Simple Summary
Multiple myeloma is an uncurable hematological malignancy, although the prognosis of myeloma patients is getting better using proteasome inhibitors (PIs), immune modulatory drugs (IMiDs), monoclonal antibodies (MoAbs), and cytotoxic agents. Drug resistance makes myeloma difficult to treat and it can be subdivided into two broad categories: de novo and acquired. De novo drug resistance is associated with the bone marrow microenvironment including bone marrow stromal cells, the vascular niche and endosteal niche. Acquired drug resistance is related to clonal evolution and non-genetic diversity. The initial treatment plays the most important role considering de novo and acquired drug resistance and should contain PIs, IMIDs, MoAbs, and autologous stem cell transplantation because these treatments improve the bone marrow microenvironment and might prevent clonal evolution via sustained deep response including minimal residual disease negativity.
Abstract
Multiple myeloma is an uncurable hematological malignancy because of obtained drug resistance. Microenvironment and clonal evolution induce myeloma cells to develop de novo and acquired drug resistance, respectively. Cell adhesion-mediated drug resistance, which is induced by the interaction between myeloma and bone marrow stromal cells, and soluble factor-mediated drug resistance, which is induced by cytokines and growth factors, are two types of de novo drug resistance. The microenvironment, including conditions such as hypoxia, vascular and endosteal niches, contributes toward de novo drug resistance. Clonal evolution was associated with acquired drug resistance and classified as branching, linear, and neutral evolutions. The branching evolution is dependent on the microenvironment and escape of immunological surveillance while the linear and neutral evolution is independent of the microenvironment and associated with aggressive recurrence and poor prognosis. Proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs), monoclonal antibody agents (MoAbs), and autologous stem cell transplantation (ASCT) have improved prognosis of myeloma via improvement of the microenvironment. The initial treatment plays the most important role considering de novo and acquired drug resistance and should contain PIs, IMIDs, MoAb and ASCT. This review summarizes the role of anti-myeloma agents for microenvironment and clonal evolution and treatment strategies to overcome drug resistance.
1. Introduction
Multiple myeloma (MM) is a type of hematological malignancy that is often uncurable because it comprises a heterogeneous group of plasma cell neoplasms which vary in terms of their morphology, phenotype, molecular biology, and clinical behavior [1]. Drug resistance is one such clinical behavior that makes MM difficult to treat, and it can be subdivided into two broad categories: de novo and acquired. De novo resistance includes environment-mediated drug resistance, which is categorized as either cell adhesion-mediated drug resistance (CAM-DR) or soluble factor-mediated drug resistance (SFM-DR). These types of drug resistance are induced rapidly via the events of a signaling pathway concerning tumor microenvironments [2,3,4]. Interactions between myeloma cells and bone marrow stromal cells (BMSC) or extracellular matrix proteins promote the growth, survival, migration, and drug resistance of tumor cells. The adhesion of myeloma cells to BMSC induces the secretion of several cytokines and growth factors, such as interleukin (IL)-6, and insulin-like growth factor-1 (IGF-1). These cytokines and growth factors are produced and secreted by myeloma cells in the bone marrow (BM) microenvironment and are regulated by autocrine and paracrine loops [5]. MM-associated microenvironments are categorized into “vascular niche” and “endosteal niche” [6]. The vascular niche is constituted via endothelial activation by mainly vascular endothelial growth factor (VEGF)/VEGF receptor (VEGFR) interaction, and contributes to the migration, proliferation, and survival of MM cells. Hypoxia contributes to the development of a vascular niche and the maintenance of stemness of MM cells. The endosteal niche, which is constituted of mainly osteoclasts and osteoblasts, contributes to the survival and dormancy of MM cells [7,8].
Acquired drug resistance develops over time as a result of sequential genetic changes that ultimately culminate in complex therapy-resistant phenotypes [2]. Clonal evolution is a form of genetic change concerning acquired drug resistance. Clonal evolution is divided into [1] branching clonal evolution, which is dependent on the microenvironment, and [2] linear and [3] neutral clonal evolution, which are both independent from the microenvironment [9]. Maintenance of a deep response, such as minimal residual disease (MRD) negativity, is essential for the prevention of aggressive clonal evolution [10,11]. Non-genetic diversity is considered to be an acquired drug resistance, such as an immunologic phenotypic change in the target molecules of monoclonal antibodies agents (MoABs) [12,13].
Finally, four classes of anti-myeloma agents are available—proteasome inhibitors (PIs), immune modulatory drugs (IMiDs), MoABs, and cytotoxic agents—but MM is an uncurable hematological malignancy due to de novo and acquired drug resistance, although the prognosis of myeloma patients is getting better using these anti-myeloma agents. Overcoming de novo and acquired drug resistance plays an important role in the search for a clinical cure in myeloma patients. High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) has been a standard of care for fit, newly diagnosed MM (NDMM) patients, since it not only relieves hematopoiesis but also affects the microenvironment. Initial treatment is extremely crucial because the microenvironment is relatively better and the frequency of clonal evolution is lower in NDMM than relapsed or refractory MM (RRMM), and should include treatment using multi-class drugs including ASCT for fit NDMM patients to help overcome de novo drug resistance and prevent acquired drug resistance. Here, we discuss treatment strategies for MM concerning de novo and acquired drug resistance. The relationship between MM cells and BMSC, the vascular niche, and endosteal niche and treatment for microenvironment are summarized in Figure 1. Clinical trials of new agents for target concerning bone marrow microenvironments in MM are shown in Table 1.
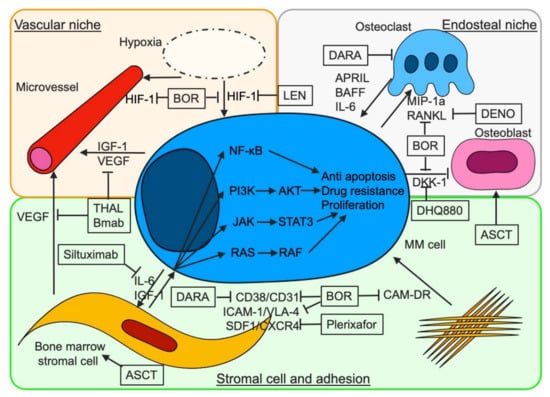
Figure 1.
Therapeutics for bone marrow microenvironment associated with the progression and survival of myeloma cells. Stromal cells contribute to the proliferation, survival, and resistance against therapeutics in myeloma cells through the activation of several signaling pathways, cell adhesion-mediated drug resistance (CAM-DR), and soluble factor-mediated drug resistance (SFM-DR). BOR, DARA, and plerixafor overcome CAM-DR by inhibiting VLA-4, CD38, and CXCR4, respectively. BOR inhibits CD38-CD31-mediated adhesion of myeloma cells to bone marrow stromal cells (BMSCs) and increases CD38 expression on myeloma cells by decreasing BMSC CD31 expression. Under hypoxic conditions, HIF-1 promotes VEGF transcription, which induces the growth of microvessels and contributes to the proliferation of myeloma cells. IGF-1 contributes to the growth of microvessels through the activation of HIF-1alpha. Hypoxia is associated with stemness of myeloma cells. BOR suppresses transcription of HIF-1 alfa and indirectly inhibits myeloma angiogenesis via decreasing levels of VEGF. THAL inhibits angiogenesis by suppressing transcription of the VEGF gene. Bevacizumab suppresses the interaction between VEGF and VEGFR. Myeloma cells activate osteoclasts and inhibit osteoblasts, which constitute the endosteal niche. In addition, several cytokines from osteoclasts contribute to the proliferation of myeloma cells. BOR suppresses osteogenesis via inhibition of RANKL and DKK-1. DENO and BHQ088 inhibit RANKL and DDK-1, respectively. ASCT contributes to the improvement of BM environment by supplying mesenchymal cells and remodeling the endosteal niche. Mesenchymal stem cells, which are provided from autografts, contribute to the remodeling of bone marrow stromal cells and the activation of osteoblasts. BOR, bortezomib; THAL, thalidomide; IMiDs, immunomodulatory drugs; LEN, lenalidomide; DARA, daratumumab; DENO, denosumab; ASCT, autologous stem cell transplantation; Bmab, bevacizumab; CAM-DR, cell-adhesion mediated drug resistance; VEGF, vascular endothelial growth factor; IGF-1, insulin-like growth factor-1; HIF-1, hypoxia inducible factor-1; VLA-4, very late antigen 4; ICAM-1, intercellular adhesion molecule-1; CXCR4, C-X-C chemokine receptor type 4; SDF-1, stromal cell-derived factor-1; IL, interleukin; APRIL, a proliferation-inducing ligand; BAFF, B cell activating factor; RANKL, receptor activator of nuclear factor kappa-B ligand; MIP-1alpha, macrophage inflammatory protein 1alpha; DKK-1, Dickkopf-1.

Table 1.
Clinical trials of new agents for target concerning bone marrow microenvironments in multiple myeloma (MM). BOR, bortezomib; THAL, thalidomide; DEX, dexamethasone; PCB, placebo; CXCR4, C-X-C chemokine receptor type 4; IL-6, interleukin-6; IGF-1R, insulin-like growth factor-1 receptor; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; RANKL, receptor activator of nuclear factor kappa-B ligand; DKK-1, Dickkopf-1; BAFF, B cell activating factor; NDMM, newly diagnosed multiple myeloma; RRMM, relapsed or refractory multiple myeloma; ORR, overall response rate; CBR, clinical benefit rate; PFS, progression free survival; EFS, event free survival; mo, months, NS = not significant.
