Aberrant Expression of and Cell Death Induction by Engagement of the MHC-II Chaperone CD74 in Anaplastic Large Cell Lymphoma (ALCL)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. RNA Preparation and RT-PCR Analyses
2.3. Immunofluorescence and Flow Cytometry, Analysis of Apoptosis and Light Microscopy
2.4. Western Blot Analyses
2.5. Immunohistochemistry (IHC)
2.6. DNA Methylation Analyses Using Illumina Infinium Arrays
2.7. Statistics
3. Results
3.1. Aberrant CD74 Expression in ALCL Cell Lines
3.2. CD74 Expression in Human Lymphocytes and Primary ALCL Cases
3.3. DNA Methylation Analyses of the CD74 Locus in ALCL
3.4. Induction of Apoptosis in ALCL Following CD74 Ligation
3.5. CD74 Ligation Sensitizes ALCL Cell Lines for Various Apoptosis-Inducing Agents
3.6. Expression of MET in ALCL
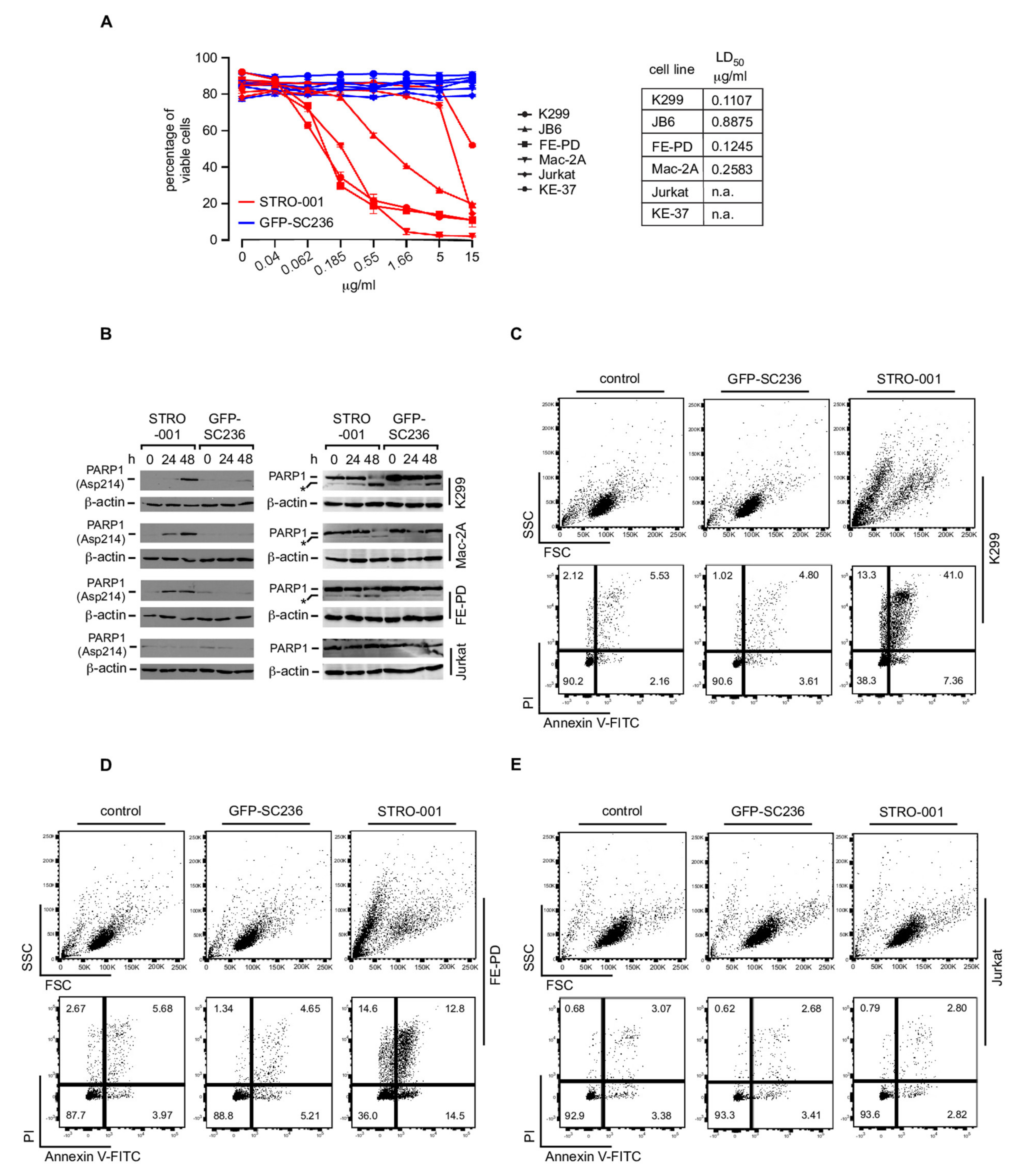
3.7. The CD74-Targeting Antibody-Drug Conjugate STRO-001 Efficiently Kills ALCL Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stein, H.; Foss, H.D.; Dürkop, H.; Marafioti, T.; Delsol, G.; Pulford, K.; Pileri, S.; Falini, B. CD30(+) anaplastic large cell lymphoma: A review of its histopathologic, genetic, and clinical features. Blood 2000, 96, 3681–3695. [Google Scholar]
- Ferreri, A.J.M.; Govi, S.; Pileri, S.A.; Savage, K.J. Anaplastic large cell lymphoma, ALK-positive. Crit. Rev. Oncol. Hematol. 2012, 83, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, A.J.M.; Govi, S.; Pileri, S.A.; Savage, K.J. Anaplastic large cell lymphoma, ALK-negative. Crit. Rev. Oncol. Hematol. 2013, 85, 206–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crescenzo, R.; Abate, F.; Lasorsa, E.; Tabbo’, F.; Gaudiano, M.; Chiesa, N.; Di Giacomo, F.; Spaccarotella, E.; Barbarossa, L.; Ercole, E.; et al. Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell 2015, 27, 516–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, A.L.; Dogan, A.; Smith, D.I.; Law, M.E.; Ansell, S.M.; Johnson, S.H.; Porcher, J.C.; Ozsan, N.; Wieben, E.D.; Eckloff, B.W.; et al. Discovery of recurrent t(6;7)(p25.3;q32.3) translocations in ALK-negative anaplastic large cell lymphomas by massively parallel genomic sequencing. Blood 2011, 117, 915–919. [Google Scholar] [CrossRef] [Green Version]
- Eckerle, S.; Brune, V.; Döring, C.; Tiacci, E.; Bohle, V.; Sundström, C.; Kodet, R.; Paulli, M.; Falini, B.; Klapper, W.; et al. Gene expression profiling of isolated tumour cells from anaplastic large cell lymphomas: Insights into its cellular origin, pathogenesis and relation to Hodgkin lymphoma. Leukemia 2009, 23, 2129–2138. [Google Scholar] [CrossRef] [Green Version]
- Parrilla Castellar, E.R.; Jaffe, E.S.; Said, J.W.; Swerdlow, S.H.; Ketterling, R.P.; Knudson, R.A.; Sidhu, J.S.; Hsi, E.D.; Karikehalli, S.; Jiang, L.; et al. ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014, 124, 1473–1480. [Google Scholar] [CrossRef] [Green Version]
