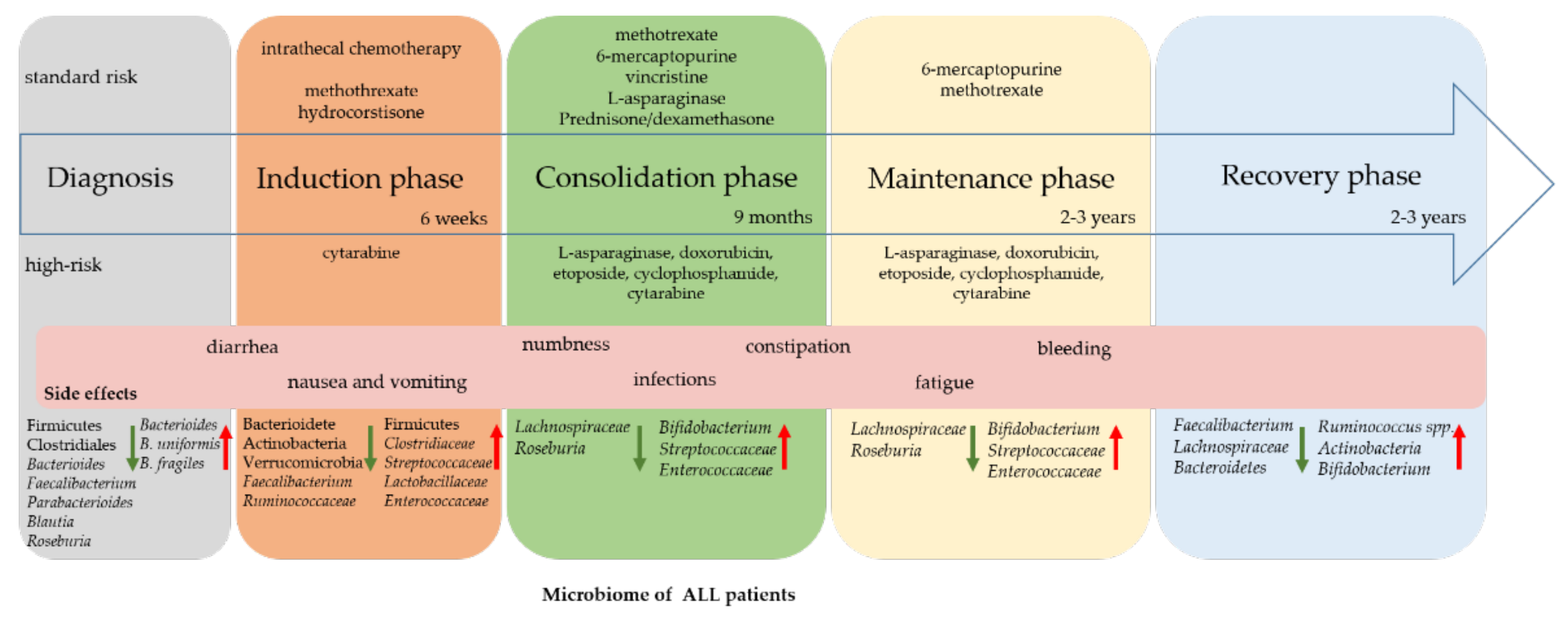
2.2. Changes during Therapy
2.3. Complications, Infections
2.4. Reconstitution after Therapy
3. Outlook: Modulation of the Microbiome
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef]
- De Luca, F.; Shoenfeld, Y. The microbiome in autoimmune diseases. Clin. Exp. Immunol. 2019, 195, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.F.; Wang, H. Environmental Exposures and Autoimmune Diseases: Contribution of Gut Microbiome. Front. Immunol. 2019, 10, 3094. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Jin, R.; Chen, H. Interactions Between Gut Microbiota and Acute Childhood Leukemia. Front. Microbiol. 2019, 10, 1300. [Google Scholar] [CrossRef] [PubMed]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holler, E.; Butzhammer, P.; Schmid, K.; Hundsrucker, C.; Koestler, J.; Peter, K.; Zhu, W.; Sporrer, D.; Hehlgans, T.; Kreutz, M.; et al. Metagenomic analysis of the stool microbiome in patients receiving allogeneic stem cell transplantation: Loss of diversity is associated with use of systemic antibiotics and more pronounced in gastrointestinal graft-versus-host disease. Biol. Blood Marrow Transplant. 2014, 20, 640–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopala, S.V.; Yooseph, S.; Harkins, D.M.; Moncera, K.J.; Zabokrtsky, K.B.; Torralba, M.G.; Tovchigrechko, A.; Highlander, S.K.; Pieper, R.; Sender, L.; et al. Gastrointestinal microbial populations can distinguish pediatric and adolescent Acute Lymphoblastic Leukemia (ALL) at the time of disease diagnosis. BMC Genom. 2016, 17, 635. [Google Scholar] [CrossRef] [Green Version]
- Cullen, C.M.; Aneja, K.K.; Beyhan, S.; Cho, C.E.; Woloszynek, S.; Convertino, M.; McCoy, S.J.; Zhang, Y.; Anderson, M.Z.; Alvarez-Ponce, D.; et al. Emerging Priorities for Microbiome Research. Front. Microbiol. 2020, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Vicente-Dueñas, C.; Janssen, S.; Oldenburg, M.; Auer, F.; González-Herrero, I.; Casado-García, A.; Isidro-Hernández, M.; Raboso-Gallego, J.; Westhoff, P.; Pandyra, A.A.; et al. An intact gut microbiome protects genetically predisposed mice against leukemia. Blood 2020, 136, 2003–2017. [Google Scholar] [CrossRef]
- Greaves, M.; Cazzaniga, V.; Ford, A. Can we prevent childhood Leukaemia? Leukemia 2021, 35, 1258–1264. [Google Scholar] [CrossRef]
- Nycz, B.T.; Dominguez, S.R.; Friedman, D.; Hilden, J.M.; Ir, D.; Robertson, C.E.; Frank, D.N. Evaluation of bloodstream infections, Clostridium difficile infections, and gut microbiota in pediatric oncology patients. PLoS ONE 2018, 13, e0191232. [Google Scholar] [CrossRef] [Green Version]
- Poore, G.D.; Kopylova, E.; Zhu, Q.; Carpenter, C.; Fraraccio, S.; Wandro, S.; Kosciolek, T.; Janssen, S.; Metcalf, J.; Song, S.J.; et al. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature 2020, 579, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Neis, E.P.; Dejong, C.H.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hauer, J.; Fischer, U.; Borkhardt, A. Towards prevention of childhood ALL by early-life immune training. Blood 2021. [Google Scholar] [CrossRef] [PubMed]