2. Interaction with Bone Marrow Stromal Cell
2.1. Cell Adhesion-Mediated Drug Resistance and Soluble Factor-Mediated Drug Resistance
Cell adhesion-mediated drug resistance (CAM-DR) is induced by the adhesion of tumor cell integrins to stromal fibroblasts or to components of the extracellular matrix, such as fibronectin, laminin, and collagen [2]. The adhesion molecules, such as very late angine-4 (VLA-4) plays an important role for CAM-DR [26]. VLA-4 is made of a heterodimer of CD49d/CD29 on MM cells. Interaction between VLA-4 and vascular cell adhesion molecule-1 (VCAM-1) binds between MM cells and BMSC, contributing to the survival of MM cells via activation of phosphoinositide 3-kinase (PI3K)/(protein kinase B) AKT pathway and CAM-DR [14]. The epigenetic mechanism is associated with CAM-DR as well. The phosphorylation-mediated enhancer of zeste homolog 2 (EZH2) inactivation and subsequent decreases in H3K27me3 levels are related to CAM-DR in MM cells [15]. Thus, EZH2 is a target of treatment for the apoptosis of myeloma cells and release of CAM-DR [27,28]. Inhibition of EZH is known to inactivate CAM-DR in vitro [27]. CAM-DR is induced in fibroblasts derived from MM patients, but not in healthy individuals [29,30]. CAM-DR may be strong in minimal residual myeloma cells compared to newly diagnosed myeloma cells because of the rich presence of adhesion molecules on the minimal residual myeloma cells [31].
Soluble factor-mediated drug resistance (SFM-DR) is induced by cytokines, chemokines and growth factors secreted by fibroblast-like tumor stromal cells [2]. BMSC-derived soluble factors, such as IL-6, IGF-1, and stromal cell-derived factor-1 (SDF-1), induce the development, proliferation, and survival of MM cells via the adhesion of the microenvironment by signal transduction such as nuclear factor-kappa B (NK-kB), Janus kinase (JAK)/signal transduces and activator of transcription 3 (STAT3), RAS/RAF, and PI3K/AKT pathway [2,32]. IL-6 and IGF-1 cooperate to enhance growth of MM cells [33]. IL-6 can trigger the binding of the membrane IL-6 receptor (IL-6R) to the IGF-1 receptor (IGF-1R) and can induce IGF-1R phosphorylation independently of the addition of IGF-1 [33].
IL-6 is an inflammatory cytokine with the ability to induce tumor growth, metastasis, and resistance to chemotherapy in a variety of tumor cells [34]. IL-6 acts as an antiapoptotic mediator in myeloma cells, whereas in normal cells, its functions seem to mainly involve cell differentiation and development [35]. The level of serum IL-6 is significantly higher in NDMM patients than RRMM [36]. A high expression of IL-6 receptors can indicate a shorter survival time in the NDMM patients treated with the Total Therapy 2 protocol [37]. The ratio of IL-6 present was higher in the patients with high-risk cytogenetic abnormality (HRCA), such as t(4;14), del13q, and 1q21 gain, than those without HRCA. In contrast, the expression of IL-6 receptors was lower in the patients with t(11;14) than those without t(11;14) [38].
IGF-I is single chain polypeptide made of 70 amino acids that functions as the primary ligand for IGF-IR [39]. IGF-1, which are secreted from BMSC and autocrined by MM cells, binds with IGF-1R, contributing to the proliferation of myeloma cells and CAM-DR [27,40]. IGF-1 promotes growth and proliferation of myeloma cells via activation of the IGF-1 receptor in addition to IL-6 [41,42]. However, the serum IGF-1 levels were similar in NDMM patients and healthy donors despite high serum IGF-1 levels being associated with shorter overall survival (OS) before proteasome inhibitors (PIs), immune modulatory drugs (IMiDs) were available [43]. IGF-1 induces protein synthesis, enhances endoplasmic reticulum (ER) stress in myeloma cells, and enhances the activity of proteasome inhibitors (PIs) [44]. The presence of IGF-1 receptors has predicted short survival times in the NDMM patients treated with the Total Therapy 2 protocol [37]. The ratio of IGF-1R present was higher in the patients with HRCA, such as t(4;14), del13q, del17p, and 1q21 gain, than those without HRCA. The ratio of IGF-1R present was similar between the patients with and without t(11;14) [36]. IGF-1 increases the expression of hypoxia-inducible factor-1 (HIF-1) via the activation of the AKT and mitogen-activated protein kinase (MAPK) pathways [45], and results in the secretion of VEGF [34]. IGF-1 regulates anti-apoptotic protein B cell lymphoma 2 (BCL2) in myeloma [38]. Thus, interaction between IGF-1 and IGF-1R is associated with angiogenesis and survival of myeloma cells, and IGF-1R is considered a therapeutic target.
2.2. Treatment for Interaction with CAM-DR and SFM-DR
CAM-DR and SFM-DR are therapeutic targets to overcome drug resistance and to suppress the proliferation and survival of MM cells via the inhibition of signaling pathways.
Bortezomib (BOR) overcomes CAM-DR by inhibiting VLA-4 [26], and shows a synergistic effect with various drugs in co-culturing human myeloma cell lines with BMSC [46]. Proteasome inhibition suppresses cytokine-mediated survival and proliferation advantages by inhibiting the NF-kB pathway in BMSCs [47]. Interactions between MM cells and BMSCs induce resistance for immunomodulatory drugs (IMiDs) by decreasing the expression of IKAROS family zinc finger 1 (IKZF1), which is a biomarker for sensitivity to IMiDs [48,49]. BOR enhances the activity of IMiDs by releasing CAM-DR and elevating IKZF1 expression.
Cluster of differentiation (CD)38 expression on the surface of myeloma cells is down-regulated when co-cultured with BMSCs due to the CD38 on myeloma cells binding to CD31 on BMSCs [50]. BOR inhibits CD38-CD31-mediated adhesion of myeloma cells to BMSCs and increases CD38 expression on myeloma cells by decreasing CD31 expression on BMSC [51]. Thus, BOR might enhance the anti-myeloma effect of anti-CD38 MoAb such as daratumumab (DARA) via the inhibition of adhesion between myeloma cells and BMSCs. DARA releases CAM-DR by inhibiting adhesion to BMSCs by internalizing CD38 in myeloma cells [52].
3. Myeloma Cells in Hypoxia and Vascular Niche
3.1. Vascular Niche in Myeloma
MM-associated niches are categorized into the “vascular niche” and “endosteal niche”, which are associated with the vasculature and osteopoiesis, respectively [53]. The endothelium plays an important role in formation of the vascular niche and maintenance of hematopoiesis [54,55]. The vascular niche enhances the metastatic outgrowth not only by delivery of oxygen, nutrients, and several growth factors but also via interaction with tumor cells [54]. In the early stages of bone metastasis, the vascular microenvironment could fail in the homing and colonization of circulating myeloma cells in the BM. In the later stage, the vasculature reduces metastatic growth, but does not induce the disappearance of BM metastases. BM blood has a lower oxygen partial pressure than venous blood, and the vascular niche may even have a much lower oxygen content [56,57]. Hypoxia plays an important role in myeloma pathogenesis [58].
The interaction between VEGFs, which is composed of six members, and VEGFRs promotes endothelium regeneration [59]. In MM, interaction VEGF-A/VEGFR-2 induces migration, proliferation, and survival of MM cells via autocrine and paracrine of VEGF [60,61,62,63]. VEGF promotes micro-angiogenesis of BM via activation of PI3K/AKT pathway, and is associated with poor prognosis in myeloma [64]. VEGF is mainly regulated by HIF, which is transcriptional factor activating in hypoxia [65,66,67].
Hypoxia in Myeloma
Hypoxic stress is considered to give stem cell-like properties to cancer cells [68]. Mean oxygen saturation in bone marrow is 87.5% ± 1.1% when that in peripheral blood is 99% in healthy volunteers [69]. A specific marker for cancer stem cells is still unclear, but it may be present in the CD138-negative fraction in myeloma cells. Hypoxic stress increases stem cell markers, such as octamer-binding transcription factor 4 (Oct-4), NANOG and (sex determining region Y (SRY)-box 2) SOX2, in CD138-negative myeloma cells [68]. Myeloma stem cells are considered to be present in hypoxic BM, and to have drug resistance [70].
Hypoxia prevents myeloma cells from maturing and might reduce the expression of signaling lymphocytic activation molecule family member 7 (SLAMF7) and CD38, which are the target of elotuzumab (ELO) and DARA or isatuximab (ISA), depending on non-genetic diversity, which is described in detail in Section 5.6, “Nongenetic clonal diversity” [71,72,73]. Myeloma cells without CD138 expression show resistance to BOR [70]. These changes in myeloma cells that prevent maturation might be induced by the suppression of interferon regulatory factor 4 (IRF4) by hypoxic stress [68,74].
Hypoxia decreases E-cadherin expression, making MM cells move from adhesion of BMSC into peripheral blood (PB) [75]. In addition, hypoxia increases C-X-C chemokine receptor type 4 (CXCR4) expression on MM cell and decreases SDF-1 from stromal cells, inducing MM cells to migrate to new BM niches [75,76,77]. Finally, MM cells lose the dependence on the microenvironment, engraft extramedullary, and form extramedullary disease (EMD) [78,79].
HIF-1alfa is the master regulator of oxygen homeostasis [46,59,67,70,80]. The HIF-1alfa protein is degraded by the ubiquitin-proteasome pathway under hypoxic condition [65]. HIF-1a is downstream of the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway and is associated with the progression of myeloma cells through the transcription of several important genes concerning myeloma pathogenesis, such as VEGF and IGF-1 [81]. HIF-1alfa is known to have formed positive feedback with MYC [82] and elevated expression of IRF4 [83]. A high level of HIF-1alfa derived from endothelial cells is a poor prognostic factor in MM [84]. HIF-1alfa increases suppressive TIL (tumor infiltrating lymphocytes) such as Treg [85]. Thus, HIF-1alfa is a target molecule for myeloma treatment.
3.2. Treatment for Vascular Niche and Hypoxia
Treatment for hypoxia and vascular niche plays an important role in preventing the occurrence of EMD and maintaining the immature MM cells.
BOR suppresses transcription of HIF-1alfa [86] and indirectly inhibits MM angiogenesis by decreasing the level of VEGF [87]. IMiDs, especially thalidomide (THAL), inhibit angiogenesis by suppressing transcription of the VEGF gene [88,89,90]. Lenalidomide (LEN) inhibits VEGF-induced PI3K/Akt pathway signaling and HIF-1alfa expression on endothelial cells [91].