- Boi, M.; Rinaldi, A.; Kwee, I.; Bonetti, P.; Todaro, M.; Tabbò, F.; Piva, R.; Rancoita, P.M.V.; Matolcsy, A.; Timar, B.; et al. PRDM1/BLIMP1 is commonly inactivated in anaplastic large T-cell lymphoma. Blood 2013, 122, 2683–2693. [Google Scholar] [CrossRef] [Green Version]
- Merkel, O.; Hamacher, F.; Laimer, D.; Sifft, E.; Trajanoski, Z.; Scheideler, M.; Egger, G.; Hassler, M.R.; Thallinger, C.; Schmatz, A.; et al. Identification of differential and functionally active miRNAs in both anaplastic lymphoma kinase (ALK)+ and ALK− anaplastic large-cell lymphoma. Proc. Natl. Acad. Sci. USA 2010, 107, 16228–16233. [Google Scholar] [CrossRef] [Green Version]
- Vose, J.; Armitage, J.; Weisenburger, D.; International T-Cell Lymphoma Project. International peripheral T-cell and natural killer/T-cell lymphoma study: Pathology findings and clinical outcomes. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 4124–4130. [Google Scholar]
- Bonzheim, I.; Geissinger, E.; Roth, S.; Zettl, A.; Marx, A.; Rosenwald, A.; Müller-Hermelink, H.K.; Rüdiger, T. Anaplastic large cell lymphomas lack the expression of T-cell receptor molecules or molecules of proximal T-cell receptor signaling. Blood 2004, 104, 3358–3360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathas, S.; Jöhrens, K.; Joos, S.; Lietz, A.; Hummel, F.; Janz, M.; Jundt, F.; Anagnostopoulos, I.; Bommert, K.; Lichter, P.; et al. Elevated NF-kappaB p50 complex formation and Bcl-3 expression in classical Hodgkin, anaplastic large-cell, and other peripheral T-cell lymphomas. Blood 2005, 106, 4287–4293. [Google Scholar] [CrossRef] [PubMed]
- Mathas, S.; Kreher, S.; Meaburn, K.J.; Jöhrens, K.; Lamprecht, B.; Assaf, C.; Sterry, W.; Kadin, M.E.; Daibata, M.; Joos, S.; et al. Gene deregulation and spatial genome reorganization near breakpoints prior to formation of translocations in anaplastic large cell lymphoma. Proc. Natl. Acad. Sci. USA 2009, 106, 5831–5836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weilemann, A.; Grau, M.; Erdmann, T.; Merkel, O.; Sobhiafshar, U.; Anagnostopoulos, I.; Hummel, M.; Siegert, A.; Hayford, C.; Madle, H.; et al. Essential role of IRF4 and MYC signaling for survival of anaplastic large cell lymphoma. Blood 2015, 125, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Hassler, M.R.; Pulverer, W.; Lakshminarasimhan, R.; Redl, E.; Hacker, J.; Garland, G.D.; Merkel, O.; Schiefer, A.-I.; Simonitsch-Klupp, I.; Kenner, L.; et al. Insights into the Pathogenesis of Anaplastic Large-Cell Lymphoma through Genome-wide DNA Methylation Profiling. Cell Rep. 2016, 17, 596–608. [Google Scholar] [CrossRef] [Green Version]
