Type 2 Diabetes Mellitus and Clinicopathological Tumor Characteristics in Women Diagnosed with Breast Cancer: A Systematic Review and Meta-Analysis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Exposure and Outcomes
2.2. Systematic Search
2.3. Study Selection
2.4. Data Extraction
2.5. Methodological Quality Assessment
2.6. Statistical Analysis
2.7. Stratified Analyses and Sensitivity Analyses
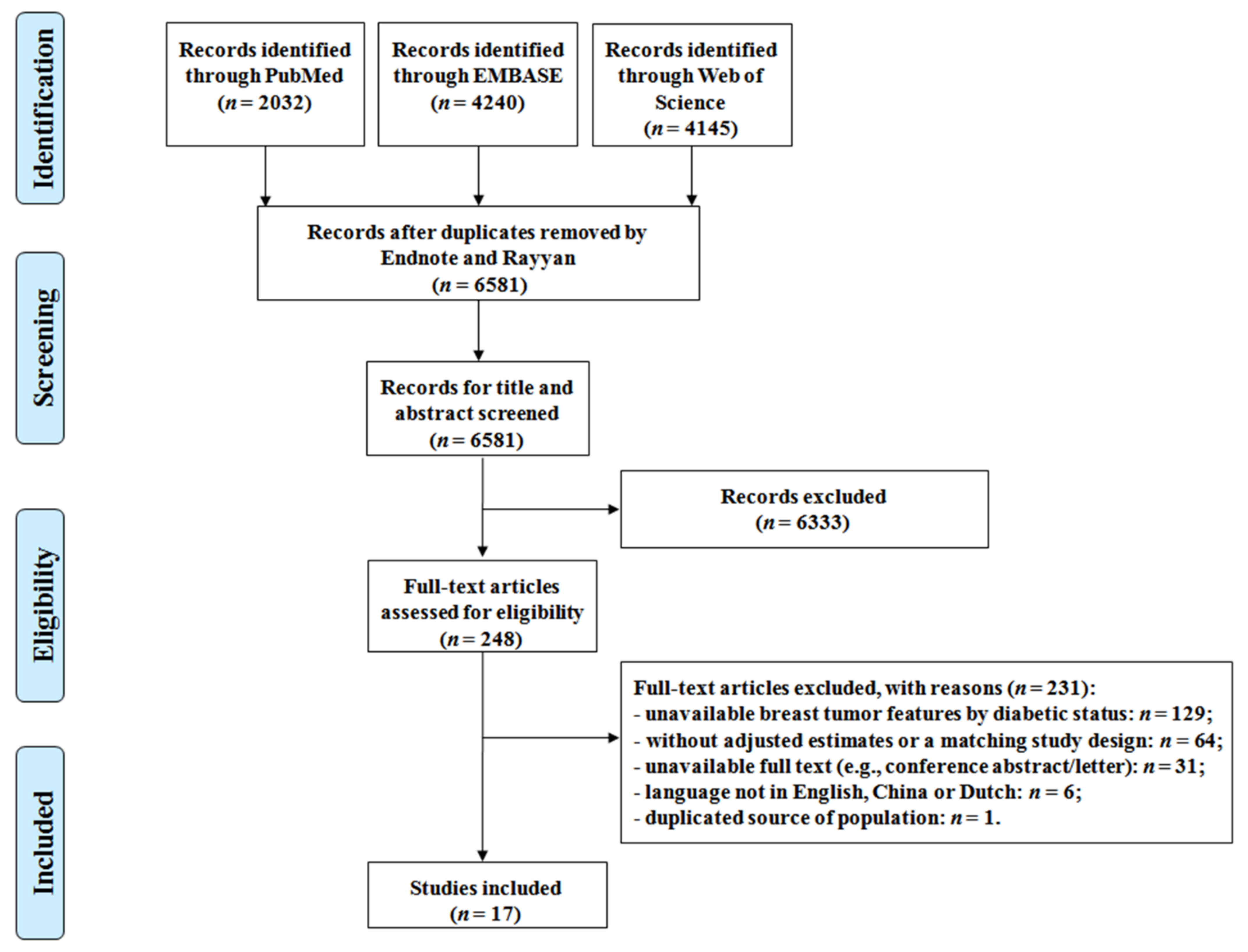
3. Results
3.1. Description of the Included Studies
3.2. Risk of Bias (ROB) of Eligible Studies
3.3. Associations between T2DM and Tumor Characteristics
3.3.1. Primary Outcome
3.3.2. Secondary Outcomes: Tumor Size, Lymph Node Status, and Tumor Grade
3.3.3. Secondary Outcomes: ER, PR, Her2, and TNBC
3.3.4. Publication Bias
4. Discussion
4.1. Summary of the Pooled Results
4.2. Tumor Stage, Tumor Size, Lymph Node Status, and Tumor Grade
4.3. ER, PR and Her2 Status
4.4. Confounding Effect
4.5. Strengths and Limitations
4.6. Future Research Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, K.; Ding, P.; Wu, Y.; Tian, W.; Pan, T.; Zhang, S. Global patterns and trends in the breast cancer incidence and mortality according to sociodemographic indices: An observational study based on the global burden of diseases. BMJ Open 2019, 9, e028461. [Google Scholar] [CrossRef]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021. [Google Scholar] [CrossRef]
- Jatoi, I.; Miller, A.B. Why is breast-cancer mortality declining? Lancet Oncol. 2003, 4, 251–254. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; et al.; Global Burden of Disease Cancer Collaboration Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar] [CrossRef]
- Ferlay, J.; Laversanne, M.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow (accessed on 12 June 2021).