Hypoxia plays an important role in EMD formation [75] and is shown as an uptake using positron emission tomography-computed tomography (PET-CT) [92]. THAL plus dexamethasone (DEX) has not been proven effective for EMD in RRMM [93]. VTD (BOR + THAL + DEX) and VRd (BOR + LEN + DEX) followed by ASCT were evaluated in the CASIOPEIA and IFM2009 trials, although there has not yet been a clinical trial that has made a direct comparison between VTD and VRd [94,95]. Complete remission (CR) ratio of VTD was lower than those of VRd (38.5% vs. 59%), while the imaging CR ratio of VTD was similar to that of VRd using PET-CT (63.9% vs. 62%) [96,97]. Thus, VTD is considered to be effective for myeloma in hypoxia due to the release of EMD by THAL although hypoxia induces resistance for BOR [98].
Finally, VDT-PACE (BOR, THAL, DEX, cisplatin, doxorubicin, cyclophosphamide, plus etoposide) might be effective for patients with HRCA and extramedullary plasmacytoma retrospectively [99]. In this regimen, THAL enhances the sensitivity of BOR and cytotoxic agents by improving hypoxia, and BOR, in turn, increases the sensitivity of doxorubicin and etoposide, whose activities are dependent on the cell cycle, through the release of CAM-DR.
4. Endosteal Niche Contributes Survival of Myeloma Cells
4.1. Endosteal Niche is Constituted by Osteoclasts and Osteoblasts
The endosteal niche is constituted by osteoclasts and osteoblasts, and is a reasonable environment to enable the survival of myeloma cells [100]. Osteoclasts contribute to the survival and proliferation of myeloma cells by releasing the receptor activator of nuclear factor kappa-B ligand (RANKL), which is secreted by myeloma cells [101], osteoclasts [102], and BMSCs [103], and the abnormality of RANΚL-osteoprotegerin (OPG) pathway [104,105,106]. An elevated level of RANKL is associated with increasing bone lytic lesions and short survival of myeloma patients [107]. Osteoclasts suppress the immune effect of cytotoxic T cells (CTL) [108]. The acidic condition induced by osteoclasts activate the PI3K/AKT pathway in MM cells [109].
Mature osteoblasts suppress the survival and proliferation of MM cells. Soluble inhibitors of the canonical Wnt pathway such as Dickkopf-1 (DKK-1) produced by MM cells suppress the differentiation of osteoblasts [110]. DKK-1 regulates osteoclastgenesis [111] and osteoblastgenesis directly [112]. The serum level of DKK-1 is high in MM patients with bone lytic lesions, and decreased when treatment including ASCT and PIs was effective [113,114,115]. Finally, osteoblasts contribute to maintaining the dormancy of MM cells, inducing resistance to melphalan and BOR [7,116].
The endosteal niche parallels the vascular niche in regulating hematopoiesis and tumor survival or dormancy during the metastatic cascade [7,8,116]. Residual MM cells, after ASCT, escape from the vascular niche in order to protect themselves from high concentrations of melphalan [117]. Thus, the endosteal niche is one of important therapeutic targets to eradicate MM cells.
4.2. Treatment for Endosteal Niche
Bone remodeling by treatment for the endosteal niche is essential for MM cells. BOR enhances osteogenesis via the inhibition of several factors concerning osteolytic disease, such as RANKL and DKK-1 and activation of osteoblasts by stabilizing β-catenin, which is essential for the differentiation of osteoblasts [115]. DARA suppresses osteolysis by osteoclasts [118]. ASCT contributes to bone remodeling as well [119].
RANKL and DKK-1 are therapeutic targets. Denosumab, which is an anti-RANKL MoAb, prevents skeletal-related events and prolongs PFS in the MM patients with bone lytic lesion compared with zoledronic acid [23]. BHQ880, which is an anti-DKK-1 MoAb, suppresses the progression of myeloma cells in vitro and in vivo [120].
5. Clonal Evolution
5.1. Two Types of Driver Mutation
Myeloma stem cells are considered to be generated due to the acquisition of 14q chromosomal translocation or hyperdiploidy when class switch and somatic mutation occur in post-germinal center cells. The 14q chromosomal translocations are commonly associated with aberrant gene expression of the cyclin D family, which confers a growth advantage to dormant plasma cells or post germinal center (post-GC) B-lymphocytes. Approximately 10% of cases overlap 14q translocation and hyperdiploidy [121].
Multiple ancestor clones are first produced from myeloma stem cells by the ‘big bang’, which means tumors grow accompanied by not only the main clone but also by numerous subclones without stringent clonal selection [122]. In each clone, genetic abnormalities are added to the reservoir myeloma clones by selective pressures by treatment, causing branching clonal evolution [123]. The type of driver mutation affects the clonal evolution in MM. MM cells carrying HRCA, such as t(4;14), acquire more copy number abnormalities than those with standard-risk abnormalities, such as t(11;14), during the course of disease progression [124,125].
5.2. Three Types of Clonal Evolution
Clonal evolution is a process of clonal expansion and clonal selection through genetic change within the adaptive condition. Clonal evolution can be divided into branching, linear, and neutral clonal evolutions [9].
Branching clonal evolution is defined as different clones having no superiority or inferiority, as one clone does not destroy the other clone, and each clone progresses independently when abnormalities of driver genes occur in different clones at the same time. In the branching evolution, driver genes associated with tumor development show heterogeneity in the local site of myeloma. The incidence of branching clonal evolution is associated with the fitness of different clones to BM microenvironments including immune systems and treatment pressure [123]. For example, clones undergo enhancement of IL-6 receptor and programmed death 1-ligand 1 (PD-L1) genes, resulting in growth and proliferation of myeloma cells and escape from immune surveyance [123].
The linear clonal evolution is defined as clones with established mutations growing and coexisting without any apparent selective pressure. The linear clonal evolution is called the “big bang model” clonal evolution, and related to genomic instability [122,123]. In RRMM, especially plasma cell leukemia (PCL), with linear clonal evolution, genes affecting tumor phenotypes such as cell adhesion or tumor suppressor genes is pointed out [126,127,128,129].
Neutral evolution is a kind of clonal evolution as stated above. The majority of mutations are not beneficial but are evolutionarily neutral, and do not contribute to variation at the molecular level due to their rapid elimination by natural selection. Therefore, mutant alleles can be accidentally modified as a result of “survival of the luckiest” rather than by a selective advantage [123,130]. The frequency of neutral evolution was higher in the patients with IgH translocation than those with hyperdiploidy [131].
5.3. Genetic Change during Treatment in PCL and EMD
The terminal stage of myeloma is independent from BMSC, which results in the formation of extramedullary lesions and leukemic change. EMD including PCL is a less frequent manifestation of myeloma, which is defined as the infiltration organ or circulation of PB independent from BMSC [78]. EMD is more frequent in patients with RRMM than those with NDMM [78], and is considered to be more frequent in the terminal stage of myeloma and due to genetic changes [79]. The frequency of IgH loci translocations is similar between the patient with NDMM and EMD, while additional cytogenetic abnormalities, such as del17p and 1q21 gain, and gene mutation, such as TP53 and RAS, increased in the patients with EMD compared with those with NDMM [79]. The incidence of EMD as a recurrence was higher in the patients with HRCA than those without HRCA [132]. There was no consensus regarding the association between the incidence of EMD and the selection of initial treatment [79].
In the terminal stage of myeloma, homozygous deletion of the genes associated with inhibition for the NF-kB pathways is detected (10–15% in total), including baculoviral IAP repeat containing protein 2/3 (BIRC2/3) on chromosome 11 (~7%), TNF receptor-associated factor 3 (TRAF3) on chromosome 14 (~3%) and cylindromatosis (CYLD) on chromosome 16 (~3%) [133].
The loss of 17p is observed in approximately 10% of patients with NDMM and increases in frequency along with disease progression in the 50–70% of MM cases with EMD and PCL. TP53, a tumor suppressor gene, is located at 17p13 and is monoallelic in the majority of MM patients. p53 haploinsufficiency induces failure of DNA repair, which is related to acquired point mutations in the other allele of TP53 during disease progression. TP53 mutations generally is observed in patients with del17p in MM [134]. TP53 mutations are detected in around 33% of patients with del17p in NDMM, and about 50% of patients with del17p in RRMM, respectively [135,136]. Finally, the frequency of TP53 mutations is 5–8% in NDMM [136,137] and up to 25% in PCL [132,138]. In addition, mutant p53 usually has oncogenic functions, such as the upregulation of c-Myc and genes encoding proteasome subunits [139], which can induce anti-cancer drug resistance [140,141,142]. As far has reports go, there has been no incidence of a treatment that could truly overcome the poor prognosis of del17p, although several new anti-myeloma agents improved the clinical outcome in the patients with del17p compared with old agents, and it remains an unmet need for the treatment of myeloma [143].
5.4. HRCA is Associated with Clonal Evolution
HRCA was identified from different BM sites at the same time in 25% of patients diagnosed with non HRCA [144]. High uptake using fluorodeoxyglucose (FDG)-PET/CT was associated with HRCA [145]. Genomic instability is associated with increasing driver mutations, loss of heterozygosity (LOH), and APOBEC signatures and predicts poor prognosis [144,146,147]. In particular, t(14;16) and t(14;20) is associated with the APOBEC signature. LOH is associated with the APOBEC signature and P53 deletion [146,148].
DIS3 mutations are associated with t(4;14), and a large number of DIS3 mutations in minor clones can predict a poor prognosis [135]. It is considered that this is because the clone with the DIS3 gene mutation was selected by the clone tide, and the growth advantage was obtained [148]. The incidence of neutral evolution in the MM cases with 14q translocations, especially t(4;14) and t(4;16), was significantly higher than that of MM cases driven by hyperdiploidy as well [131] because t(4;14) and t(14;16) accelerate clonal heterogeneity and lead to a clonal dominance of selected clones [124,149,150]. Neutral evolution was associated with TP53 gene mutation and 1q21 gain, and predicts poor prognosis in patients treated with IMiDs [123,131]. THAL induced clone selection more frequently than BOR [150]. ASCT might overcome the negative impact of neutral clonal evolution [131]. Thus, PIs-containing chemotherapy and ASCT might be essential for patients with HRCA considering the incidence of neutral clonal evolution.