- Coates, M.; Lee, M.J.; Norton, D.; MacLeod, A.S. The Skin and Intestinal Microbiota and Their Specific Innate Immune Systems. Front. Immunol. 2019, 10, 2950. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Švec, P.; Franz, C.M.A.P. The genus Enterococcus. In Lactic Acid Bacteria: Biodiversity and Taxonomy; Holzapfel, W.H., Wood, B.J.B., Eds.; © 2021 by John Wiuley & Sons, Ltd. Registered Office: Chichester, West Sussex, UK, 2014; pp. 175–211. [Google Scholar]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-Utilizing Bacteria, Isolated from Human Feces, That Produce Butyrate as a Major Fermentation Product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briliute, J.; Urbanowicz, P.A.; Luis, A.S.; Basle, A.; Paterson, N.; Rebello, O.; Hendel, J.; Ndeh, D.A.; Lowe, E.C.; Martens, E.C.; et al. Complex N-glycan breakdown by gut Bacteroides involves an extensive enzymatic apparatus encoded by multiple co-regulated genetic loci. Nat. Microbiol. 2019, 4, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced Abundance of Butyrate-Producing Bacteria Species in the Fecal Microbial Community in Crohn’s Disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Lenoir, M.; Martin, R.; Torres-Maravilla, E.; Chadi, S.; Gonzalez-Davila, P.; Sokol, H.; Langella, P.; Chain, F.; Bermudez-Humaran, L.G. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes 2020, 12, 1826748. [Google Scholar] [CrossRef]
- Tsai, H.F.; Hsu, P.N. Interplay between Helicobacter pylori and immune cells in immune pathogenesis of gastric inflammation and mucosal pathology. Cell Mol. Immunol. 2010, 7, 255–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittana, H.; Gomes-Neto, J.C.; Heck, K.; Geis, A.L.; Segura Munoz, R.R.; Cody, L.A.; Schmaltz, R.J.; Bindels, L.B.; Sinha, R.; Hostetter, J.M.; et al. Commensal Escherichia coli Strains Can Promote Intestinal Inflammation via Differential Interleukin-6 Production. Front. Immunol. 2018, 9, 2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christofi, T.; Panayidou, S.; Dieronitou, I.; Michael, C.; Apidianakis, Y. Metabolic output defines Escherichia coli as a health-promoting microbe against intestinal Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 14463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van den Elsen, L.W.J.; Garssen, J.; Burcelin, R.; Verhasselt, V. Shaping the Gut Microbiota by Breastfeeding: The Gateway to Allergy Prevention? Front. Pediatr. 2019, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.J.; Zhao, D.D.; Liu, H.; Chen, H.T.; Li, J.J.; Mu, X.Q.; Liu, Z.; Li, X.; Tang, L.; Zhao, Z.Y.; et al. Cancer killers in the human gut microbiota: Diverse phylogeny and broad spectra. Oncotarget 2017, 8, 49574–49591. [Google Scholar] [CrossRef] [PubMed]
- Galloway-Pena, J.R.; Shi, Y.; Peterson, C.B.; Sahasrabhojane, P.; Gopalakrishnan, V.; Brumlow, C.E.; Daver, N.G.; Alfayez, M.; Boddu, P.C.; Khan, M.A.W.; et al. Gut Microbiome Signatures Are Predictive of Infectious Risk Following Induction Therapy for Acute Myeloid Leukemia. Clin. Infect. Dis. 2020, 71, 63–71. [Google Scholar] [CrossRef]