- Li, M.; Roder, D.; D’Onise, K.; Walters, D.; Farshid, G.; Buckley, E.; Karapetis, C.; Joshi, R.; Price, T.; Townsend, A.; et al. Monitoring TNM stage of female breast cancer and survival across the South Australian population, with national and international TNM benchmarking: A population-based cohort study. BMJ Open 2020, 10, e037069. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Meisel, J.; Jiang, R.; Behera, M.; Peng, L. Validation of the newly proposed American Joint Committee on Cancer (AJCC) breast cancer prognostic staging group and proposing a new staging system using the National Cancer Database. Breast Cancer Res. Treat. 2018, 171, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Sant, M.; Allemani, C.; Capocaccia, R.; Hakulinen, T.; Aareleid, T.; Coebergh, J.W.; Coleman, M.P.; Grosclaude, P.; Martinez, C.; Bell, J.; et al. Stage at diagnosis is a key explanation of differences in breast cancer survival across Europe. Int. J. Cancer 2003, 106, 416–422. [Google Scholar] [CrossRef]
- Weiss, A.; Chavez-MacGregor, M.; Lichtensztajn, D.Y.; Yi, M.; Tadros, A.; Hortobagyi, G.N.; Giordano, S.H.; Hunt, K.K.; Mittendorf, E.A. Validation Study of the American Joint Committee on Cancer Eighth Edition Prognostic Stage Compared With the Anatomic Stage in Breast Cancer. JAMA Oncol. 2018, 4, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Rakha, E.A.; Reis-Filho, J.S.; Baehner, F.; Dabbs, D.J.; Decker, T.; Eusebi, V.; Fox, S.B.; Ichihara, S.; Jacquemier, J.; Lakhani, S.R.; et al. Breast cancer prognostic classification in the molecular era: The role of histological grade. Breast Cancer Res. 2010, 12, 207. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.M.; Henson, D.E.; Chen, D.; Rajamarthandan, S. Histologic grade remains a prognostic factor for breast cancer regardless of the number of positive lymph nodes and tumor size: A study of 161 708 cases of breast cancer from the SEER Program. Arch. Pathol. Lab. Med. 2014, 138, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thurlimann, B.; Senn, H.J.; Panel, M. Strategies for subtypes--dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Howlader, N.; Cronin, K.A.; Kurian, A.W.; Andridge, R. Differences in Breast Cancer Survival by Molecular Subtypes in the United States. Cancer Epidemiol. Biomark. Prev. 2018, 27, 619–626. [Google Scholar] [CrossRef]
- Helvie, M.A.; Chang, J.T.; Hendrick, R.E.; Banerjee, M. Reduction in late-stage breast cancer incidence in the mammography era: Implications for overdiagnosis of invasive cancer. Cancer 2014, 120, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Tabar, L.; Chen, T.H.; Yen, A.M.; Chen, S.L.; Fann, J.C.; Chiu, S.Y.; Ku, M.M.S.; Wu, W.Y.; Hsu, C.Y.; Chen, Y.Y.; et al. Effect of Mammography Screening on Mortality by Histological Grade. Cancer Epidemiol. Biomark. Prev. 2018, 27, 154–157. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Bae, S.; Choi, M.Y.; Lee, J.; Jung, S.P.; Kim, S.; Choe, J.H.; Kim, J.H.; Kim, J.S.; et al. Comparison between screen-detected and symptomatic breast cancers according to molecular subtypes. Breast Cancer Res. Treat. 2012, 131, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Falck, A.K.; Rome, A.; Ferno, M.; Olsson, H.; Chebil, G.; Bendahl, P.O.; Ryden, L. St Gallen molecular subtypes in screening-detected and symptomatic breast cancer in a prospective cohort with long-term follow-up. Br. J. Surg. 2016, 103, 513–523. [Google Scholar] [CrossRef]
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019; Available online: https://www.diabetesatlas.org (accessed on 12 June 2021).