5.5. Relation between Deep Response and Clonal Evolution
In MRC Myeloma XI, the incidence of gene mutation in the patients with PR was significantly higher than those with very good partial response (VGPR) and CR. The incidences of linear evolution and branching evolution were almost similar, regardless of the therapeutic effect. OS after recurrence was not associated with the type of clonal evolution [151]. According to analysis of clone selection using fluorescence in situ hybridization (FISH) from China, the recurrences accompanied with clonal evolution are observed frequently in HRCA (especially del17p, 1q21gain) [150]. Clone selection was pointed out infrequently in the patients with relapsed myeloma from MRD-negativity and CR. These results may be opposite to those of MRC Myeloma XI concerning the relation between the incidence of clonal evolution and the depth of response.
These two reports mention that the incidence of clonal evolution depends on a deep response, while there is no evidence about association between clonal evolution and the period during MRD negativity. MRD-negativity was identified as a surrogate marker for PFS prolongation [10,11]. Several reports showed that a long interval of MRD-negativity predicted long PFS, while aggressive relapse, such as the presence of EMD, might occur after MRD-negativity to clonal evolution [152]. Considering the incidence of developing clonal evolution, we believe that it is necessary to continue treatment to prevent recurrence even after achieving MRD-negativity especially in the patients with HRCA.
5.6. Nongenetic Clonal Diversity
Clonal evolution induces clonal diversity and is a cause of acquired drug resistance, while conversion of phenotype is a cause of drug resistance such as nongenetic clonal change in myeloma. Chaidos et al. demonstrated that the ratios of CD19−CD138− pre-PC (plasma cell) myeloma cell, CD19−CD138low myeloma cell, and CD19-CD138high myeloma cell were changed via therapeutic selection and pre-PC myeloma showed drug resistance [153]. Paiva et al. demonstrated that CD19−CD81+ myeloma cell differentiated into CD19−CD81− mature myeloma cell in 5% of patients, while CD19−CD81− mature myeloma cell changed into CD19−CD81+ myeloma cell in 20% of patients via therapeutic selection in the myeloma patients treated with bortezomib, melphalan, and prednisone (VMP) [12]. The CD38 expression, which was associated with a clinical response of DARA, was down after the start of DARA, and elevated 6 months after the conclusion of DARA [13]. Thus, the phenotype should be analyzed before a change in chemotherapy, because the nongenetic clonal change often occurs.
IMiDs might be effective dormant myeloma cells under the BM niche because dormant MM cells, which adhere to the BM niche, highly express IKZF1 [154]. LEN is active for immature myeloma cells and side population [155,156,157]. In contrast, PIs are effective mature myeloma cells which have ER stress [68,71]. Thus, IMiDs and PIs work for myeloma cells in different differentiation stages.
CD38 and CD319, which is identified with SLAMF-7, are expressed independently of maturation of myeloma cells [153]. Thus, anti CD38 and SLAMF-7 MoAbs can be active for myeloma cells in various differentiation stages.
6. Overall Treatment Strategy Considering Microenvironment and Clonal Evolution
6.1. Concept of Treatment Concerning Microenvironment and Clonal Evolution
MM is considered to be uncurable because MM cells are protected by the BM microenvironment, including CAM-DR, SFM-DR, vascular and endosteal niches, and clonal evolution leads to refractoriness for chemotherapy.
Initial treatment should be given the most importance in treatments approaching de novo and acquired drug resistance. First, the majority of molecules concerning SFM-DR increase, dependent on disease progression from MGUS, NDMM, and RRMM to EMM. For example, IL-6 increases in RRMM, and suppresses CD38 expression on MM cells, inducing resistance to DARA. Second, the maintenance of a deep response should be essential to prevent clonal evolution. Several clinical trials have demonstrated that a short interval of MRD-negativity is not adequate for long survival [10,11]. Meanwhile, how long an interval of MRD-negativity is enough to achieve a clinical cure has been open to discussion until now. In addition, the incidence of EMD was higher in the patients with RRMM than NDMM after MRD-negativity was failed [152]. Third, immune reconstitution can predict a good prognosis in the patients with MM independently from MRD and HRCA [157,158,159,160,161,162,163,164]. Finally, the microenvironment, such as CAM-DR, hypoxia, and the endosteal niche, contributes to the survival of MRD and MM stem cells. Treatment of the microenvironment is necessary to kill all myeloma cells. Associations between the activity of treatments, including PIs, IMiDs, anti CD38 MoAb, and ASCT, cytogenetic risk and maturation of MM cells are summarized in Figure 2 [123,165,166]. Treatment strategies for fit and unfit patients with NDMM, and RRMM, terminal phase MM are shown in Figure 3.
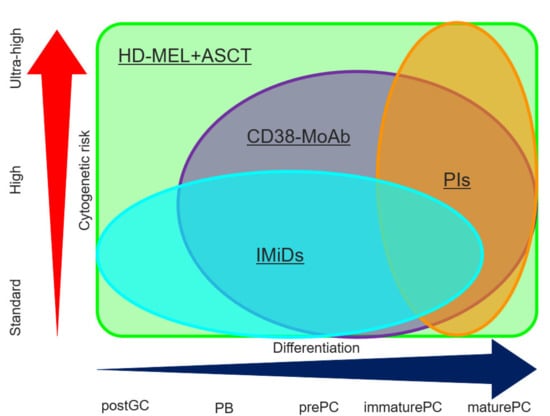
Figure 2.
Activity of PIs, IMiDs, CD38 MoAB, and ASCT in terms of the differentiation of MM cells and cytogenetic risk. PIs contribute to anti-myeloma activity in more mature MM cells relatively independent from cytogenetic risk. IMiDs attack immature MM cells, but this is not enough to treat high-risk cytogenetic abnormality. CD38 MoAb can be used for CD38-positive MM cells excluding post GC cells and improves clinical outcome in the patients with HRCA through long-interval administration. A high dose of melphalan followed by ASCT kills MM cells independent of the differentiation of MM cells and cytogenetic risk. PIs, proteasome inhibitors; IMiDs, immunomodulatory drugs; MoAbs, monoclonal antibody agents; HD-MEL + ASCT, high dose melphalan followed by autologous stem cell transplantation; postGC, post germinal center; PB, plasmablast; PC, plasma cell.
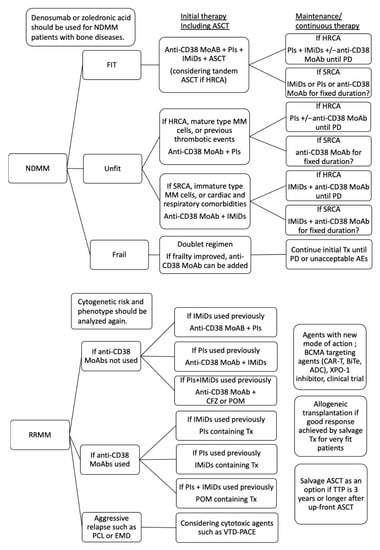
Figure 3.
Treatment strategies for fit and unfit patients with NDMM, and RRMM, terminal phase MM. Multi-drug treatment including PIs, IMiDs, CD38 MoAb, and ASCT is essential for fit patients with NDMM. For unfit patients, who are eligible for intensive treatment including ASCT, CD38 MoAb plus PIs or IMiDs should be selected according to the differentiation of MM cells, cytogenetic risk and comorbidities. Maintenance or continuous therapy using two or three class agents until PD might be essential for patients with HRCA. Maintenance or continuous therapy using one class agents for fixed duration might be possible for patients with SRCA if MRD-negativity was achieved. For frail patients, doublet regimes might be selected considering general condition, comorbidity, and social background rather than cytogenetic risk. If frailty improved via the efficacy of chemotherapy, anti-CD38 MoAb can be added. Initial treatment should be continued until PD or unacceptable adverse events. When the disease is relapsed or refractory, cytogenetics and phenotype should be analyzed again. Anti-CD38 MoAbs should be used as second line of therapy if anti-CD38 MoAbs has not been administered yet. A high dose of carfilzomib or pomalidomide containing therapy might be active for patients with refractoriness for PIs and IMiDs. PIs containing therapy are selected as a second line of therapy if PIs have not been administered as initial therapy yet. IMiDs containing therapy are selected as a second line of therapy if IMiDs have not been administered as initial therapy yet. Salvage ASCT is considered when the time to progression from up-front ASCT is 3 years or more. Allogeneic transplantation is considered for very fit patients who achieved a good response using salvage chemotherapy. Cytotoxic agents-containing therapy, such as VTD-PACE, might be active for aggressive relapse, such as extramedullary disease and plasma cell leukemia. Agents with a new mode of action, BCMA-targeting therapy, XPO-1 inhibitors, and clinical trials are considered when they are available. NDMM, newly diagnose multiple myeloma; RRMM relapse or refractory multiple myeloma, HRCA, high risk cytogenetic abnormality; SRCA, standard risk cytogenetic abnormality; PD, progressive disease; PIs, proteasome inhibitors; IMiDs, immunomodulatory drugs; MoAbs, monoclonal antibody agents; ASCT, autologous stem cell transplantation; CFZ, carfilzomib; POM, pomalidomide; EMD, extramedullary disease; PCL, plasma cell leukemia; TTNT, time to progression; XPO-1, exportin-1; BCMA, B cell mature antigen, CAR-T, chimeric antigen receptor-T cell; ADC, antibody-drug conjugate; bispecific T-cell engager; Tx, treatment.
PIs release CAM-DR via the inhibition of VLA-4, induce ER stress for M protein rich mature myeloma cells, improve hypoxic condition, and promote bone remodeling as above [68]. High expression of XBP1, which is associated with the differentiation of plasma cells and independent from cytogenetic risk in MM, predicted good clinical outcome in MM patients treated with BOR via ER stress [167,168,169]. Thus, PIs might be active for MM independently from cytogenetic risk. In addition, PIs enhance ADCC activity by suppression of HLA class 1 expression on MM cells [170].
IMiDs enhance both adaptive and innate immune system via co-stimulation of T cells and enhancement of NK and NKT cells in vitro [171]. IMiDs suppress Treg function by suppression of Foxp3 gene expression in Treg [172]. Finally, IMiDs suppress PD-1 expression on T and NK cells [173], and LEN suppresses PD-L1 expression in MM cells [174]. Thus, LEN and pomalidomide (POM) are good partners of some MoAbs because they enhance antibody-dependent cellular cytotoxicity (ADCC). IMiDs contributes to immune reconstitution compared with PIs [164]. High IKZF1 expression, which was higher in non-HRCA than HRCA, predicted a good treatment response of IMiDs [175]. In addition, IMiDs tended to be active for immature MM cells from the results of genetic analyses and clinical trials compared with PIs [68].