- Mancini, N.; Greco, R.; Pasciuta, R.; Barbanti, M.C.; Pini, G.; Morrow, O.B.; Morelli, M.; Vago, L.; Clementi, N.; Giglio, F.; et al. Enteric Microbiome Markers as Early Predictors of Clinical Outcome in Allogeneic Hematopoietic Stem Cell Transplant: Results of a Prospective Study in Adult Patients. Open Forum Infect. Dis. 2017, 4, ofx215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xue, J.; Zhou, X.; You, M.; Du, Q.; Yang, X.; He, J.; Zou, J.; Cheng, L.; Li, M.; et al. Oral microbiota distinguishes acute lymphoblastic leukemia pediatric hosts from healthy populations. PLoS ONE 2014, 9, e102116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, L.; Zhou, P.; Li, D.; Ju, X. Changes in the gastrointestinal microbiota of children with acute lymphoblastic leukaemia and its association with antibiotics in the short term. J. Med. Microbiol. 2017, 66, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zou, Y.; Ruan, M.; Chang, L.; Chen, X.; Wang, S.; Yang, W.; Zhang, L.; Guo, Y.; Chen, Y.; et al. Pediatric Acute Lymphoblastic Leukemia Patients Exhibit Distinctive Alterations in the Gut Microbiota. Front. Cell Infect. Microbiol. 2020, 10, 558799. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, W.; Liu, H.; Duan, J.; Zhang, Y.; Liu, M.; Li, H.; Hou, Z.; Wu, K.K. Effect of high-dose methotrexate chemotherapy on intestinal Bifidobacteria, Lactobacillus and Escherichia coli in children with acute lymphoblastic leukemia. Exp. Biol. Med. 2012, 237, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Hakim, H.; Dallas, R.; Wolf, J.; Tang, L.; Schultz-Cherry, S.; Darling, V.; Johnson, C.; Karlsson, E.A.; Chang, T.C.; Jeha, S.; et al. Gut Microbiome Composition Predicts Infection Risk During Chemotherapy in Children With Acute Lymphoblastic Leukemia. Clin. Infect. Dis. 2018, 67, 541–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajagopala, S.V.; Singh, H.; Yu, Y.; Zabokrtsky, K.B.; Torralba, M.G.; Moncera, K.J.; Frank, B.; Pieper, R.; Sender, L.; Nelson, K.E. Persistent Gut Microbial Dysbiosis in Children with Acute Lymphoblastic Leukemia (ALL) During Chemotherapy. Microb. Ecol. 2020, 79, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.L.; Rajasuriar, R.; Lim, Y.A.L.; Woo, Y.L.; Loke, P.; Ariffin, H. Temporal changes in gut microbiota profile in children with acute lymphoblastic leukemia prior to commencement-, during-, and post-cessation of chemotherapy. BMC Cancer 2020, 20, 151. [Google Scholar] [CrossRef] [Green Version]
- De Pietri, S.; Ingham, A.C.; Frandsen, T.L.; Rathe, M.; Krych, L.; Castro-Mejia, J.L.; Nielsen, D.S.; Nersting, J.; Wehner, P.S.; Schmiegelow, K.; et al. Gastrointestinal toxicity during induction treatment for childhood acute lymphoblastic leukemia: The impact of the gut microbiota. Int. J. Cancer 2020, 147, 1953–1962. [Google Scholar] [CrossRef]