- Larsson, S.C.; Mantzoros, C.S.; Wolk, A. Diabetes mellitus and risk of breast cancer: A meta-analysis. Int. J. Cancer 2007, 121, 856–862. [Google Scholar] [CrossRef]
- Liao, S.; Li, J.; Wei, W.; Wang, L.; Zhang, Y.; Li, J.; Wang, C.; Sun, S. Association between diabetes mellitus and breast cancer risk: A meta-analysis of the literature. Asian Pac. J. Cancer Prev. 2011, 12, 1061–1065. [Google Scholar]
- Starup-Linde, J.; Karlstad, O.; Eriksen, S.A.; Vestergaard, P.; Bronsveld, H.K.; de Vries, F.; Andersen, M.; Auvinen, A.; Haukka, J.; Hjellvik, V.; et al. CARING (CAncer Risk and INsulin analoGues): The association of diabetes mellitus and cancer risk with focus on possible determinants—a systematic review and a meta-analysis. Curr. Drug Saf. 2013, 8, 296–332. [Google Scholar] [CrossRef] [PubMed]
- Barone, B.B.; Yeh, H.C.; Snyder, C.F.; Peairs, K.S.; Stein, K.B.; Derr, R.L.; Wolff, A.C.; Brancati, F.L. Long-term all-cause mortality in cancer patients with preexisting diabetes mellitus: A systematic review and meta-analysis. JAMA 2008, 300, 2754–2764. [Google Scholar] [CrossRef]
- Zhao, X.B.; Ren, G.S. Diabetes mellitus and prognosis in women with breast cancer: A systematic review and meta-analysis. Medicine 2016, 95, e5602. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, X.; Gu, C.; Xia, J. Influence of diabetes mellitus on mortality in breast cancer patients. ANZ J. Surg. 2015, 85, 972–978. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Lega, I.C.; Wu, W.; Lipscombe, L.L. Breast, cervical and colorectal cancer screening in adults with diabetes: A systematic review and meta-analysis. Diabetologia 2020, 63, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Flores-Lopez, L.A.; Martinez-Hernandez, M.G.; Viedma-Rodriguez, R.; Diaz-Flores, M.; Baiza-Gutman, L.A. High glucose and insulin enhance uPA expression, ROS formation and invasiveness in breast cancer-derived cells. Cell Oncol. 2016, 39, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Takatani-Nakase, T.; Matsui, C.; Maeda, S.; Kawahara, S.; Takahashi, K. High glucose level promotes migration behavior of breast cancer cells through zinc and its transporters. PLoS ONE 2014, 9, e90136. [Google Scholar] [CrossRef]
- Peairs, K.S.; Barone, B.B.; Snyder, C.F.; Yeh, H.C.; Stein, K.B.; Derr, R.L.; Brancati, F.L.; Wolff, A.C. Diabetes mellitus and breast cancer outcomes: A systematic review and meta-analysis. J. Clin. Oncol. 2011, 29, 40–46. [Google Scholar] [CrossRef]
- Khanh, V.C.; Fukushige, M.; Moriguchi, K.; Yamashita, T.; Osaka, M.; Hiramatsu, Y.; Ohneda, O. Type 2 Diabetes Mellitus Induced Paracrine Effects on Breast Cancer Metastasis Through Extracellular Vesicles Derived from Human Mesenchymal Stem Cells. Stem Cells Dev. 2020, 29, 1382–1394. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Hayden, J.A.; van der Windt, D.A.; Cartwright, J.L.; Cote, P.; Bombardier, C. Assessing bias in studies of prognostic factors. Ann. Intern. Med. 2013, 158, 280–286. [Google Scholar] [CrossRef]
- Scala, C.; Leone Roberti Maggiore, U.; Candiani, M.; Venturini, P.L.; Ferrero, S.; Greco, T.; Cavoretto, P. Aberrant right subclavian artery in fetuses with Down syndrome: A systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2015, 46, 266–276. [Google Scholar] [CrossRef]
- Stinson, J.N.; Hayden, J.A.; Ahola Kohut, S.; Soobiah, C.; Cartwright, J.; Weiss, S.K.; Witmans, M.B. Sleep problems and associated factors in children with juvenile idiopathic arthritis: A systematic review. Pediatr. Rheumatol. Online J. 2014, 12, 19. [Google Scholar] [CrossRef]
- Boles, A.; Kandimalla, R.; Reddy, P.H. Dynamics of diabetes and obesity: Epidemiological perspective. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1026–1036. [Google Scholar] [CrossRef]
- Kann, S.; Schmid, S.M.; Eichholzer, M.; Huang, D.J.; Amann, E.; Guth, U. The impact of overweight and obesity on breast cancer: Data from Switzerland, so far a country little affected by the current global obesity epidemic. Gland Surg. 2014, 3, 181–197. [Google Scholar] [CrossRef] [PubMed]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast cancer in young women: An overview. Updates Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Schut, L.; Wangensteen, A.; Maaskant, J.; Tol, J.L.; Bahr, R.; Moen, M. Can Clinical Evaluation Predict Return to Sport after Acute Hamstring Injuries? A Systematic Review. Sports Med. 2017, 47, 1123–1144. [Google Scholar] [CrossRef]
- Ubachs, J.; Ziemons, J.; Minis-Rutten, I.J.G.; Kruitwagen, R.; Kleijnen, J.; Lambrechts, S.; Olde Damink, S.W.M.; Rensen, S.S.; Van Gorp, T. Sarcopenia and ovarian cancer survival: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 1165–1174. [Google Scholar] [CrossRef] [PubMed]
- Sidik, K.; Jonkman, J.N. A comparison of heterogeneity variance estimators in combining results of studies. Stat. Med. 2007, 26, 1964–1981. [Google Scholar] [CrossRef]
- Alsaeed, E.; Albeeshi, M.; Alsari, M.; Alowaini, F.; Alsaawi, A.; Bahabri, I. Diabetes Mellitus, Hypertension, Hyperlipidemia and Obesity do not Affect Tumor Expression of Estrogen and Progesterone Receptors in Saudi Breast Cancer Patients. Kuwait Med. J. 2017, 49, 17–21. [Google Scholar]
- Bronsveld, H.K.; Jensen, V.; Vahl, P.; De Bruin, M.L.; Cornelissen, S.; Sanders, J.; Auvinen, A.; Haukka, J.; Andersen, M.; Vestergaard, P.; et al. Diabetes and Breast Cancer Subtypes. PLoS ONE 2017, 12, e0170084. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.J.; Lu, L.J.; Jin, L.B.; Li, H.Y.; Ren, G.S.; Wu, K.N.; Liu, S.C.; Kong, L.Q. Clinicopathologic features of breast cancer patients with type 2 diabetes mellitus in southwest of China. Med. Oncol. 2014, 31, 788. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, S.; Sendur, M.A.; Altundag, K. Demographic and clinico-pathological characteristics in patients with invasive breast cancer receiving metformin. Med. Oncol. 2013, 30, 590. [Google Scholar] [CrossRef]
- Hamling, J.; Lee, P.; Weitkunat, R.; Ambuhl, M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat. Med. 2008, 27, 954–970. [Google Scholar] [CrossRef]
- Kuiper, J.S.; Zuidersma, M.; Zuidema, S.U.; Burgerhof, J.G.; Stolk, R.P.; Oude Voshaar, R.C.; Smidt, N. Social relationships and cognitive decline: A systematic review and meta-analysis of longitudinal cohort studies. Int. J. Epidemiol. 2016, 45, 1169–1206. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.; Altman, D.G. Chapter 10: Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (Updated February 2021); Higgins, J., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M., Welch, V., Eds.; Cochrane: London, UK, 2021; Available online: www.training.cochrane.org/handbook (accessed on 12 June 2021).
