Remodeling of the Lymph Node High Endothelial Venules Reflects Tumor Invasiveness in Breast Cancer and is Associated with Dysregulation of Perivascular Stromal Cells
Abstract
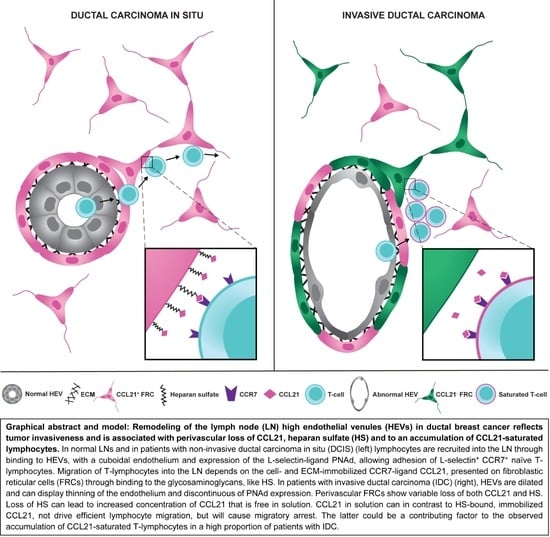
Simple Summary
Abstract
1. Introduction
2. Results
2.1. HEV Remodeling in the Paracortex (T-Cell Zone) Is Associated with Tumor Invasiveness But Is Independent of Nodal Metastasis
2.2. Thinning of the Endothelium and Discontinous Expression of PNAd in Highly Dilated HEVs
2.3. Partial and Complete Loss of CCL21 in Perivascular αSMA+ FRCs of Highly Dilated HEVs
2.4. LN Metastasis Induce Local Effects on HEVs and Perivascular FRCs
2.5. Accumulation of CCL21-Saturated Lymphocytes around Highly Dilated HEVs
2.6. Downregulation of CCL21-Binding Heparan Sulfate
3. Discussion
4. Materials and Methods
4.1. Biobank Material and Ethical Considerations
4.2. Immunofluorescence Staining
4.3. Imaging
4.4. Image Analysis
4.4.1. Vessel Morphology
4.4.2. CCL21-Expression and Perivascular αSMA+ Cells
4.4.3. Metastasis Associated (MA) and in Metastasis (IM) HEVs
4.4.4. Metastatic Burden
4.4.5. CCL21+ Immune Cells
4.4.6. Heparan Sulfate
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Disibio, G.; French, S.W. Metastatic patterns of cancers: Results from a large autopsy study. Arch. Pathol. Lab. Med. 2008, 132, 931–939. [Google Scholar] [CrossRef]
- Weigelt, B.; Peterse, J.L.; van’t Veer, L.J. Breast cancer metastasis: Markers and models. Nat. Rev. Cancer 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Carter, C.L.; Allen, C.; Henson, D.E. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989, 63, 181–187. [Google Scholar] [CrossRef]
- Nathanson, S.D.; Krag, D.; Kuerer, H.M.; Newman, L.A.; Brown, M.; Kerjaschki, D.; Pereira, E.R.; Padera, T.P. Breast cancer metastasis through the lympho-vascular system. Clin. Exp. Metastasis 2018. [Google Scholar] [CrossRef]
- Ullah, I.; Karthik, G.M.; Alkodsi, A.; Kjallquist, U.; Stalhammar, G.; Lovrot, J.; Martinez, N.F.; Lagergren, J.; Hautaniemi, S.; Hartman, J.; et al. Evolutionary history of metastatic breast cancer reveals minimal seeding from axillary lymph nodes. J. Clin. Invest. 2018, 128, 1355–1370. [Google Scholar] [CrossRef]
- Chang, J.E.; Turley, S.J. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol. 2015, 36, 30–39. [Google Scholar] [CrossRef]
- Ulvmar, M.H.; Makinen, T. Heterogeneity in the lymphatic vascular system and its origin. Cardiovasc. Res. 2016, 111, 310–321. [Google Scholar] [CrossRef]
- Chen, D.S.; Mellman, I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013, 39, 1–10. [Google Scholar] [CrossRef]
- Ager, A.; May, M.J. Understanding high endothelial venules: Lessons for cancer immunology. Oncoimmunology 2015, 4, e1008791. [Google Scholar] [CrossRef]
- Eckert, N.; Permanyer, M.; Yu, K.; Werth, K.; Forster, R. Chemokines and other mediators in the development and functional organization of lymph nodes. Immunol. Rev. 2019, 289, 62–83. [Google Scholar] [CrossRef]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.N.; Berghuis, B.; Tsarfaty, G.; Bruch, M.; Kort, E.J.; Ditlev, J.; Tsarfaty, I.; Hudson, E.; Jackson, D.G.; Petillo, D.; et al. Preparing the “soil”: the primary tumor induces vasculature reorganization in the sentinel lymph node before the arrival of metastatic cancer cells. Cancer Res. 2006, 66, 10365–10376. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Chao-Nan, Q.; Seng, O.A.; Peiyi, C.; Bernice, W.H.; Swe, M.S.; Chii, W.J.; Jacqueline, H.S.; Chee, S.K. Changes in specialized blood vessels in lymph nodes and their role in cancer metastasis. J. Transl. Med. 2012, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Ager, A. High Endothelial Venules and Other Blood Vessels: Critical Regulators of Lymphoid Organ Development and Function. Front. Immunol. 2017, 8, 45. [Google Scholar] [CrossRef] [PubMed]