- Schleussner, N.; Merkel, O.; Costanza, M.; Liang, H.-C.; Hummel, F.; Romagnani, C.; Durek, P.; Anagnostopoulos, I.; Hummel, M.; Jöhrens, K.; et al. The AP-1-BATF and -BATF3 module is essential for growth, survival and TH17/ILC3 skewing of anaplastic large cell lymphoma. Leukemia 2018, 32, 1994–2007. [Google Scholar] [CrossRef]
- Roukos, V.; Mathas, S. The origins of ALK translocations. Front. Biosci. 2015, 7, 260–268. [Google Scholar]
- Schröder, B. The multifaceted roles of the invariant chain CD74—More than just a chaperone. Biochim. Biophys. Acta 2016, 1863, 1269–1281. [Google Scholar] [CrossRef]
- Quaranta, V.; Majdic, O.; Stingl, G.; Liszka, K.; Honigsmann, H.; Knapp, W. A human Ia cytoplasmic determinant located on multiple forms of invariant chain (gamma, gamma 2, gamma 3). J. Immunol. 1984, 132, 1900–1905. [Google Scholar]
- Dörken, B.; Möller, P.; Pezzutto, A.; Schwartz-Albiez, R.M.G. Leukocyte Typing IV; Oxford University Press: Oxford, UK, 1989; pp. 106–109. [Google Scholar]
- Knapp, W.; Dörken, B.; Rieber, P.; Schmidt, R.E.; Stein, H.; von dem Borne, A.E. CD antigens 1989. Blood 1989, 74, 1448–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wraight, C.J.; van Endert, P.; Möller, P.; Lipp, J.; Ling, N.R.; MacLennan, I.C.; Koch, N.; Moldenhauer, G. Human major histocompatibility complex class II invariant chain is expressed on the cell surface. J. Biol. Chem. 1990, 265, 5787–5792. [Google Scholar] [CrossRef]
- Ong, G.L.; Goldenberg, D.M.; Hansen, H.J.; Mattes, M.J. Cell surface expression and metabolism of major histocompatibility complex class II invariant chain (CD74) by diverse cell lines. Immunology 1999, 98, 296–302. [Google Scholar] [CrossRef]
- Degener, T.; Momburg, F.; Möller, P. Differential expression of HLA-DR, HLA-DP, HLA-DQ and associated invariant chain (Ii) in normal colorectal mucosa, adenoma and carcinoma. Virchows Arch. A Pathol. Anat. Histopathol. 1988, 412, 315–322. [Google Scholar] [CrossRef]
- Pawlak-Bvczkowska, E.J.; Hansen, H.J.; Dion, A.S. Two New Monoclonal Antibodies, EPB-1 and EPB-2, Reactive with Human Lymphoma1. Cancer Res. 1989, 49, 4568–4577. [Google Scholar]
- Keppler, O.T.; Tibroni, N.; Venzke, S.; Rauch, S.; Fackler, O.T. Modulation of specific surface receptors and activation sensitization in primary resting CD4+ T lymphocytes by the Nef protein HIV-1. J. Leukoc. Biol. 2006, 79, 616–627. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Molina, A.; Yu, A.; Hanson, J.; Cheung, H.; Li, X.; Natkunam, Y. High frequency of CD74 expression in lymphomas: Implications for targeted therapy using a novel anti-CD74-drug conjugate. J. Pathol. Clin. Res. 2019, 5, 12–24. [Google Scholar] [CrossRef]
- Gore, Y.; Starlets, D.; Maharshak, N.; Becker-Herman, S.; Kaneyuki, U.; Leng, L.; Bucala, R.; Shachar, I. Macrophage Migration Inhibitory Factor Induces B Cell Survival by Activation of a CD74-CD44 Receptor Complex. J. Biol. Chem. 2008, 283, 2784–2792. [Google Scholar] [CrossRef] [Green Version]