- Carmichael, A.R.; Bates, T. Obesity and breast cancer: A review of the literature. Breast 2004, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, A.M.; Edwards, S.A.; Sheppard, A.J.; Mirea, L.; Chong, N.; Paszat, L.; Shumak, R.S.; O’Malley, F.P.; Breast Screening Study Group. Favourable prognostic factors of subsequent screen-detected breast cancers among women aged 50–69. Eur. J. Cancer Prev. 2012, 21, 499–506. [Google Scholar] [CrossRef]
- Coughlin, S.S. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res. Treat. 2019, 177, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Yasmeen, S.; Hubbard, R.A.; Romano, P.S.; Zhu, W.; Geller, B.M.; Onega, T.; Yankaskas, B.C.; Miglioretti, D.L.; Kerlikowske, K. Risk of advanced-stage breast cancer among older women with comorbidities. Cancer Epidemiol. Biomark. Prev. 2012, 21, 1510–1519. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis, 1st ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2009. [Google Scholar]
- Fahim, S.M.; Hsu, C.H.; Lin, F.J.; Qian, J.; Chou, C. Association between prior use of anti-diabetic medication and breast cancer stage at diagnosis. Expert Opin. Drug Saf. 2021, 20, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, J.A.; van Herk-Sukel, M.P.P.; Vissers, P.A.J.; van der Heijden, A.; Bronsveld, H.K.; Herings, R.M.C.; Schmidt, M.K.; Nijpels, G. Type 2 Diabetes, but Not Insulin (Analog) Treatment, Is Associated With More Advanced Stages of Breast Cancer: A National Linkage of Cancer and Pharmacy Registries. Diabetes Care 2019, 42, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Cook, L.S.; Tang, M.C.; Hill, D.A.; Wiggins, C.L.; Li, C.I. Relationship between Diabetes and Diabetes Medications and Risk of Different Molecular Subtypes of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Ghanem, H.B.; Elsheikh, M.; El-Benhawy, S.A.; Shahba, A. Adipocytokines, inflammatory, epigenetic instability & angiogenesis biomarkers in type 2 diabetic Egyptian women with breast cancer. Diabetes Metab. Syndr. 2019, 13, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Murto, M.O.; Artama, M.; Pukkala, E.; Visvanathan, K.; Murtola, T.J. Breast cancer extent and survival among diabetic women in a Finnish nationwide cohort study. Int. J. Cancer 2018, 142, 2227–2233. [Google Scholar] [CrossRef]
- Mu, L.; Zhu, N.; Zhang, J.; Xing, F.; Li, D.; Wang, X. Type 2 diabetes, insulin treatment and prognosis of breast cancer. Diabetes Metab. Res. Rev. 2017, 33. [Google Scholar] [CrossRef]
- Samson, M.E.; Adams, S.A.; Orekoya, O.; Hebert, J.R. Understanding the Association of Type 2 Diabetes Mellitus in Breast Cancer Among African American and European American Populations in South Carolina. J. Racial Ethn. Health Disparities 2016, 3, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Lipscombe, L.L.; Fischer, H.D.; Austin, P.C.; Fu, L.; Jaakkimainen, R.L.; Ginsburg, O.; Rochon, P.A.; Narod, S.; Paszat, L. The association between diabetes and breast cancer stage at diagnosis: A population-based study. Breast Cancer Res. Treat. 2015, 150, 613–620. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Guo, X.; Cui, S.; Liu, H. Expression of HIF-1alpha and correlation with angiogenesis in tissue of breast cancer complicated with diabetes. Zhonghua Yi Xue Za Zhi 2015, 95, 252–255. [Google Scholar] [PubMed]
- Karlin, N.J.; Dueck, A.C.; Reddy, S.K.N.; Verona, P.M.; Cook, C.B. Implications of breast cancer with diabetes mellitus on patient outcomes and care. Diabetes Manag. 2014, 4, 411–419. [Google Scholar] [CrossRef]
- Ferro, A.; Goyal, S.; Kim, S.; Wu, H.; Taunk, N.K.; Schiff, D.; Pirlamarla, A.; Haffty, B.G. Evaluation of Diabetic Patients with Breast Cancer Treated with Metformin during Adjuvant Radiotherapy. Int. J. Breast Cancer 2013, 2013, 659723. [Google Scholar] [CrossRef] [PubMed]
- Yerrabothala, S.; Shaaban, H.; Capo, G.; Maroules, M.; Debari, V.A. The impact of diabetes mellitus on breast cancer outcomes: A single center retrospective study. Pathol. Oncol. Res. 2014, 20, 209–214. [Google Scholar] [CrossRef]