Anti CD38 MoAbs have several anti-myeloma and microenvironment activities, such as complement-dependent cytotoxicity (CDC), ADCC, direct myeloma killing, enhancement of the immune system, inhibition of CAM-DR, and enhancement of bone remodeling as above. CD38 expression is independent of the maturation of MM cells’ cytogenetic abnormality [153,176], but is dependent on serum IL-6 level, which increases in disease progression [177]. Anti-CD38 MoAbs contribute to the enhancement of the immune system [178]. The activity of anti CD38 MoAbs in NDMM should be higher than RRMM because the immune system in NDMM is better than those in RRMM [179]. The efficacy of DARA for HRCA is still controversial. DRd (DARA + LEN + DEX) contributed to the longer progression-free survival (PFS) compared with Rd in a POLLUX trial, which is a phase 3 trial about DRd versus Rd for RRMM; the hazard ratio for PFS of DRd compared with Rd decreased as the follow-up interval increased [180,181,182]. DRd can be active for HRCA, excluding the patients at ultra-high risk, whose diseases progress in the early stages after DRd started. It was suggested that DRd continuous treatment might be effective for HRCA patients. Thus, anti-CD38 MoAbs contribute to anti-myeloma activity in early phase MM cells.
6.2. Autologous Stem Cell Transplantation for Microenvironment and Clonal Evolution
High-dose melphalan followed by ASCT is still a standard of care. High dose melphalan kills MM cells nonspecifically via deoxyribonucleic acid (DNA) damage and may attack the subclonal structure, potentially reducing the impact of a neutral or non-neutral evolutional tumor history [123,131]. In addition, the autograft plays an important role for the therapeutic effect on not only the recovery of myelosuppression but also the improvement of microenvironment in the patients received ASCT. Mesenchymal stem cells (MSC), which are non-hematopoietic undifferentiated cells, are able to differentiate into not only components of BMSC such as hematopoietic tissues, but also various mesodermal-type tissues such as osteoblasts, chondrocytes, and myocytes [183]. BMSC are a major component of the hematopoietic microenvironment, and contribute to maintaining long-term hematopoietic maintenance. In addition, osteoblasts work as the actual state of endosteal niches. Thus, MSC play an important role as one of the major members responsible for the hematopoietic mechanism. MSC are difficult to collect from the PB of healthy individuals, while they are also mobilized in PB when mobilized with granulocyte colony stimulating factor (G-CSF) [9].
In FORTE trial, PFS in the patients treated with four cycles of KRd (carfilzomib (CFZ) + LEN + DEX) followed by ASCT followed by four cycles of KRd (KRd-ASCT group) was significantly longer than those with 12 cycles KRd (KRd12), although the MRD negativity ratio was similar using multicolor flow cytometry (cut-off = 10−5) [184,185]. In addition, in a long follow-up, the MRD ratios were similar between the KRd12 and KCd (CFZ + cyclophosphamide + DEX)-ASCT groups. The reason why KRd12 was inferior compared with KRd-ASCT and similar with KCd-ASCT is a deeper response (MRD negativity rate using next generation sequencing, 23% vs. 34%; cut-off = 10−6) and immune reconstitution in the patients that received ASCT. Thus, up-front ASCT is a standard of care from the point of view concerning the results of clinical trials but also the improvement of the microenvironment.
6.3. Clinical Significance of Immune Reconstitution
Immune reconstitution is important for the patients with HRCA instead of MRD status. Continuous IMiD treatments induce immune reconstitution, and play an important role for the treatment for myeloma patients with HRCA. However, survival time in the HRCA patients treated with IMiDs was short in the majority of clinical trials. Continuous therapy with IMiDs combined with PIs or monoclonal antibodies might be essential for myeloma patients with HRCA. In addition, ASCT contributes to a deeper response and the improvement of the microenvironment instead of neutral evolution and HRCA [131]. In the patients that received ASCT, normal plasma cells increase in patients with MRD-negativity compared with those with MRD-positivity, leading to immune reconstitution [105]. Because the benign BM niche provides support to the survival of normal plasma cells, regulates the number of normal plasma cells [186], and promotes immune reconstitution, it is suggested that the benign BM niche might increase via the eradication of MM cells in niche by intensive chemotherapy, increasing the niche available for normal plasma cells [117,187]. Therefore, multi-drugs approaches, PIs, IMiDs, DARA, and ASCT, might be essential for fit NDMM patients from the point of view of the microenvironment. In fact, DARA + VRd followed by ASCT was tolerable and effective for NDMM in GRIFFIN trial [188].
6.4. Treatment for Unfit and Frail NDMM, RRMM, and EMDs
In the case of unfit or frail NDMM patients who are ineligible for intensive treatment including ASCT, DARA-based treatment is a good option because CD38 expresses on MM cells independently from cytogenetics [189], and a relatively better immune system contributes the activity of anti-CD38 MoAbs [190,191,192,193]. Partners of DARA, such as PIs and IMiDs, are selected considering cytogenetic abnormality, clinical symptom, and comorbidity. Differentiation of MM cells and the cancer panel should contribute to classifying which MM cells are suitable for treatment with PIs or IMiDs [68,194,195]. For frail patients, doublet regimens, such as VD (BOR + DEX) and Rd might be selected considering the general condition, comorbidity, and social background rather than cytogenetic risk. There is no evidence of the efficacy and tolerability of anti-CD38 MoAbs plus PIs or IMiDs as initial chemotherapy for frail patients up to now, although PFS in the patients treated with DARA + VMP and DARA + Rd were significantly longer than those in those treated with VMP and Rd among the elderly patients according to ALCYONE and MAIA trials, respectively [196]. If frailty improved via the efficacy of chemotherapy, anti-CD38 MoAb can be added. Initial treatment should be continued until PD or unacceptable adverse events, because the possibility of receiving the next chemotherapy is low [197].
In the RRMM patients after the multi-drug approach, the reason why treatment failed should be evaluated. Fluorescence in situ hybridization (FISH) for del17p, 1q21 gain, and MYC containing a cytogenetic abnormality should be repeated for each relapse because these cytogenetic abnormalities can be added. The phenotype of MM cells using flow cytometry should be repeated to analyze non-genetic changes to MM cells. Thereafter, the suitable treatment for cytogenetic abnormality and phenotype should be selected.
In the terminal phase of MM, EMD is often detected. The treatment for EMD is challenging. Cytotoxic agents containing chemotherapy including ASCT might be effective for EMD [79,198,199]. VTD-PACE is a good option for EMD considering CAM-DR and hypoxia, but might be intolerable for the majority of patients with a terminal phase of MM [200].
6.5. Future Direction
Several new drugs are developed to overcome de novo drug resistance, such as proteasome inhibitors and EZH2 inhibitors to overcome CAM-DR, and anti-IL-6 monoclonal antibodies and IGF-1R inhibitors to overcome SFM-DR, in a preclinical study, although several of those have not shown efficacy in clinical trials and thus are not available in clinical practice. In addition, treatments to prevent and overcome acquired drug resistance are already one of unmet medical needs as well. In future, treatment strategies to prevent clonal evolution and overcome acquired drug resistance including the terminal phase of myeloma will be necessary. Treatment with new modes of action, such as chimeric antigen receptor-T cell therapy (CAR-T) [201], antibody-drug conjugate (ADC) [202], bispecific T-cell engager (BiTE) for B cell mature antigen (BCMA) [203], exportin-1 (XPO-1) inhibitor [204] and melflufen [205], may be active for patients with a terminal phase of MM.
7. Conclusions
This review summarizes myeloma pathogenesis and drug resistance from the perspectives of the adhesion of BMSCs, hypoxia, and vascular and endosteal niches, and describes treatment strategies for each microenvironment in myeloma. The research and development of specific inhibitors for the BM microenvironment are progressing, while the majority of these drugs have been evaluated in preclinical studies and are not available in clinical practice. Currently available anti-myeloma agents, such as PIs, IMiDs, and anti-CD38 MoAb, work for the BM microenvironment and show an antimyeloma effect. ASCT is expected to not only have an antitumor effect through high-dose chemotherapy, but also help in the improvement of the BM microenvironment including immune reconstitution. There is no specific treatment for the inhibition of clonal evolution that shows acquired treatment resistance as yet, although treatment strategy, guided by the myeloma phenotype using flowcytometry, can be selected. In addition, maintenance of a deep response should be essential in preventing clonal evolution. The initial treatment strategies containing PIs, IMiDs, MoAbs, and ASCT should be necessary to overcome de novo drug resistance and prevent acquired drug resistance. It may be possible to select personalized treatment strategies through the analysis of clonal variation in each case using next generation sequencing. In the future, it is important not only to treat the myeloma cells themselves but also to develop a treatment strategy that considers the microenvironment and clonal evolution.
Funding
This research received no external funding.
Conflicts of Interest
Kazuhito Suzuki received personal fees from Takeda Pharmaceutical Company, Janssen Pharmaceutical K.K., Celgene, outside the submitted work; Kaichi Nishiwaki reports personal fees from Kyowa Hakko Kirin Co, Ltd., outside the submitted work. The other authors declare that they have no conflict of interest.