- Cyster, J.G. Chemokines and Cell Migration in Secondary Lymphoid Organs. Science 1999, 286, 2098–2102. [Google Scholar] [CrossRef] [PubMed]
- Eom, J.; Park, S.M.; Feisst, V.; Chen, C.J.; Mathy, J.E.; McIntosh, J.D.; Angel, C.E.; Bartlett, A.; Martin, R.; Mathy, J.A.; et al. Distinctive Subpopulations of Stromal Cells Are Present in Human Lymph Nodes Infiltrated with Melanoma. Cancer Immunol. Res. 2020. [Google Scholar] [CrossRef] [PubMed]
- Bardi, G.; Lipp, M.; Baggiolini, M.; Loetscher, P. The T cell chemokine receptor CCR7 is internalized on stimulation with ELC, but not with SLC. Eur. J. Immunol. 2001, 31, 3291–3297. [Google Scholar] [CrossRef]
- Woolf, E.; Grigorova, I.; Sagiv, A.; Grabovsky, V.; Feigelson, S.W.; Shulman, Z.; Hartmann, T.; Sixt, M.; Cyster, J.G.; Alon, R. Lymph node chemokines promote sustained T lymphocyte motility without triggering stable integrin adhesiveness in the absence of shear forces. Nat. Immunol. 2007, 8, 1076–1085. [Google Scholar] [CrossRef]
- Schumann, K.; Lammermann, T.; Bruckner, M.; Legler, D.F.; Polleux, J.; Spatz, J.P.; Schuler, G.; Forster, R.; Lutz, M.B.; Sorokin, L.; et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity 2010, 32, 703–713. [Google Scholar] [CrossRef]
- Jones, D.; Pereira, E.R.; Padera, T.P. Growth and Immune Evasion of Lymph Node Metastasis. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Rotman, J.; Koster, B.D.; Jordanova, E.S.; Heeren, A.M.; de Gruijl, T.D. Unlocking the therapeutic potential of primary tumor-draining lymph nodes. Cancer Immunol. Immunother. 2019, 68, 1681–1688. [Google Scholar] [CrossRef] [PubMed]
- Mionnet, C.; Sanos, S.L.; Mondor, I.; Jorquera, A.; Laugier, J.P.; Germain, R.N.; Bajenoff, M. High endothelial venules as traffic control points maintaining lymphocyte population homeostasis in lymph nodes. Blood 2011, 118, 6115–6122. [Google Scholar] [CrossRef] [PubMed]
- Riedel, A.; Shorthouse, D.; Haas, L.; Hall, B.A.; Shields, J. Tumor-induced stromal reprogramming drives lymph node transformation. Nat. Immunol. 2016, 17, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Carrière, V.; Colisson, R.; Jiguet-Jiglaire, C.; Bellard, E.; Bouche, G.; Al Saati, T.; Amalric, F.; Girard, J.-P.; M’Rini, C. Cancer Cells Regulate Lymphocyte Recruitment and Leukocyte-Endothelium Interactions in the Tumor-Draining Lymph Node. Cancer Res. 2005, 65, 11639–11648. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.S.; Haraldsen, G.; Brandtzaeg, P.; Baekkevold, E.S. Disparate lymphoid chemokine expression in mice and men: No evidence of CCL21 synthesis by human high endothelial venules. Blood 2005, 106, 444–446. [Google Scholar] [CrossRef]
- Takeda, A.; Hollmen, M.; Dermadi, D.; Pan, J.; Brulois, K.F.; Kaukonen, R.; Lonnberg, T.; Bostrom, P.; Koskivuo, I.; Irjala, H.; et al. Single-Cell Survey of Human Lymphatics Unveils Marked Endothelial Cell Heterogeneity and Mechanisms of Homing for Neutrophils. Immunity 2019, 51, 561–572. [Google Scholar] [CrossRef]
- Stein, J.V.; Rot, A.; Luo, Y.; Narasimhaswamy, M.; Nakano, H.; Gunn, M.D.; Matsuzawa, A.; Quackenbush, E.J.; Dorf, M.E.; von Andrian, U.H. The CC chemokine thymus-derived chemotactic agent 4 (TCA-4, secondary lymphoid tissue chemokine, 6Ckine, exodus-2) triggers lymphocyte function-associated antigen 1-mediated arrest of rolling T lymphocytes in peripheral lymph node high endothelial venules. J. Exp. Med. 2000, 191, 61–76. [Google Scholar] [CrossRef]
- Hirose, J.; Kawashima, H.; Swope Willis, M.; Springer, T.A.; Hasegawa, H.; Yoshie, O.; Miyasaka, M. Chondroitin sulfate B exerts its inhibitory effect on secondary lymphoid tissue chemokine (SLC) by binding to the C-terminus of SLC. Biochim. Biophys. Acta 2002, 1571, 219–224. [Google Scholar] [CrossRef]