- Liao, S.; Li, J.; Wang, L.; Zhang, Y.; Wang, C.; Hu, M.; Ma, B.; Wang, G.; Sun, S. Type 2 diabetes mellitus and characteristics of breast cancer in China. Asian Pac. J. Cancer Prev. 2010, 11, 933–937. [Google Scholar] [PubMed]
- Kheirandish, M.; Mahboobi, H.; Yazdanparast, M.; Kamal, W.; Kamal, M.A. Anti-cancer Effects of Metformin: Recent Evidences for its Role in Prevention and Treatment of Cancer. Curr. Drug Metab. 2018, 19, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; Negalha, G.; Azevedo, A.; Martel, F. Metformin and Breast Cancer: Molecular Targets. J. Mammary Gland Biol. Neoplasia 2019, 24, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Jalving, M.; Gietema, J.A.; Lefrandt, J.D.; de Jong, S.; Reyners, A.K.; Gans, R.O.; de Vries, E.G. Metformin: Taking away the candy for cancer? Eur. J. Cancer 2010, 46, 2369–2380. [Google Scholar] [CrossRef]
- Hill-Briggs, F.; Adler, N.E.; Berkowitz, S.A.; Chin, M.H.; Gary-Webb, T.L.; Navas-Acien, A.; Thornton, P.L.; Haire-Joshu, D. Social Determinants of Health and Diabetes: A Scientific Review. Diabetes Care 2020. [Google Scholar] [CrossRef]
- Kolb, H.; Martin, S. Environmental/lifestyle factors in the pathogenesis and prevention of type 2 diabetes. BMC Med. 2017, 15, 131. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, X.F.; Chen, J.; Xia, L.; Cao, A.; Zhang, Y.; Wang, J.; Li, H.; Yang, K.; Guo, K.; et al. Combined lifestyle factors and risk of incident type 2 diabetes and prognosis among individuals with type 2 diabetes: A systematic review and meta-analysis of prospective cohort studies. Diabetologia 2020, 63, 21–33. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Barbosa, A.M.; Martel, F. Targeting Glucose Transporters for Breast Cancer Therapy: The Effect of Natural and Synthetic Compounds. Cancers 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Joung, K.H.; Jeong, J.W.; Ku, B.J. The association between type 2 diabetes mellitus and women cancer: The epidemiological evidences and putative mechanisms. Biomed Res. Int. 2015, 2015, 920618. [Google Scholar] [CrossRef]
- Matou-Nasri, S.; Sharaf, H.; Wang, Q.; Almobadel, N.; Rabhan, Z.; Al-Eidi, H.; Yahya, W.B.; Trivilegio, T.; Ali, R.; Al-Shanti, N.; et al. Biological impact of advanced glycation endproducts on estrogen receptor-positive MCF-7 breast cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2808–2820. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zielinska, H.A.; Arshad, A.; Shield, J.P.; Bahl, A.; Holly, J.M.; Perks, C.M. Hyperglycaemia-induced chemoresistance in breast cancer cells: Role of the estrogen receptor. Endocr. Relat. Cancer 2016, 23, 125–134. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Jang, S.Y.; Kim, C.; Choi, Y.; Kim, A. Anticancer effect of metformin on estrogen receptor-positive and tamoxifen-resistant breast cancer cell lines. Oncol. Rep. 2016, 35, 2553–2560. [Google Scholar] [CrossRef][Green Version]
- Park, Y.M.; Bookwalter, D.B.; O’Brien, K.M.; Jackson, C.L.; Weinberg, C.R.; Sandler, D.P. A prospective study of type 2 diabetes, metformin use, and risk of breast cancer. Ann. Oncol. 2021, 32, 351–359. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, C.; Choi, J.; Kim, A. Anti-cancer effect of metformin by suppressing signaling pathway of HER2 and HER3 in tamoxifen-resistant breast cancer cells. Tumour Biol. 2016, 37, 5811–5819. [Google Scholar] [CrossRef]
- Gutierrez, C.; Schiff, R. HER2: Biology, detection, and clinical implications. Arch. Pathol. Lab. Med. 2011, 135, 55–62. [Google Scholar] [CrossRef]
- Glovaci, D.; Fan, W.; Wong, N.D. Epidemiology of Diabetes Mellitus and Cardiovascular Disease. Curr. Cardiol. Rep. 2019, 21, 21. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Salehiniya, H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer 2019, 11, 151–164. [Google Scholar] [CrossRef]