- Lue, H.; Dewor, M.; Leng, L.; Bucala, R.; Bernhagen, J. Activation of the JNK signalling pathway by macrophage migration inhibitory factor (MIF) and dependence on CXCR4 and CD74. Cell. Signal. 2011, 23, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Klasen, C.; Ohl, K.; Sternkopf, M.; Shachar, I.; Schmitz, C.; Heussen, N.; Hobeika, E.; Levit-Zerdoun, E.; Tenbrock, K.; Reth, M.; et al. MIF Promotes B Cell Chemotaxis through the Receptors CXCR4 and CD74 and ZAP-70 Signaling. J. Immunol. 2014, 192, 5273–5284. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Leng, L.; Wang, T.; Wang, W.; Du, X.; Li, J.; McDonald, C.; Chen, Z.; Murphy, J.W.; Lolis, E.; et al. CD44 Is the Signaling Component of the Macrophage Migration Inhibitory Factor-CD74 Receptor Complex. Immunity 2006, 25, 595–606. [Google Scholar] [CrossRef] [Green Version]
- Gordin, M.; Tesio, M.; Cohen, S.; Gore, Y.; Lantner, F.; Leng, L.; Bucala, R.; Shachar, I. c-Met and Its Ligand Hepatocyte Growth Factor/Scatter Factor Regulate Mature B Cell Survival in a Pathway Induced by CD74. J. Immunol. 2010, 185, 2020–2031. [Google Scholar] [CrossRef]
- Lantner, F.; Starlets, D.; Gore, Y.; Flaishon, L.; Yamit-Hezi, A.; Dikstein, R.; Leng, L.; Bucala, R.; Machluf, Y.; Oren, M.; et al. CD74 induces TAp63 expression leading to B-cell survival. Blood 2007, 110, 4303–4311. [Google Scholar] [CrossRef] [Green Version]
- Starlets, D.; Gore, Y.; Binsky, I.; Haran, M.; Harpaz, N.; Shvidel, L.; Becker-Herman, S.; Berrebi, A.; Shachar, I. Cell-surface CD74 initiates a signaling cascade leading to cell proliferation and survival. Blood 2006, 107, 4807–4816. [Google Scholar] [CrossRef]
- Stein, R.; Qu, Z.; Cardillo, T.M.; Chen, S.; Rosario, A.; Horak, I.D.; Hansen, H.J.; Goldenberg, D.M. Antiproliferative activity of a humanized anti-CD74 monoclonal antibody, hLL1, on B-cell malignancies. Blood 2004, 104, 3705–3711. [Google Scholar] [CrossRef] [PubMed]
- Frölich, D.; Blaβfeld, D.; Reiter, K.; Giesecke, C.; Daridon, C.; Mei, H.E.; Burmester, G.R.; Goldenberg, D.M.; Salama, A.; Dörner, T. The anti-CD74 humanized monoclonal antibody, milatuzumab, which targets the invariant chain of MHC II complexes, alters B-cell proliferation, migration, and adhesion molecule expression. Arthritis Res. Ther. 2012, 14, R54. [Google Scholar] [CrossRef] [Green Version]