References
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Meads, M.B.; Gatenby, R.A.; Dalton, W.S. Environment-mediated drug resistance: A major contributor to minimal residual disease. Nat. Rev. Cancer 2009, 9, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 1999, 93, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Meads, M.B.; Hazlehurst, L.A.; Dalton, W.S. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin. Cancer Res. 2008, 14, 2519–2526. [Google Scholar] [CrossRef] [PubMed]
- Podar, K.; Tai, Y.T.; Lin, B.K.; Narsimhan, R.P.; Sattler, M.; Kijima, T.; Salgia, R.; Gupta, D.; Chauhan, D.; Anderson, K.C. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta 1 integrin and phosphatidylinositol 3-kinase-dependent PKC alpha activation. J. Biol. Chem. 2002, 277, 7875–7881. [Google Scholar] [CrossRef]
- Ria, R.; Vacca, A. Bone Marrow Stromal Cells-Induced Drug Resistance in Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 613. [Google Scholar] [CrossRef]
- Boulais, P.E.; Frenette, P.S. Making sense of hematopoietic stem cell niches. Blood 2015, 125, 2621–2629. [Google Scholar] [CrossRef]
- Bussard, K.M.; Gay, C.V.; Mastro, A.M. The bone microenvironment in metastasis; what is special about bone? Cancer Metastasis Rev. 2008, 27, 41–55. [Google Scholar] [CrossRef]
- Greaves, M.; Maley, C.C. Clonal evolution in cancer. Nature 2012, 481, 306–313. [Google Scholar] [CrossRef]
- Takamatsu, H. Clinical value of measurable residual disease testing for multiple myeloma and implementation in Japan. Int. J. Hematol. 2020, 111, 519–529. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Rawstron, A.C.; Owen, R.G.; Child, J.A.; Thakurta, A.; Sherrington, P.; Samur, M.K.; Georgieva, A.; Anderson, K.C.; et al. Association of Minimal Residual Disease With Superior Survival Outcomes in Patients With Multiple Myeloma: A Meta-analysis. JAMA Oncol. 2017, 3, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Paiva, B.; Puig, N.; Cedena, M.T.; de Jong, B.G.; Ruiz, Y.; Rapado, I.; Martinez-Lopez, J.; Cordon, L.; Alignani, D.; Delgado, J.A.; et al. Differentiation stage of myeloma plasma cells: Biological and clinical significance. Leukemia 2017, 31, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Nijhof, I.S.; Casneuf, T.; van Velzen, J.; van Kessel, B.; Axel, A.E.; Syed, K.; Groen, R.W.; van Duin, M.; Sonneveld, P.; Minnema, M.C.; et al. CD38 expression and complement inhibitors affect response and resistance to daratumumab therapy in myeloma. Blood 2016, 128, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Ghobrial, I.M.; Liu, C.J.; Zavidij, O.; Azab, A.K.; Baz, R.; Laubach, J.P.; Mishima, Y.; Armand, P.; Munshi, N.C.; Basile, F.; et al. Phase I/II trial of the CXCR4 inhibitor plerixafor in combination with bortezomib as a chemosensitization strategy in relapsed/refractory multiple myeloma. Am. J. Hematol. 2019, 94, 1244–1253. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Manges, R.F.; Sonneveld, P.; Jagannath, S.; Somlo, G.; Krishnan, A.; Lentzsch, S.; Frank, R.C.; Zweegman, S.; Wijermans, P.W.; et al. A phase 2 multicentre study of siltuximab, an anti-interleukin-6 monoclonal antibody, in patients with relapsed or refractory multiple myeloma. Br. J. Haematol. 2013, 161, 357–366. [Google Scholar] [CrossRef]
- Orlowski, R.Z.; Gercheva, L.; Williams, C.; Sutherland, H.; Robak, T.; Masszi, T.; Goranova-Marinova, V.; Dimopoulos, M.A.; Cavenagh, J.D.; Špička, I.; et al. A phase 2, randomized, double-blind, placebo-controlled study of siltuximab (anti-IL-6 mAb) and bortezomib versus bortezomib alone in patients with relapsed or refractory multiple myeloma. Am. J. Hematol. 2015, 90, 42–49. [Google Scholar] [CrossRef]
- Lacy, M.Q.; Alsina, M.; Fonseca, R.; Paccagnella, M.L.; Melvin, C.L.; Yin, D.; Sharma, A.; Enriquez Sarano, M.; Pollak, M.; Jagannath, S.; et al. Phase I, pharmacokinetic and pharmacodynamic study of the anti-insulinlike growth factor type 1 Receptor monoclonal antibody CP-751,871 in patients with multiple myeloma. J. Clin. Oncol. 2008, 26, 3196–3203. [Google Scholar] [CrossRef]
- Somlo, G.; Lashkari, A.; Bellamy, W.; Zimmerman, T.M.; Tuscano, J.M.; O’Donnell, M.R.; Mohrbacher, A.F.; Forman, S.J.; Frankel, P.; Chen, H.X.; et al. Phase II randomized trial of bevacizumab versus bevacizumab and thalidomide for relapsed/refractory multiple myeloma: A California Cancer Consortium trial. Br. J. Haematol. 2011, 154, 533–535. [Google Scholar] [CrossRef]
- White, D.; Kassim, A.; Bhaskar, B.; Yi, J.; Wamstad, K.; Paton, V.E. Results from AMBER, a randomized phase 2 study of bevacizumab and bortezomib versus bortezomib in relapsed or refractory multiple myeloma. Cancer 2013, 119, 339–347. [Google Scholar] [CrossRef]
- Yordanova, A.; Hose, D.; Neben, K.; Witzens-Harig, M.; Gütgemann, I.; Raab, M.S.; Moehler, T.; Goldschmidt, H.; Schmidt-Wolf, I.G. Sorafenib in patients with refractory or recurrent multiple myeloma. Hematol. Oncol. 2013, 31, 197–200. [Google Scholar] [CrossRef]
- Srkalovic, G.; Hussein, M.A.; Hoering, A.; Zonder, J.A.; Popplewell, L.L.; Trivedi, H.; Mazzoni, S.; Sexton, R.; Orlowski, R.Z.; Barlogie, B. A phase II trial of BAY 43-9006 (sorafenib) (NSC-724772) in patients with relapsing and resistant multiple myeloma: SWOG S0434. Cancer Med. 2014, 3, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, M.J.; Reece, D.E.; Marcellus, D.; Meyer, R.M.; Mathews, S.; Dong, R.P.; Eisenhauer, E. A phase II study of ZD6474 (Zactima, a selective inhibitor of VEGFR and EGFR tyrosine kinase in patients with relapsed multiple myeloma—NCIC CTG IND.145. Investig. New Drugs 2006, 24, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.; Terpos, E.; Willenbacher, W.; Shimizu, K.; García-Sanz, R.; Durie, B.; Legieć, W.; Krejčí, M.; Laribi, K.; Zhu, L.; et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018, 19, 370–381. [Google Scholar] [CrossRef]
- Iyer, S.P.; Beck, J.T.; Stewart, A.K.; Shah, J.; Kelly, K.R.; Isaacs, R.; Bilic, S.; Sen, S.; Munshi, N.C. A Phase IB multicentre dose-determination study of BHQ880 in combination with anti-myeloma therapy and zoledronic acid in patients with relapsed or refractory multiple myeloma and prior skeletal-related events. Br. J. Haematol. 2014, 167, 366–375. [Google Scholar] [CrossRef] [PubMed]
- Raje, N.S.; Moreau, P.; Terpos, E.; Benboubker, L.; Grząśko, N.; Holstein, S.A.; Oriol, A.; Huang, S.Y.; Beksac, M.; Kuliczkowski, K.; et al. Phase 2 study of tabalumab, a human anti-B-cell activating factor antibody, with bortezomib and dexamethasone in patients with previously treated multiple myeloma. Br. J. Haematol. 2017, 176, 783–795. [Google Scholar] [CrossRef]
- Noborio-Hatano, K.; Kikuchi, J.; Takatoku, M.; Shimizu, R.; Wada, T.; Ueda, M.; Nobuyoshi, M.; Oh, I.; Sato, K.; Suzuki, T.; et al. Bortezomib overcomes cell-adhesion-mediated drug resistance through downregulation of VLA-4 expression in multiple myeloma. Oncogene 2009, 28, 231–242. [Google Scholar] [CrossRef]
- Kikuchi, J.; Koyama, D.; Wada, T.; Izumi, T.; Hofgaard, P.O.; Bogen, B.; Furukawa, Y. Phosphorylation-mediated EZH2 inactivation promotes drug resistance in multiple myeloma. J. Clin. Investig. 2015, 125, 4375–4390. [Google Scholar] [CrossRef]
- Alzrigat, M.; Jernberg-Wiklund, H.; Licht, J.D. Targeting EZH2 in Multiple Myeloma-Multifaceted Anti-Tumor Activity. Epigenomes 2018, 2, 16. [Google Scholar] [CrossRef]
- Reagan, M.R.; Ghobrial, I.M. Multiple myeloma mesenchymal stem cells: Characterization, origin, and tumor-promoting effects. Clin. Cancer Res. 2012, 18, 342–349. [Google Scholar] [CrossRef]
- Frassanito, M.A.; Rao, L.; Moschetta, M.; Ria, R.; Di Marzo, L.; De Luisi, A.; Racanelli, V.; Catacchio, I.; Berardi, S.; Basile, A.; et al. Bone marrow fibroblasts parallel multiple myeloma progression in patients and mice: In vitro and in vivo studies. Leukemia 2014, 28, 904–916. [Google Scholar] [CrossRef]
- Paiva, B.; Corchete, L.A.; Vidriales, M.B.; Puig, N.; Maiso, P.; Rodriguez, I.; Alignani, D.; Burgos, L.; Sanchez, M.L.; Barcena, P.; et al. Phenotypic and genomic analysis of multiple myeloma minimal residual disease tumor cells: A new model to understand chemoresistance. Blood 2016, 127, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Kikuchi, J. Epigenetic mechanisms of cell adhesion-mediated drug resistance in multiple myeloma. Int. J. Hematol. 2016, 104, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Abroun, S.; Ishikawa, H.; Tsuyama, N.; Liu, S.; Li, F.J.; Otsuyama, K.; Zheng, X.; Obata, M.; Kawano, M.M. Receptor synergy of interleukin-6 (IL-6) and insulin-like growth factor-I in myeloma cells that highly express IL-6 receptor alpha [corrected]. Blood 2004, 103, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.; Chauhan, D.; Teoh, G.; Treon, S.P.; Urashima, M.; Schlossman, R.L.; Anderson, K.C. IL-6 triggers cell growth via the Ras-dependent mitogen-activated protein kinase cascade. J. Immunol. 1997, 159, 2212–2221. [Google Scholar] [PubMed]