- Pearson-Stuttard, J.; Zhou, B.; Kontis, V.; Bentham, J.; Gunter, M.J.; Ezzati, M. Worldwide burden of cancer attributable to diabetes and high body-mass index: A comparative risk assessment. Lancet Diabetes Endocrinol. 2018, 6, 95–104. [Google Scholar] [CrossRef]
- Torres-de la Roche, L.A.; Steljes, I.; Janni, W.; Friedl, T.W.P.; De Wilde, R.L. The Association between Obesity and Premenopausal Breast Cancer According to Intrinsic Subtypes—a Systematic Review. Geburtshilfe Frauenheilkd 2020, 80, 601–610. [Google Scholar] [CrossRef]
- Tatulashvili, S.; Fagherazzi, G.; Dow, C.; Cohen, R.; Fosse, S.; Bihan, H. Socioeconomic inequalities and type 2 diabetes complications: A systematic review. Diabetes Metab. 2020, 46, 89–99. [Google Scholar] [CrossRef]
- Belbasis, L.; Bellou, V. Introduction to Epidemiological Studies. Methods Mol. Biol. 2018, 1793, 1–6. [Google Scholar] [CrossRef]
- Jung, S.M.; Kang, D.; Guallar, E.; Yu, J.; Lee, J.E.; Kim, S.W.; Nam, S.J.; Cho, J.; Lee, S.K. Impact of Serum Lipid on Breast Cancer Recurrence. J. Clin. Med. 2020, 9, 2846. [Google Scholar] [CrossRef]



| First Author (Publication Year) | Study Design | Calendar Year of BC Diagnosis (Region) | Sample Size and Age (Years) | Original Comparisons: OR, 95% CI | Adjusted Factors 6 | Matched Factors 6 | |
|---|---|---|---|---|---|---|---|
| Breast Cancer with DM | Breast Cancer without DM | ||||||
| Fahim (2021) [55] | CS | 2007~2013 (USA) | sample size: 1719; age 1: <55, 55–64, 65–74, 75–84, >85: 45, 153, 657, 643, 221. | sample size: 6084; age 1: <55, 55–64, 65–74, 75–84, >85: 193, 328, 2238, 2172, 1153. | stages III/IV vs. cancers in situ and stages I/II: 0.97 (0.83–1.14). | age, race, geographic location, metropolitan status, comorbidity index, marital status, and year of BC diagnosis. | |
| Overbeek (2019) [56] | nested CC | 2002~2014 (the Netherlands) | sample size: 1567; age: mean ± SD: 71 ± 11; ≤53/>53 1: 85/1482. | sample size: 6267; age: mean ± SD: 71 ± 11; ≤53/>53 1: 330/5937. | more advanced tumor stage: 1.28 (1.13–1.44); larger tumor size: 1.22 (1.08–1.38); more advanced lymph node status: 1.31 (1.12–1.53); more advanced grade: 1.22 (1.08,1.39); ER− vs. ER+: 1.04 (0.86–1.25); PR− vs. PR+: 0.77 (0.67–0.89); Her2+ vs. Her2-: 0.93 (0.75–1.16); more aggressive subtype: 1.10 (0.94–1.27). | age, year of BC diagnosis, SES, chronic disease score, and use of glucocorticoids, estrogen-progestogen contraceptives, and hormone replacement therapy in the year prior to the BC diagnosis. | age at BC diagnosis. |
| Chen (2019) [57] | CC | 2004~2015; 2004~2012 (USA) | sample size: ER+/HER2-: 129; ER+/HER2+: 11; TN: 124; H2E: 46. | sample size: ER+/HER2-: 1863; ER+/HER2+: 313; TN: 1322; H2E: 532. | ER+/Her2+ vs. ER+/Her2-: 0.77 (0.40, 1.48); TNBC vs. ER+/Her2-: 1.38 (1.10, 1.89); H2E vs. ER+/Her2-: 1.38 (0.93, 2.06) | study site, year of BC diagnosis, BMI, age of BC diagnosis and race/ethnicity. | |
| Age 1: <40, 40–49, 50–59, 60–69: ER+/Her2-: 268, 555, 639, 530; ER+/Her2+: 74, 119, 88, 43; TN: 206, 409, 457, 374; H2E: 70, 133, 220, 155. | |||||||
| Ghanem (2019) [58] | CS | 2016~2017 (Egypt) | sample size: 20; age: mean ± SD: 59.67 ± 4.03. | sample size: 20; age: mean ± SD: 57.27 ± 4.48 | stage III vs. stages I/II: 2.45 (0.64–9.39); tumor size: >50 mm vs. ≤50 mm: 1.23 (0.35–4.31); lymph node invasion: N1–3 vs. N0: 2.45 (0.64–9.39); grade 3 vs. grade 1 and 2: 2.45 (0.64–9.39); grades 2 and 3 vs. grade 1: 1.89 (0.38–9.27); ER− vs. ER+: 0.46 (0.11–1.94); PR− vs. PR+: 0.66 (0.18–2.35). | menstrual state and SES. | |
| Murto (2018) [59] | CS | 1995~2013 (Finland) | sample size: 5469 age 1: ≤39, 40–55, ≥56:5, 207, 5257. | sample size: 67,701 age 1: ≤39, 40–55, ≥56: 570, 8311, 58, 820. | tumor stage: locally advanced vs. localized: 1.26 (1.18, 1.35); distant metastases vs. localized: 1.59 (1.44, 1.75). | age, number of mammography, screening rounds attended before the BC diagnosis, hypercholesterolemia, hypertension, coronary artery disease and obesity. | |