- Matza, D.; Wolstein, O.; Dikstein, R.; Shachar, I. Invariant Chain Induces B Cell Maturation by Activating a TAF II 105-NF-κB-dependent Transcription Program. J. Biol. Chem. 2001, 276, 27203–27206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stein, R.; Mattes, M.J.; Cardillo, T.M.; Hansen, H.J.; Chang, C.-H.; Burton, J.; Govindan, S.; Goldenberg, D.M. CD74: A New Candidate Target for the Immunotherapy of B-Cell Neoplasms. Clin. Cancer Res. 2007, 13, 5556s–5563s. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, B.A.; Poi, M.; Jones, J.A.; Porcu, P.; Maddocks, K.; Flynn, J.M.; Benson, D.M.; Phelps, M.A.; Wei, L.; Byrd, J.C.; et al. The combination of milatuzumab, a humanized anti-CD74 antibody, and veltuzumab, a humanized anti-CD20 antibody, demonstrates activity in patients with relapsed and refractory B-cell non-Hodgkin lymphoma. Br. J. Haematol. 2015, 169, 701–710. [Google Scholar] [CrossRef]
- Kaufman, J.L.; Niesvizky, R.; Stadtmauer, E.A.; Chanan-Khan, A.; Siegel, D.; Horne, H.; Wegener, W.A.; Goldenberg, D.M. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br. J. Haematol. 2013, 163, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Abrahams, C.L.; Li, X.; Embry, M.; Yu, A.; Krimm, S.; Krueger, S.; Greenland, N.Y.; Wen, K.W.; Jones, C.; DeAlmeida, V.; et al. Targeting CD74 in multiple myeloma with the novel, site-specific antibody-drug conjugate STRO-001. Oncotarget 2018, 9, 37700–37714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gil-Yarom, N.; Radomir, L.; Sever, L.; Kramer, M.P.; Lewinsky, H.; Bornstein, C.; Blecher-Gonen, R.; Barnett-Itzhaki, Z.; Mirkin, V.; Friedlander, G.; et al. CD74 is a novel transcription regulator. Proc. Natl. Acad. Sci. USA 2017, 114, 562–567. [Google Scholar] [CrossRef] [Green Version]
- Cappuzzo, F.; Moro-Sibilot, D.; Gautschi, O.; Boleti, E.; Felip, E.; Groen, H.J.M.; Germonpré, P.; Meldgaard, P.; Arriola, E.; Steele, N.; et al. Management of crizotinib therapy for ALK-rearranged non-small cell lung carcinoma: An expert consensus. Lung Cancer 2015, 87, 89–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gambacorti Passerini, C.; Farina, F.; Stasia, A.; Redaelli, S.; Ceccon, M.; Mologni, L.; Messa, C.; Guerra, L.; Giudici, G.; Sala, E.; et al. Crizotinib in Advanced, Chemoresistant Anaplastic Lymphoma Kinase–Positive Lymphoma Patients. JNCI: J. Natl. Cancer Inst. 2014, 106, djt378. [Google Scholar] [CrossRef]
- Schwartz, V.; Lue, H.; Kraemer, S.; Korbiel, J.; Krohn, R.; Ohl, K.; Bucala, R.; Weber, C.; Bernhagen, J. A functional heteromeric MIF receptor formed by CD74 and CXCR4. FEBS Lett. 2009, 583, 2749–2757. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.; Karnad, A.; Zhao, S.; Freeman, J.W. Roles of c-Met and RON kinases in tumor progression and their potential as therapeutic targets. Oncotarget 2015, 6, 3507–3518. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Plattel, W.; van den Berg, A.; Ruther, N.; Huang, X.; Wang, M.; de Jong, D.; Vos, H.; van Imhoff, G.; Viardot, A.; et al. Expression of the c-Met oncogene by tumor cells predicts a favorable outcome in classical Hodgkin’s lymphoma. Haematologica 2012, 97, 572–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, K.M.; Labeta, M.O.; Pawelec, G.; Fernandez, N. Cell-surface expression of human histocompatibility leucocyte antigen (HLA) class II-associated invariant chain (CD74) does not always correlate with cell-surface expression of HLA class II molecules. Immunology 1993, 79, 331–335. [Google Scholar] [PubMed]