- Nearing, J.T.; Connors, J.; Whitehouse, S.; Van Limbergen, J.; Macdonald, T.; Kulkarni, K.; Langille, M.G.I. Infectious Complications Are Associated With Alterations in the Gut Microbiome in Pediatric Patients With Acute Lymphoblastic Leukemia. Front. Cell Infect. Microbiol. 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zeng, X.; Yang, X.; Que, J.; Du, Q.; Zhang, Q.; Zou, J. Oral Health, Caries Risk Profiles, and Oral Microbiome of Pediatric Patients with Leukemia Submitted to Chemotherapy. BioMed Res. Int. 2021, 2021, 6637503. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.L.; Rajasuriar, R.; Azanan, M.S.; Abdullah, N.K.; Tang, M.S.; Lee, S.C.; Woo, Y.L.; Lim, Y.A.; Ariffin, H.; Loke, P. Reduced microbial diversity in adult survivors of childhood acute lymphoblastic leukemia and microbial associations with increased immune activation. Microbiome 2017, 5, 35. [Google Scholar] [CrossRef] [Green Version]
- Thomas, R.; Wong, W.S.W.; Saadon, R.; Vilboux, T.; Deeken, J.; Niederhuber, J.; Hourigan, S.K.; Yang, E. Gut microbial composition difference between pediatric ALL survivors and siblings. Pediatr. Hematol. Oncol. 2020, 37, 475–488. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, H.C.; Kim, C.S. Oral signs of acute leukemia for early detection. J. Periodontal. Implant. Sci. 2014, 44, 293–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauritano, D.; Petruzzi, M.; Fumagalli, T.; Giacomello, M.S.; Caccianiga, G. Oral Manifestations in Children with Acute Lymphoblastic Leukemia. Eur. J. Inflamm. 2012, 10, 65–68. [Google Scholar] [CrossRef]
- Vacca, M.; Celano, G.; Calabrese, F.M.; Portincasa, P.; Gobbetti, M.; De Angelis, M. The Controversial Role of Human Gut Lachnospiraceae. Microorganisms 2020, 8, 573. [Google Scholar] [CrossRef]
- Inaba, H.; Pei, D.; Wolf, J.; Howard, S.C.; Hayden, R.T.; Go, M.; Varechtchouk, O.; Hahn, T.; Buaboonnam, J.; Metzger, M.L.; et al. Infection-related complications during treatment for childhood acute lymphoblastic leukemia. Ann. Oncol. 2017, 28, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Tidjani Alou, M.; Lagier, J.-C.; Raoult, D. Diet influence on the gut microbiota and dysbiosis related to nutritional disorders. Hum. Microbiome J. 2016, 1, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, 20121173. [Google Scholar] [CrossRef] [Green Version]
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Smilowitz, J.T. Colonization by B. infantis EVC001 modulates enteric inflammation in exclusively breastfed infants. Pediatr. Res. 2019, 86, 749–757. [Google Scholar] [CrossRef]
- Fattizzo, B.; Cavallaro, F.; Folino, F.; Barcellini, W. Recent insights into the role of the microbiome in malignant and benign hematologic diseases. Crit. Rev. Oncol. Hematol. 2021, 160, 103289. [Google Scholar] [CrossRef]
- Blijlevens, N.M.; Donnelly, J.P.; De Pauw, B.E. Mucosal barrier injury: Biology, pathology, clinical counterparts and consequences of intensive treatment for haematological malignancy: An overview. Bone Marrow Transplant. 2000, 25, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Shi, L.; Sun, T.; Guo, K.; Geng, S. Dysbiosis of gut microbiota and its correlation with dysregulation of cytokines in psoriasis patients. BMC Microbiol. 2021, 21, 78. [Google Scholar] [CrossRef] [PubMed]