| Alsaeed (2017) [42] | CS | 2000~2006 (Saudi) | sample size: 24; | sample size: 86; | ER+ vs. ER−: 0.72 (0.22, 2.32); Her2+ vs. Her2-: 0.40 (0.12, 1.13). | obesity, hypertension and dyslipidemia. | |
| Age 1:<25, 25–35, 36–45, >45: 2, 13, 29, 66. | |||||||
| Mu (2017) [60] | CS | 2005~2010 (China) | sample size: 462; age 1: <50, ≥50-<65, ≥65: 61, 256, 145. | sample size: 1644; age 1:<50, ≥50-<65, ≥65:237, 896, 511. | tumor size: >20 mm vs. ≤20 mm: 1.09 (0.85–1.40) ;>50 mm vs. ≤50 mm: 1.47 (1.07–2.02); lymph node status: N1–3 vs. N0: 1.04 (0.85–1.28); grades 2 and 3 vs. grade 1: 1.45 (1.08–1.94); ER− vs. ER+: 1.177 (0.96–1.45); PR− vs. PR+: 1.06 (0.86–1.31); Her2+ vs. Her2-: 1.13 (0.90–1.42). | the same recruiting period and matched with age. | |
| Bronsveld (2016) [43] | CS | 2000~2010 (Denmark) | sample size: 211; age: median, IQR in two strata of age (≤50, >50): 47.0 (43.0, 50.0); 67.0 (60.0, 75.0). | sample size: 101; age: median, IQR in two strata of age (≤50, >50): 47.0 (43.0, 50.0); 67.0 (62.0, 73.0). | tumor size 2: >20 mm vs. ≤20 mm: 0.98 (0.61–1.59); >50 mm vs. ≤50 mm: 1.97 (0.54–7.14); lymph node status 2: N1–3 vs. N0: 1.16 (0.72–1.88); N2/N3 vs. N0/N1: 0.83 (0.45–1.53); grade 2 vs. grade 1 (pre-, post-menopausal):0.56 (0.22,1.42), 0.80 (0.31,2.03); grade 3 vs. grade 1 (pre-, post-menopausal): 1.08 (0.41, 2.86), 1.97 (0.72, 5.39); ER− vs. ER+ (pre-, post-menopausal): 2.32 (0.86, 6.31), 1.33 (0.52, 3.40); PR− vs. PR+ (pre-, post-menopausal):2.18 (0.92, 5.17), 1.06 (0.51, 2.19); Her2- vs. Her2+ (pre-, post-menopausal) :2.94 (1.08, 8.02), 1.20 (0.40, 3.59); luminal B-like, Her2- vs. luminal A-like (pre-, post-menopausal): 1.05 (0.40–2.73), 0.58 (0.25–1.35); Her2+ vs. luminal A-like (pre-, post-menopausal): 0.41 (0.14, 1.20), 0.88 (0.28, 2.71); TNBC vs. luminal A-like (pre-, post-menopausal): 2.21 (0.71, 6.69), 1.30 (0.40, 4.20). | age and BMI, except for grade, which was adjusted for age only. | year of birth and age at diagnosis (both in 10-year categories). |
| Samson (2016) [61] | CS | 1993~2002; 1996~2001 (USA) | sample size: African-American: 170; European-American: 73. | sample size: African-American: 509; European-American: 619. | Stage 3: localized vs. in situ: 1.23 (0.33, 4.54); regional vs. in situ: 1.34 (0.36, 5.05); distant metastasis vs. in situ: 1.36 (0.22, 8.59). | diabetes medications, and menopausal (deduced by age). | |
| age: mean ± SD: African-American: 61 ± 12; European-American: 63 ± 13. | |||||||
| Lipscombe (2015) [62] | CS | 2007~2012 (Canada) | sample size: 6115; age: median, IQR: 68 (60, 77). | sample size: 32,292; age: median, IQR: 59 (50, 69). | stage II vs. stage I: 1.14 (1.07, 1.22); stage III vs. stage I: 1.21 (1.11, 1.33); stage IV vs. stage I: 1.16 (1.01, 1.33); tumor size: > 20 mm vs. ≤20 mm: 1.16 (1.06, 1.28); lymph node status: N1–3 vs. N0: 1.16 (1.06–1.27); ER+ vs. ER−: 1.01 (0.93, 1.10). | prior screening mammogram, age, neighborhood income quintile, rural residence, number of primary care visits, weighted ADG comorbidity score, renal dialysis, and history of acute myocardial infarction, stroke, or congestive heart failure. | |
| Li (2015) [63] | CS | 2009~2012 (China) | sample size: 98; age: mean ± SD: 57.3 ± 10.3. | sample size: 107; age: mean ± SD: 56.6 ± 11.1. | tumor size: >20 mm vs. ≤20 mm: 1.83 (1.03–3.23); lymph node status: N1–3 vs. N0: 1.85 (1.06–3.22); grade 3 vs. grade 1–2: 1.67 (0.81–3.47); ER− vs. ER+: 1.05 (0.59–1.87); PR− vs. PR+: 1.31 (0.75–2.29); Her2+ vs. Her2-: 1.40 (0.73–2.71); | the same admitting period and age. | |
| Karlin (2014) [64] | CS | 2007~2011 (USA) | sample size: 109; age: median, range: 68 (28–91). | sample size: 109; age: median, range: 68 (28–91). | stages III/IV vs. stages I/II: 1.011 (0.466–2.195); grades 2 and 3 vs. grade 1: 1.06 (0.54–2.11); grade 3 vs. grades 1 and 2: 1.41 (0.76–2.60); ER− vs. ER+: 0.978 (0.461–2.074); PR− vs. PR+: 0.86 (0.48–1.55); Her2+ vs. Her2-: 1.710 (0.737–3.971). | age at diagnosis of BC, race, ethnicity, and year of BC diagnosis. | |