- Miles, R.R.; Cairo, M.S.; Satwani, P.; Zwick, D.L.; Lones, M.A.; Sposto, R.; Abromovitch, M.; Tripp, S.; Angiolillo, A.L.; Roman, E.; et al. Immunophenotypic identification of possible therapeutic targets in paediatric non-Hodgkin lymphomas: A children’s oncology group report. Br. J. Haematol. 2007, 138, 506–512. [Google Scholar] [CrossRef]
- Alinari, L.; Yu, B.; Christian, B.A.; Yan, F.; Shin, J.; Lapalombella, R.; Hertlein, E.; Lustberg, M.E.; Quinion, C.; Zhang, X.; et al. Combination anti-CD74 (milatuzumab) and anti-CD20 (rituximab) monoclonal antibody therapy has in vitro and in vivo activity in mantle cell lymphoma. Blood 2011, 117, 4530–4541. [Google Scholar] [CrossRef] [Green Version]
- Shah, N.N.; Krishnan, A.Y.; Shah, N.D.; Burke, J.M.; Melear, J.M.; Spira, A.I.; Popplewell, L.L.; Andreadis, C.B.; Chhabra, S.; Sharman, J.P.; et al. Preliminary Results of a Phase 1 Dose Escalation Study of the First-in-Class Anti-CD74 Antibody Drug Conjugate (ADC), STRO-001, in Patients with Advanced B-Cell Malignancies. Blood 2019, 134, 5329. [Google Scholar] [CrossRef]
- Pro, B.; Advani, R.; Brice, P.; Bartlett, N.L.; Rosenblatt, J.D.; Illidge, T.; Matous, J.; Ramchandren, R.; Fanale, M.; Connors, J.M.; et al. Brentuximab Vedotin (SGN-35) in Patients With Relapsed or Refractory Systemic Anaplastic Large-Cell Lymphoma: Results of a Phase II Study. J. Clin. Oncol. 2012, 30, 2190–2196. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.L.; Christensen, J.H.; Morschhauser, F.; Domingo-Domenech, E.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2019, 393, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, T.I.M.; Villarese, P.; Fairbairn, C.J.; Lamant, L.; Trinquand, A.; Hook, C.E.; Burke, G.A.A.; Brugières, L.; Hughes, K.; Payet, D.; et al. Anaplastic large cell lymphoma arises in thymocytes and requires transient TCR expression for thymic egress. Nat. Commun. 2016, 7, 10087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Shokrollahi, K.; Rozen, W.M.; Conyers, R.; Wright, P.; Kenner, L.; Turner, S.D.; Whitaker, I.S. Anaplastic large cell lymphoma (ALCL) and breast implants: Breaking down the evidence. Mutat. Res. Rev. Mutat. Res. 2014, 762, 123–132. [Google Scholar] [CrossRef] [Green Version]
- Lamant, L.; Pileri, S.; Sabattini, E.; Brugieres, L.; Jaffe, E.S.; Delsol, G. Cutaneous presentation of ALK-positive anaplastic large cell lymphoma following insect bites: Evidence for an association in five cases. Haematologica 2010, 95, 449–455. [Google Scholar] [CrossRef] [Green Version]