- De Ryck, T.; Vanlancker, E.; Grootaert, C.; Roman, B.I.; De Coen, L.M.; Vandenberghe, I.; Stevens, C.V.; Bracke, M.; Van de Wiele, T.; Vanhoecke, B. Microbial inhibition of oral epithelial wound recovery: Potential role for quorum sensing molecules? AMB Express 2015, 5, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fijlstra, M.; Ferdous, M.; Koning, A.M.; Rings, E.H.H.M.; Harmsen, H.J.M.; Tissing, W.J.E. Substantial decreases in the number and diversity of microbiota during chemotherapy-induced gastrointestinal mucositis in a rat model. Support. Care Cancer 2014, 23, 1513–1522. [Google Scholar] [CrossRef]
- Kwong, Y.L.; Yeung, D.Y.; Chan, J.C. Intrathecal chemotherapy for hematologic malignancies: Drugs and toxicities. Ann. Hematol. 2009, 88, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xia, X.; Wang, P.; Chen, S.; Yu, C.; Huang, R.; Zhang, R.; Wang, Y.; Lu, L.; Yuan, F.; et al. Induction and Amelioration of Methotrexate-Induced Gastrointestinal Toxicity are Related to Immune Response and Gut Microbiota. EBioMedicine 2018, 33, 122–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.H.; Qian, L.Y.; Pang, J.; Lin, J.Y.; Xu, Q.; Wang, L.H.; Huang, D.S.; Zou, H. The regulation of immune cells by Lactobacilli: A potential therapeutic target for anti-atherosclerosis therapy. Oncotarget 2017, 8, 59915–59928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Sanchez, B.; Margolles, A. Bifidobacteria and Their Molecular Communication with the Immune System. Front. Microbiol. 2017, 8, 2345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzin, M.; Stefancic, K.; Lucafo, M.; Decorti, G.; Stocco, G. Microbiota and Drug Response in Inflammatory Bowel Disease. Pathogens 2021, 10, 211. [Google Scholar] [CrossRef]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef]
- Fakhoury, M.; de Beaumais, T.; Medard, Y.; Jacqz-Aigrain, E.; Suivi Thérapeutique Pharmacologique de la Société Française de Pharmacologie et de Thérapeutique. Therapeutic drug monitoring of 6-thioguanine nucleotides in paediatric acute lymphoblastic leukaemia: Interest and limits. Therapie 2010, 65, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Ma, R.; Riordan, S.M.; Grimm, M.C.; Liu, L.; Wang, Y.; Zhang, L. Azathioprine, Mercaptopurine, and 5-Aminosalicylic Acid Affect the Growth of IBD-Associated Campylobacter Species and Other Enteric Microbes. Front. Microbiol. 2017, 8, 527. [Google Scholar] [CrossRef] [Green Version]
- Rigby, R.J.; Carr, J.; Orgel, K.; King, S.L.; Lund, P.K.; Dekaney, C.M. Intestinal bacteria are necessary for doxorubicin-induced intestinal damage but not for doxorubicin-induced apoptosis. Gut Microbes 2016, 7, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, A.; Culp, E.; Perry, J.; Lau, J.T.; MacNeil, L.T.; Surette, M.G.; Wright, G.D. Transformation of the Anticancer Drug Doxorubicin in the Human Gut Microbiome. ACS Infect. Dis. 2018, 4, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Lim, S.H. Bidirectional interaction between intestinal microbiome and cancer: Opportunities for therapeutic interventions. Biomark. Res. 2020, 8, 31. [Google Scholar] [CrossRef]