| Wang (2014) [44] | CS | 2007~2013 (China) | sample size: 164; age: mean: 60.7. | sample size: the first control group (nondiabetic patients with breast cancer): 328; age: unknown. | tumor size 4: >20 mm vs. ≤20 mm: 0.82 (0.54, 1.23); >50 mm vs. ≤50 mm: 1.44 (0.78, 2.64); lymph node status 4: N1–3 vs. N0:1.69 (1.12–2.56); N2/N3 vs. N1/N2: 1.85 (1.14–3.01); grade 3 vs. grades 1 and 2 4: 1.02 (0.46–2.27);grades 2 and 3 vs. grade 1 4: 0.86 (0.42–1.79);ER− vs. ER+ 4: 1.26 (0.84–1.88); PR− vs. PR+ 4: 0.79 (0.54–1.17); Her2+ vs. Her2- 4,5: 1.10 (0.74–1.63). | age at the time of BC diagnosis (±5 years), and year of diagnosis (±5 years). | |
| Aksoy (2013) [45] | CS | 2000~2012 (Turkey) | sample size: 148; age 1: <50/≥50: 37/111. | sample size: 636; age 1: <50/≥50: 148/488. | stages III/IV vs. stages I/II: 1.15 (0.78–1.68);tumor size: >50 mm vs. ≤5 mm: 1.23 (0.77, 1.96); lymph node invasion: N1–3 vs. N0: 1.07 (0.72–1.59); N2/N3 vs. N0: 1.34 (0.89–2.03); grade 3 vs. grades 1 and 2: 0.61 (0.40–0.92); grades 2/3 vs. grade 1: 1.05 (0.61–1.80); ER− vs. ER+: 0.55 (0.34–0.89); PR− vs. PR+: 0.58 (0.37–0.91); Her2+ vs. Her2-: 0.77 (0.47–1.26); TNBC: yes vs. no: 0.41 (0.19–0.86). | age. | |
| Ferro (2013) [65] | CS | 2004~2012 (USA) | sample size: 51; age: mean: 60.02;>50/≤50 1: 44/7. | sample size: 51; age: mean: 57.75; >50/≤50 1: 39/12. | tumor size: >20 mm vs. ≤20 mm: 1.18 (0.50–2.76); >50 mm vs. ≤50 mm: 0.32 (0.06–1.67); grade 3 vs. grades 1 and 2: 1.38 (0.56–3.41); grades 2 and 3 vs. grade 1: 0.43 (0.10–1.80); ER− vs. ER+: 1.21 (0.50–2.94); PR− vs. PR+: 1.10 (0.47–2.56); Her2+ vs. Her2-: 0.32 (0.11–0.91); | age (±5 years), surgical procedure, presence of adjuvant chemotherapy, radiation field design, and radiation dose. | |
| Yerrabothala (2013) [66] | CS | 2001~2010 (USA) | sample size: 85; age: median, IQR: 61 (55, 71). | sample size: 170; age: median, IQR: 62 (55, 71). | stages III/IV vs. cancers in situ and stages I/II: 1.51 (0.81–2.80); ER− vs. ER+: 2.63 (1.36–5.10); PR− vs. PR+: 2.22 (1.30–3.79); Her2+ vs. Her2-: 1.47 (0.71–3.01); TNBC: yes vs. no: 3.69 (1.70–8.04). | age (±2 years). | |
| Liao (2010) [67] | CS | 2005~2009 (China) | sample size: 143; age: mean ± SD: 58.3 ± 10.0. | sample size: 143; age: mean ± SD: 49.1 ± 11.7. | tumor size: >50 mm vs. ≤50 mm: 1.42 (0.67, 2.98); lymph node status: N2/N3 vs. N0/N1: 16.32 (5.54, 48.0); ER− vs. ER+: 1.40 (0.77, 2.53); PR− vs. PR+: 2.10 (1.13, 3.89); Her2- vs. Her2+: 1.76 (0.98, 3.14). | age. | the same admitting period. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; de Haan-Du, J.; Sidorenkov, G.; Landman, G.W.D.; Jalving, M.; Zhang, Q.; de Bock, G.H. Type 2 Diabetes Mellitus and Clinicopathological Tumor Characteristics in Women Diagnosed with Breast Cancer: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 4992. https://doi.org/10.3390/cancers13194992
Zhang F, de Haan-Du J, Sidorenkov G, Landman GWD, Jalving M, Zhang Q, de Bock GH. Type 2 Diabetes Mellitus and Clinicopathological Tumor Characteristics in Women Diagnosed with Breast Cancer: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(19):4992. https://doi.org/10.3390/cancers13194992
Chicago/Turabian StyleZhang, Fan, Jing de Haan-Du, Grigory Sidorenkov, Gijs W. D. Landman, Mathilde Jalving, Qingying Zhang, and Geertruida H. de Bock. 2021. "Type 2 Diabetes Mellitus and Clinicopathological Tumor Characteristics in Women Diagnosed with Breast Cancer: A Systematic Review and Meta-Analysis" Cancers 13, no. 19: 4992. https://doi.org/10.3390/cancers13194992
APA StyleZhang, F., de Haan-Du, J., Sidorenkov, G., Landman, G. W. D., Jalving, M., Zhang, Q., & de Bock, G. H. (2021). Type 2 Diabetes Mellitus and Clinicopathological Tumor Characteristics in Women Diagnosed with Breast Cancer: A Systematic Review and Meta-Analysis. Cancers, 13(19), 4992. https://doi.org/10.3390/cancers13194992