- God, J.M.; Cameron, C.; Figueroa, J.; Amria, S.; Hossain, A.; Kempkes, B.; Bornkamm, G.W.; Stuart, R.K.; Blum, J.S.; Haque, A. Elevation of c-MYC Disrupts HLA Class II–Mediated Immune Recognition of Human B Cell Tumors. J. Immunol. 2015, 194, 1434–1445. [Google Scholar] [CrossRef] [Green Version]
- Chornoguz, O.; Gapeev, A.; O’Neill, M.C.; Ostrand-Rosenberg, S. Major Histocompatibility Complex Class II + Invariant Chain Negative Breast Cancer Cells Present Unique Peptides that Activate Tumor-specific T Cells from Breast Cancer Patients. Mol. Cell. Proteom. 2012, 11, 1457–1467. [Google Scholar] [CrossRef] [Green Version]
- Thompson, J.A.; Srivastava, M.K.; Bosch, J.J.; Clements, V.K.; Ksander, B.R.; Ostrand-Rosenberg, S. The absence of invariant chain in MHC II cancer vaccines enhances the activation of tumor-reactive type 1 CD4+ T lymphocytes. Cancer Immunol. Immunother. 2008, 57, 389–398. [Google Scholar] [CrossRef] [Green Version]
- Park, W.S. T cell expression of CIITA represses Th1 immunity. Int. Immunol. 2004, 16, 1355–1364. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Shoshana, O.; Zelman-Toister, E.; Maharshak, N.; Binsky-Ehrenreich, I.; Gordin, M.; Hazan-Halevy, I.; Herishanu, Y.; Shvidel, L.; Haran, M.; et al. The Cytokine Midkine and Its Receptor RPTPζ Regulate B Cell Survival in a Pathway Induced by CD74. J. Immunol. 2012, 188, 259–269. [Google Scholar] [CrossRef] [PubMed]




| Lymphoma Entity | No. of Cases | IHC Staining Intensity | |||||
|---|---|---|---|---|---|---|---|
| Absent | + | +/++ | ++ | ++/+++ | +++ | ||
| ALCL ALK+ | 35 | 0 | 8 | 15 | 11 | 1 | 0 |
| ALCL ALK− | 21 | 0 | 3 | 8 | 5 | 4 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wurster, K.D.; Costanza, M.; Kreher, S.; Glaser, S.; Lamprecht, B.; Schleussner, N.; Anagnostopoulos, I.; Hummel, M.; Jöhrens, K.; Stein, H.; et al. Aberrant Expression of and Cell Death Induction by Engagement of the MHC-II Chaperone CD74 in Anaplastic Large Cell Lymphoma (ALCL). Cancers 2021, 13, 5012. https://doi.org/10.3390/cancers13195012
Wurster KD, Costanza M, Kreher S, Glaser S, Lamprecht B, Schleussner N, Anagnostopoulos I, Hummel M, Jöhrens K, Stein H, et al. Aberrant Expression of and Cell Death Induction by Engagement of the MHC-II Chaperone CD74 in Anaplastic Large Cell Lymphoma (ALCL). Cancers. 2021; 13(19):5012. https://doi.org/10.3390/cancers13195012
Chicago/Turabian StyleWurster, Kathrin D., Mariantonia Costanza, Stephan Kreher, Selina Glaser, Björn Lamprecht, Nikolai Schleussner, Ioannis Anagnostopoulos, Michael Hummel, Korinna Jöhrens, Harald Stein, and et al. 2021. "Aberrant Expression of and Cell Death Induction by Engagement of the MHC-II Chaperone CD74 in Anaplastic Large Cell Lymphoma (ALCL)" Cancers 13, no. 19: 5012. https://doi.org/10.3390/cancers13195012