- Yang, J.; Liu, K.X.; Qu, J.M.; Wang, X.D. The changes induced by cyclophosphamide in intestinal barrier and microflora in mice. Eur. J. Pharmacol. 2013, 714, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Viaud, S.; Saccheri, F.; Mignot, G.; Yamazaki, T.; Daillère, R.; Hannani, D.; Enot, D.P.; Pfirschke, C.; Engblom, C.; Pittet, M.J.; et al. The Intestinal Microbiota Modulates the Anticancer Immune Effects of Cyclophosphamide. Science 2013, 342, 971–976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vital, M.; Karch, A.; Pieper, D.H. Colonic Butyrate-Producing Communities in Humans: An Overview Using Omics Data. mSystems 2017, 2, e00130-17. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.P.; Pembroke, J.T. Brevundimonas spp.: Emerging global opportunistic pathogens. Virulence 2018, 9, 480–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Vliet, M.J.; Tissing, W.J.; Rings, E.H.; Koetse, H.A.; Stellaard, F.; Kamps, W.A.; de Bont, E.S. Citrulline as a marker for chemotherapy induced mucosal barrier injury in pediatric patients. Pediatr. Blood Cancer 2009, 53, 1188–1194. [Google Scholar] [CrossRef]
- De Pietri, S.; Frandsen, T.L.; Christensen, M.; Grell, K.; Rathe, M.; Muller, K. Citrulline as a biomarker of bacteraemia during induction treatment for childhood acute lymphoblastic leukaemia. Pediatr. Blood Cancer 2021, 68, e28793. [Google Scholar] [CrossRef] [PubMed]
- Pelland-Marcotte, M.-C.; Pole, J.D.; Hwee, J.; Sutradhar, R.; Science, M.; Nathan, P.C.; Sung, L. Long-Term Risk of Infections After Treatment of Childhood Leukemia: A Population-Based Cohort Study Using Administrative Health Data. J. Clin. Oncol. 2019, 37, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Kawashima, T.; Hudson, M.M.; Meadows, A.T.; Friedman, D.L.; Marina, N.; Hobbie, W.; Kadan-Lottick, N.S.; et al. Chronic Health Conditions in Adult Survivors of Childhood Cancer. N. Engl. J. Med. 2006, 355, 1572–1582. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical Ascertainment of Health Outcomes Among Adults Treated for Childhood Cancer. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Armstrong, G.T.; Kawashima, T.; Leisenring, W.; Stratton, K.; Stovall, M.; Hudson, M.M.; Sklar, C.A.; Robison, L.L.; Oeffinger, K.C. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J. Clin. Oncol. 2014, 32, 1218–1227. [Google Scholar] [CrossRef]
- Gupta, S.; Allen-Vercoe, E.; Petrof, E.O. Fecal microbiota transplantation: In perspective. Ther. Adv. Gastroenterol. 2016, 9, 229–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Severyn, C.J.; Brewster, R.; Andermann, T.M. Microbiota modification in hematology: Still at the bench or ready for the bedside? Blood Adv. 2019, 3, 3461–3472. [Google Scholar] [CrossRef] [PubMed]
- Drekonja, D.; Reich, J.; Gezahegn, S.; Greer, N.; Shaukat, A.; MacDonald, R.; Rutks, I.; Wilt, T.J. Fecal Microbiota Transplantation for Clostridium difficile Infection: A Systematic Review. Ann. Intern. Med. 2015, 162, 630–638. [Google Scholar] [CrossRef]
- Kim, P.; Gadani, A.; Abdul-Baki, H.; Mitre, R.; Mitre, M. Fecal microbiota transplantation in recurrent Clostridium difficile infection: A retrospective single-center chart review. JGH Open 2019, 3, 4–9. [Google Scholar] [CrossRef] [Green Version]
- FMT Trials. Available online: https://clinicaltrials.gov/ct2/results?cond=fecal+microbiota+transplant&Search=Apply&age_v=&age=0&gndr=&type=&rslt= (accessed on 1 July 2021).