- Lauta, V.M. A review of the cytokine network in multiple myeloma: Diagnostic, prognostic, and therapeutic implications. Cancer 2003, 97, 2440–2452. [Google Scholar] [CrossRef]
- Jasrotia, S.; Gupta, R.; Sharma, A.; Halder, A.; Kumar, L. Cytokine profile in multiple myeloma. Cytokine 2020, 136, 155271. [Google Scholar] [CrossRef]
- Barlogie, B.; Tricot, G.; Rasmussen, E.; Anaissie, E.; van Rhee, F.; Zangari, M.; Fassas, A.; Hollmig, K.; Pineda-Roman, M.; Shaughnessy, J.; et al. Total therapy 2 without thalidomide in comparison with total therapy 1: Role of intensified induction and posttransplantation consolidation therapies. Blood 2006, 107, 2633–2638. [Google Scholar] [CrossRef]
- Sprynski, A.C.; Hose, D.; Caillot, L.; Réme, T.; Shaughnessy, J.D., Jr.; Barlogie, B.; Seckinger, A.; Moreaux, J.; Hundemer, M.; Jourdan, M.; et al. The role of IGF-1 as a major growth factor for myeloma cell lines and the prognostic relevance of the expression of its receptor. Blood 2009, 113, 4614–4626. [Google Scholar] [CrossRef]
- Vishwamitra, D.; George, S.K.; Shi, P.; Kaseb, A.O.; Amin, H.M. Type I insulin-like growth factor receptor signaling in hematological malignancies. Oncotarget 2017, 8, 1814–1844. [Google Scholar] [CrossRef]
- Chiron, D.; Maïga, S.; Surget, S.; Descamps, G.; Gomez-Bougie, P.; Traore, S.; Robillard, N.; Moreau, P.; Le Gouill, S.; Bataille, R.; et al. Autocrine insulin-like growth factor 1 and stem cell factor but not interleukin 6 support self-renewal of human myeloma cells. Blood Cancer J. 2013, 3, e120. [Google Scholar] [CrossRef]
- Georgii-Hemming, P.; Wiklund, H.J.; Ljunggren, O.; Nilsson, K. Insulin-like growth factor I is a growth and survival factor in human multiple myeloma cell lines. Blood 1996, 88, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Kirito, K.; Yoshida, K.; Mitsumori, T.; Nakajima, K.; Nozaki, Y.; Hamanaka, S.; Nagashima, T.; Kunitama, M.; Sakoe, K.; et al. Inhibition of hypoxia-inducible factor-1 function enhances the sensitivity of multiple myeloma cells to melphalan. Mol. Cancer Ther. 2009, 8, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Standal, T.; Borset, M.; Lenhoff, S.; Wisloff, F.; Stordal, B.; Sundan, A.; Waage, A.; Seidel, C. Serum insulinlike growth factor is not elevated in patients with multiple myeloma but is still a prognostic factor. Blood 2002, 100, 3925–3929. [Google Scholar] [CrossRef] [PubMed]
- Tagoug, I.; Jordheim, L.P.; Herveau, S.; Matera, E.L.; Huber, A.L.; Chettab, K.; Manié, S.; Dumontet, C. Therapeutic enhancement of ER stress by insulin-like growth factor I sensitizes myeloma cells to proteasomal inhibitors. Clin. Cancer Res. 2013, 19, 3556–3566. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, R.; Hirota, K.; Fan, F.; Jung, Y.D.; Ellis, L.M.; Semenza, G.L. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 2002, 277, 38205–38211. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, J.; Koyama, D.; Mukai, H.Y.; Furukawa, Y. Suitable drug combination with bortezomib for multiple myeloma under stroma-free conditions and in contact with fibronectin or bone marrow stromal cells. Int. J. Hematol. 2014, 99, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Adams, J. The proteasome: A suitable antineoplastic target. Nat. Rev. Cancer 2004, 4, 349–360. [Google Scholar] [CrossRef]
- Kikuchi, J.; Kuroda, Y.; Koyama, D.; Osada, N.; Izumi, T.; Yasui, H.; Kawase, T.; Ichinohe, T.; Furukawa, Y. Myeloma Cells Are Activated in Bone Marrow Microenvironment by the CD180/MD-1 Complex, Which Senses Lipopolysaccharide. Cancer Res. 2018, 78, 1766–1778. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Braggio, E.; Shi, C.X.; Kortuem, K.M.; Bruins, L.A.; Schmidt, J.E.; Chang, X.B.; Langlais, P.; Luo, M.; Jedlowski, P.; et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood 2014, 124, 536–545. [Google Scholar] [CrossRef]
- Horenstein, A.L.; Quarona, V.; Toscani, D.; Costa, F.; Chillemi, A.; Pistoia, V.; Giuliani, N.; Malavasi, F. Adenosine Generated in the Bone Marrow Niche Through a CD38-Mediated Pathway Correlates with Progression of Human Myeloma. Mol. Med. 2016, 22, 694–704. [Google Scholar] [CrossRef]
- Sung, B.; Kunnumakkara, A.B.; Sethi, G.; Anand, P.; Guha, S.; Aggarwal, B.B. Curcumin circumvents chemoresistance in vitro and potentiates the effect of thalidomide and bortezomib against human multiple myeloma in nude mice model. Mol. Cancer Ther. 2009, 8, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Ghose, J.; Viola, D.; Terrazas, C.; Caserta, E.; Troadec, E.; Khalife, J.; Gunes, E.G.; Sanchez, J.; McDonald, T.; Marcucci, G.; et al. Daratumumab induces CD38 internalization and impairs myeloma cell adhesion. Oncoimmunology 2018, 7, e1486948. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Vacca, A. Multiple myeloma as a model for the role of bone marrow niches in the control of angiogenesis. Int. Rev. Cell Mol. Biol. 2015, 314, 259–282. [Google Scholar] [CrossRef] [PubMed]
- Kusumbe, A.P. Vascular niches for disseminated tumour cells in bone. J. Bone Oncol. 2016, 5, 112–116. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silberstein, L.E.; Lin, C.P. A new image of the hematopoietic stem cell vascular niche. Cell Stem. Cell 2013, 13, 514–516. [Google Scholar] [CrossRef][Green Version]
- Martin, S.K.; Diamond, P.; Gronthos, S.; Peet, D.J.; Zannettino, A.C. The emerging role of hypoxia, HIF-1 and HIF-2 in multiple myeloma. Leukemia 2011, 25, 1533–1542. [Google Scholar] [CrossRef]
- Hu, J.; Van Valckenborgh, E.; Menu, E.; De Bruyne, E.; Vanderkerken, K. Understanding the hypoxic niche of multiple myeloma: Therapeutic implications and contributions of mouse models. Dis. Model Mech. 2012, 5, 763–771. [Google Scholar] [CrossRef]
- Hu, J.; Handisides, D.R.; Van Valckenborgh, E.; De Raeve, H.; Menu, E.; Vande Broek, I.; Liu, Q.; Sun, J.D.; Van Camp, B.; Hart, C.P.; et al. Targeting the multiple myeloma hypoxic niche with TH-302, a hypoxia-activated prodrug. Blood 2010, 116, 1524–1527. [Google Scholar] [CrossRef]
- Ria, R.; Melaccio, A.; Racanelli, V.; Vacca, A. Anti-VEGF Drugs in the Treatment of Multiple Myeloma Patients. J. Clin. Med. 2020, 9, 1765. [Google Scholar] [CrossRef]
- Vacca, A.; Ria, R.; Semeraro, F.; Merchionne, F.; Coluccia, M.; Boccarelli, A.; Scavelli, C.; Nico, B.; Gernone, A.; Battelli, F.; et al. Endothelial cells in the bone marrow of patients with multiple myeloma. Blood 2003, 102, 3340–3348. [Google Scholar] [CrossRef]
- Ria, R.; Todoerti, K.; Berardi, S.; Coluccia, A.M.; De Luisi, A.; Mattioli, M.; Ronchetti, D.; Morabito, F.; Guarini, A.; Petrucci, M.T.; et al. Gene expression profiling of bone marrow endothelial cells in patients with multiple myeloma. Clin. Cancer Res. 2009, 15, 5369–5378. [Google Scholar] [CrossRef] [PubMed]
- Vacca, A.; Ria, R.; Ribatti, D.; Semeraro, F.; Djonov, V.; Di Raimondo, F.; Dammacco, F. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica 2003, 88, 176–185. [Google Scholar] [PubMed]
- Adams, J.; Carder, P.J.; Downey, S.; Forbes, M.A.; MacLennan, K.; Allgar, V.; Kaufman, S.; Hallam, S.; Bicknell, R.; Walker, J.J.; et al. Vascular endothelial growth factor (VEGF) in breast cancer: Comparison of plasma, serum, and tissue VEGF and microvessel density and effects of tamoxifen. Cancer Res. 2000, 60, 2898–2905. [Google Scholar] [PubMed]
- Sujobert, P.; Bardet, V.; Cornillet-Lefebvre, P.; Hayflick, J.S.; Prie, N.; Verdier, F.; Vanhaesebroeck, B.; Muller, O.; Pesce, F.; Ifrah, N.; et al. Essential role for the p110delta isoform in phosphoinositide 3-kinase activation and cell proliferation in acute myeloid leukemia. Blood 2005, 106, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Witzig, T.E.; Timm, M.; Haug, J.; Wellik, L.; Kimlinger, T.K.; Greipp, P.R.; Rajkumar, S.V. Bone marrow angiogenic ability and expression of angiogenic cytokines in myeloma: Evidence favoring loss of marrow angiogenesis inhibitory activity with disease progression. Blood 2004, 104, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Mesa, R.A.; Fonseca, R.; Schroeder, G.; Plevak, M.F.; Dispenzieri, A.; Lacy, M.Q.; Lust, J.A.; Witzig, T.E.; Gertz, M.A.; et al. Bone marrow angiogenesis in 400 patients with monoclonal gammopathy of undetermined significance, multiple myeloma, and primary amyloidosis. Clin. Cancer Res. 2002, 8, 2210–2216. [Google Scholar]