- Nagarajan, A.; Malvi, P.; Wajapeyee, N. Heparan Sulfate and Heparan Sulfate Proteoglycans in Cancer Initiation and Progression. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Lindahl, U.; Kjellen, L. Pathophysiology of heparan sulphate: many diseases, few drugs. J. Intern. Med. 2013, 273, 555–571. [Google Scholar] [CrossRef]
- Browning, J.L.; Allaire, N.; Ngam-Ek, A.; Notidis, E.; Hunt, J.; Perrin, S.; Fava, R.A. Lymphotoxin-beta receptor signaling is required for the homeostatic control of HEV differentiation and function. Immunity 2005, 23, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Onder, L.; Danuser, R.; Scandella, E.; Firner, S.; Chai, Q.; Hehlgans, T.; Stein, J.V.; Ludewig, B. Endothelial cell-specific lymphotoxin-beta receptor signaling is critical for lymph node and high endothelial venule formation. J. Exp. Med. 2013, 210, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, H.R.; Eestermans, I.L. Disappearance and reappearance of high endothelial venules and immigrating lymphocytes in lymph nodes deprived of afferent lymphatic vessels: A possible regulatory role of macrophages in lymphocyte migration. Eur. J. Immunol. 1983, 13, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Mebius, R.E.; Streeter, P.R.; Breve, J.; Duijvestijn, A.M.; Kraal, G. The influence of afferent lymphatic vessel interruption on vascular addressin expression. J. Cell. Biol. 1991, 115, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Moussion, C.; Girard, J.P. Dendritic cells control lymphocyte entry to lymph nodes through high endothelial venules. Nature 2011, 479, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.Y.; Bhattacharya, N.; Mu, J.; Setiadi, A.F.; Carcamo-Cavazos, V.; Lee, G.H.; Simons, D.L.; Yadegarynia, S.; Hemati, K.; Kapelner, A.; et al. Spatial organization of dendritic cells within tumor draining lymph nodes impacts clinical outcome in breast cancer patients. J. Transl. Med. 2013, 11, 242. [Google Scholar] [CrossRef]
- Gil Del Alcazar, C.R.; Huh, S.J.; Ekram, M.B.; Trinh, A.; Liu, L.L.; Beca, F.; Zi, X.; Kwak, M.; Bergholtz, H.; Su, Y.; et al. Immune Escape in Breast Cancer During In Situ to Invasive Carcinoma Transition. Cancer Discov. 2017, 7, 1098–1115. [Google Scholar] [CrossRef]
- Blenman, K.R.M.; He, T.F.; Frankel, P.H.; Ruel, N.H.; Schwartz, E.J.; Krag, D.N.; Tan, L.K.; Yim, J.H.; Mortimer, J.E.; Yuan, Y.; et al. Sentinel lymph node B cells can predict disease-free survival in breast cancer patients. NPJ Breast Cancer 2018, 4, 28. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekkhus, T.; Martikainen, T.; Olofsson, A.; Franzén Boger, M.; Vasiliu Bacovia, D.; Wärnberg, F.; Ulvmar, M.H. Remodeling of the Lymph Node High Endothelial Venules Reflects Tumor Invasiveness in Breast Cancer and is Associated with Dysregulation of Perivascular Stromal Cells. Cancers 2021, 13, 211. https://doi.org/10.3390/cancers13020211
Bekkhus T, Martikainen T, Olofsson A, Franzén Boger M, Vasiliu Bacovia D, Wärnberg F, Ulvmar MH. Remodeling of the Lymph Node High Endothelial Venules Reflects Tumor Invasiveness in Breast Cancer and is Associated with Dysregulation of Perivascular Stromal Cells. Cancers. 2021; 13(2):211. https://doi.org/10.3390/cancers13020211
Chicago/Turabian StyleBekkhus, Tove, Teemu Martikainen, Anna Olofsson, Mathias Franzén Boger, Daniel Vasiliu Bacovia, Fredrik Wärnberg, and Maria H. Ulvmar. 2021. "Remodeling of the Lymph Node High Endothelial Venules Reflects Tumor Invasiveness in Breast Cancer and is Associated with Dysregulation of Perivascular Stromal Cells" Cancers 13, no. 2: 211. https://doi.org/10.3390/cancers13020211
APA StyleBekkhus, T., Martikainen, T., Olofsson, A., Franzén Boger, M., Vasiliu Bacovia, D., Wärnberg, F., & Ulvmar, M. H. (2021). Remodeling of the Lymph Node High Endothelial Venules Reflects Tumor Invasiveness in Breast Cancer and is Associated with Dysregulation of Perivascular Stromal Cells. Cancers, 13(2), 211. https://doi.org/10.3390/cancers13020211