- Bilinski, J.; Grzesiowski, P.; Sorensen, N.; Madry, K.; Muszynski, J.; Robak, K.; Wroblewska, M.; Dzieciatkowski, T.; Dulny, G.; Dwilewicz-Trojaczek, J.; et al. Fecal Microbiota Transplantation in Patients With Blood Disorders Inhibits Gut Colonization With Antibiotic-Resistant Bacteria: Results of a Prospective, Single-Center Study. Clin. Infect. Dis. 2017, 65, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Ward, T.; Sidiropoulos, D.; Hillmann, B.M.; Chun, C.L.; Sadowsky, M.J.; Knights, D.; Montassier, E. Fecal microbiota transplantation reverses antibiotic and chemotherapy-induced gut dysbiosis in mice. Sci. Rep. 2018, 8, 6219. [Google Scholar] [CrossRef]
- Rotz, S.J.; Dandoy, C.E. The microbiome in pediatric oncology. Cancer 2020, 126, 3629–3637. [Google Scholar] [CrossRef]
- Sharifi, M.; Moridnia, A.; Mortazavi, D.; Salehi, M.; Bagheri, M.; Sheikhi, A. Kefir: A powerful probiotics with anticancer properties. Med. Oncol. 2017, 34, 183. [Google Scholar] [CrossRef]
- Reyna-Figueroa, J.; Barrón-Calvillo, E.; García-Parra, C.; Galindo-Delgado, P.; Contreras-Ochoa, C.; Lagunas-Martínez, A.; Campos-Romero, F.H.; Silva-Estrada, J.A.; Limón-Rojas, A.E. Probiotic Supplementation Decreases Chemotherapy-induced Gastrointestinal Side Effects in Patients With Acute Leukemia. J. Pediatr. Hematol. Oncol. 2019, 41, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Reyna-Figueroa, J.; Bejarano-Juvera, A.A.; García-Parra, C.; Barrón-Calvillo, E.E.; Queipo-Garcia, G.E.; Galindo-Delgado, P. Decrease of Postchemotherapy Complications With the Use of Probiotics in Children With Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2021, 43, e457–e461. [Google Scholar] [CrossRef] [PubMed]
- Sherid, M.; Samo, S.; Sulaiman, S.; Husein, H.; Sifuentes, H.; Sridhar, S. Liver abscess and bacteremia caused by lactobacillus: Role of probiotics? Case report and review of the literature. BMC Gastroenterol. 2016, 16, 138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, S.; Fujita, H.; Shimosato, T.; Kamijo, A.; Ishiyama, Y.; Yamamoto, E.; Ishii, Y.; Hattori, Y.; Hagihara, M.; Yamazaki, E.; et al. Septicemia from Lactobacillus rhamnosus GG, from a Probiotic Enriched Yogurt, in a Patient with Autologous Stem Cell Transplantation. Probiotics AntiMicrob. Proteins 2019, 11, 295–298. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly-Y, M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.L.; Wang, S.N.; Miao, C.Y. Influence of Microbiota on Intestinal Immune System in Ulcerative Colitis and Its Intervention. Front. Immunol. 2017, 8, 1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Phylum | Genera | Species | Nutrient | Immunity | Refs. |
|---|---|---|---|---|---|
| Fusobacteria | F. nucleatum | NK | [21] | ||
| Firmicutes | Clostridium | Cluster XIVa/IV | butyrate | Treg | [22] |
| Eubacterium | lactic acid | [19] | |||
| Faecalibacterium | F. prausnitzii | butyrate | TH17/Treg | [23] | |
| Roseburia | SCFAs | Treg | [17] | ||
| Ruminococcus | butyrate | Treg | [22] | ||
| Enterococcus | lactic acid | [18] | |||
| Bacterioidetes | Bacteroides | glycans | [20] | ||
| Prevotella | carbohydrates | [16] | |||
| Actinobacteria | Bifidobacterium | SCFAs | [16] | ||
| Proteobacteria | Helicobacter | TH1 | [24] | ||
| Escherischia | protective against Salmonella typhimurium and Pseudomonas aeruginosa | [25,26] | |||
| Time Point | Sample Type | Patients | Controls | Time Points | Seq. Region | Refs. |
|---|---|---|---|---|---|---|
| At time of diagnosis | Oral | 13 | 13 | 1 | V1-V3 | [31] |
| Stool | 23+5 | 23 | 4 | V1-V3 | [8] | |
| Fecal | 30 | 33 | 1 | V3-V4 | [32] | |
| Stool | 58 | 23 | 1 | V1-V9 | [33] | |
| During therapy | Stool | 36 | 36 | patients = 3; controls = 1 | N/A | [34] |
| Stool | 199 | 0 | 4 | V1-V3 | [35] | |
| Stool | 32 | 25 | 13 | V4 | [36] | |
| Anal swabs | 7 | 7 | 3 | V4 | [37] | |
| Stool | 51 | 19 | patients = 5; controls = 2 | V3-V4 | [38] | |
| Complications during therapy | Stool | 42 (15 ALL) | 0 | 1 | V3-V4 | [12] |
| Stool | 199 | 0 | 4 | V1-V3 | [35] | |
| Stool | 16 | 0 | 2 | V4-V5 | [39] | |
| Stool | 51 | 19 | patients = 5; controls = 2 | V3-V4 | [38] | |
| Oral | 39 | 39 | 1 | V1-V3 | [40] | |
| After therapy | Anal swabs | 73 | 61 | 1 | V4 | [41] |
| Stool | 32 | 25 | 13 | V4 | [36] | |
| Stool | 38 | 16 | 1 | V4 | [42] | |
| Anal swabs | 7 | 7 | 3 | V4 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oldenburg, M.; Rüchel, N.; Janssen, S.; Borkhardt, A.; Gössling, K.L. The Microbiome in Childhood Acute Lymphoblastic Leukemia. Cancers 2021, 13, 4947. https://doi.org/10.3390/cancers13194947
Oldenburg M, Rüchel N, Janssen S, Borkhardt A, Gössling KL. The Microbiome in Childhood Acute Lymphoblastic Leukemia. Cancers. 2021; 13(19):4947. https://doi.org/10.3390/cancers13194947
Chicago/Turabian StyleOldenburg, Marina, Nadine Rüchel, Stefan Janssen, Arndt Borkhardt, and Katharina L. Gössling. 2021. "The Microbiome in Childhood Acute Lymphoblastic Leukemia" Cancers 13, no. 19: 4947. https://doi.org/10.3390/cancers13194947
APA StyleOldenburg, M., Rüchel, N., Janssen, S., Borkhardt, A., & Gössling, K. L. (2021). The Microbiome in Childhood Acute Lymphoblastic Leukemia. Cancers, 13(19), 4947. https://doi.org/10.3390/cancers13194947