- Vacca, A.; Ribatti, D.; Roncali, L.; Ranieri, G.; Serio, G.; Silvestris, F.; Dammacco, F. Bone marrow angiogenesis and progression in multiple myeloma. Br. J. Haematol. 1994, 87, 503–508. [Google Scholar] [CrossRef]
- Kawano, Y.; Kikukawa, Y.; Fujiwara, S.; Wada, N.; Okuno, Y.; Mitsuya, H.; Hata, H. Hypoxia reduces CD138 expression and induces an immature and stem cell-like transcriptional program in myeloma cells. Int. J. Oncol. 2013, 43, 1809–1816. [Google Scholar] [CrossRef]
- Harrison, J.S.; Rameshwar, P.; Chang, V.; Bandari, P. Oxygen saturation in the bone marrow of healthy volunteers. Blood 2002, 99, 394. [Google Scholar] [CrossRef]
- Asosingh, K.; De Raeve, H.; de Ridder, M.; Storme, G.A.; Willems, A.; Van Riet, I.; Van Camp, B.; Vanderkerken, K. Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematologica 2005, 90, 810–817. [Google Scholar]
- Leung-Hagesteijn, C.; Erdmann, N.; Cheung, G.; Keats, J.J.; Stewart, A.K.; Reece, D.E.; Chung, K.C.; Tiedemann, R.E. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell 2013, 24, 289–304. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z. Why proteasome inhibitors cannot ERADicate multiple myeloma. Cancer Cell 2013, 24, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, J.; Tolomelli, G.; Xu, H.; Xia, J.; Wang, H.; Zhou, W.; Zhou, Y.; Das, S.; Gu, Z.; et al. RARα2 expression confers myeloma stem cell features. Blood 2013, 122, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, S.; Kitadate, A.; Abe, F.; Saitoh, H.; Michishita, Y.; Hatano, Y.; Kawabata, Y.; Kitabayashi, A.; Teshima, K.; Kume, M.; et al. Hypoxia-inducible microRNA-210 regulates the DIMT1-IRF4 oncogenic axis in multiple myeloma. Cancer Sci. 2017, 108, 641–652. [Google Scholar] [CrossRef]
- Azab, A.K.; Hu, J.; Quang, P.; Azab, F.; Pitsillides, C.; Awwad, R.; Thompson, B.; Maiso, P.; Sun, J.D.; Hart, C.P.; et al. Hypoxia promotes dissemination of multiple myeloma through acquisition of epithelial to mesenchymal transition-like features. Blood 2012, 119, 5782–5794. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Mishima, Y.; Sacco, A.; Moschetta, M.; Tai, Y.T.; Shi, J.; Zhang, Y.; Reagan, M.R.; Huynh, D.; Kawano, Y.; et al. CXCR4 Regulates Extra-Medullary Myeloma through Epithelial-Mesenchymal-Transition-like Transcriptional Activation. Cell Rep. 2015, 12, 622–635. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Sacco, A.; Purschke, W.G.; Moschetta, M.; Buchner, K.; Maasch, C.; Zboralski, D.; Zöllner, S.; Vonhoff, S.; Mishima, Y.; et al. SDF-1 inhibition targets the bone marrow niche for cancer therapy. Cell Rep. 2014, 9, 118–128. [Google Scholar] [CrossRef]
- Bladé, J.; Fernández de Larrea, C.; Rosiñol, L.; Cibeira, M.T.; Jiménez, R.; Powles, R. Soft-tissue plasmacytomas in multiple myeloma: Incidence, mechanisms of extramedullary spread, and treatment approach. J. Clin. Oncol. 2011, 29, 3805–3812. [Google Scholar] [CrossRef]
- Bhutani, M.; Foureau, D.M.; Atrash, S.; Voorhees, P.M.; Usmani, S.Z. Extramedullary multiple myeloma. Leukemia 2020, 34, 1–20. [Google Scholar] [CrossRef]
- Galluzzo, M.; Pennacchietti, S.; Rosano, S.; Comoglio, P.M.; Michieli, P. Prevention of hypoxia by myoglobin expression in human tumor cells promotes differentiation and inhibits metastasis. J. Clin. Investig. 2009, 119, 865–875. [Google Scholar] [CrossRef]
- Rankin, E.B.; Giaccia, A.J. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008, 15, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sattler, M.; Tonon, G.; Grabher, C.; Lababidi, S.; Zimmerhackl, A.; Raab, M.S.; Vallet, S.; Zhou, Y.; Cartron, M.A.; et al. Targeting angiogenesis via a c-Myc/hypoxia-inducible factor-1alpha-dependent pathway in multiple myeloma. Cancer Res. 2009, 69, 5082–5090. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.L.; Emre, N.C.; Lamy, L.; Ngo, V.N.; Wright, G.; Xiao, W.; Powell, J.; Dave, S.; Yu, X.; Zhao, H.; et al. IRF4 addiction in multiple myeloma. Nature 2008, 454, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Ria, R.; Catacchio, I.; Berardi, S.; De Luisi, A.; Caivano, A.; Piccoli, C.; Ruggieri, V.; Frassanito, M.A.; Ribatti, D.; Nico, B.; et al. HIF-1α of bone marrow endothelial cells implies relapse and drug resistance in patients with multiple myeloma and may act as a therapeutic target. Clin. Cancer Res. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- Maj, T.; Wang, W.; Crespo, J.; Zhang, H.; Wang, W.; Wei, S.; Zhao, L.; Vatan, L.; Shao, I.; Szeliga, W.L.; et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 2017, 18, 1332–1341. [Google Scholar] [CrossRef]
- Shin, D.H.; Chun, Y.S.; Lee, D.S.; Huang, L.E.; Park, J.W. Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood 2008, 111, 3131–3136. [Google Scholar] [CrossRef]
- Roccaro, A.M.; Hideshima, T.; Raje, N.; Kumar, S.; Ishitsuka, K.; Yasui, H.; Shiraishi, N.; Ribatti, D.; Nico, B.; Vacca, A.; et al. Bortezomib mediates antiangiogenesis in multiple myeloma via direct and indirect effects on endothelial cells. Cancer Res. 2006, 66, 184–191. [Google Scholar] [CrossRef]
- Dredge, K.; Marriott, J.B.; Macdonald, C.D.; Man, H.W.; Chen, R.; Muller, G.W.; Stirling, D.; Dalgleish, A.G. Novel thalidomide analogues display anti-angiogenic activity independently of immunomodulatory effects. Br. J. Cancer 2002, 87, 1166–1172. [Google Scholar] [CrossRef][Green Version]
- De Luisi, A.; Ferrucci, A.; Coluccia, A.M.; Ria, R.; Moschetta, M.; de Luca, E.; Pieroni, L.; Maffia, M.; Urbani, A.; Di Pietro, G.; et al. Lenalidomide restrains motility and overangiogenic potential of bone marrow endothelial cells in patients with active multiple myeloma. Clin. Cancer Res. 2011, 17, 1935–1946. [Google Scholar] [CrossRef]
- Breitkreutz, I.; Raab, M.S.; Vallet, S.; Hideshima, T.; Raje, N.; Mitsiades, C.; Chauhan, D.; Okawa, Y.; Munshi, N.C.; Richardson, P.G.; et al. Lenalidomide inhibits osteoclastogenesis, survival factors and bone-remodeling markers in multiple myeloma. Leukemia 2008, 22, 1925–1932. [Google Scholar] [CrossRef]
- Lu, L.; Payvandi, F.; Wu, L.; Zhang, L.H.; Hariri, R.J.; Man, H.W.; Chen, R.S.; Muller, G.W.; Hughes, C.C.; Stirling, D.I.; et al. The anti-cancer drug lenalidomide inhibits angiogenesis and metastasis via multiple inhibitory effects on endothelial cell function in normoxic and hypoxic conditions. Microvasc. Res. 2009, 77, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Chen, J.; Gao, H.; Kong, S.N.; Deivasigamani, A.; Shi, M.; Xie, T.; Hui, K.M. Hypoxia-induced modulation of glucose transporter expression impacts 18 F-fluorodeoxyglucose PET-CT imaging in hepatocellular carcinoma. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Rosiñol, L.; Cibeira, M.T.; Bladé, J.; Esteve, J.; Aymerich, M.; Rozman, M.; Segarra, M.; Cid, M.C.; Filella, X.; Montserrat, E. Extramedullary multiple myeloma escapes the effect of thalidomide. Haematologica 2004, 89, 832–836. [Google Scholar] [PubMed]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Moreau, P.; Zweegman, S.; Perrot, A.; Hulin, C.; Caillot, D.; Facon, T.; Leleu, X.; Belhadj, K.; Karlin, L.; Benboubker, L.; et al. Evaluation of the Prognostic Value of Positron Emission Tomography-Computed Tomography (PET-CT) at Diagnosis and Follow-up in Transplant-Eligible Newly Diagnosed Multiple Myeloma (TE NDMM) Patients Treated in the Phase 3 Cassiopeia Study: Results of the Cassiopet Companion Study. Blood 2019, 134 (Suppl. 1), 692. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Caillot, D.; Macro, M.; Karlin, L.; Garderet, L.; Facon, T.; Benboubker, L.; Escoffre-Barbe, M.; Stoppa, A.M.; et al. Prospective Evaluation of Magnetic Resonance Imaging and [ 18 F]Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography at Diagnosis and Before Maintenance Therapy in Symptomatic Patients With Multiple Myeloma Included in the IFM/DFCI 2009 Trial: Results of the IMAJEM Study. J. Clin. Oncol. 2017, 35, 2911–2918. [Google Scholar] [CrossRef]
- Wu, C.; Yang, T.; Liu, Y.; Lu, Y.; Yang, Y.; Liu, X.; Liu, X.; Ye, L.; Sun, Y.; Wang, X.; et al. ARNT/HIF-1β links high-risk 1q21 gain and microenvironmental hypoxia to drug resistance and poor prognosis in multiple myeloma. Cancer Med. 2018, 7, 3899–3911. [Google Scholar] [CrossRef]
- Lakshman, A.; Singh, P.P.; Rajkumar, S.V.; Dispenzieri, A.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Dingli, D.; Hwa, Y.L.; Fonder, A.L.; et al. Efficacy of VDT PACE-like regimens in treatment of relapsed/refractory multiple myeloma. Am. J. Hematol. 2018, 93, 179–186. [Google Scholar] [CrossRef]
- Capp, J.P.; Bataille, R. Multiple Myeloma as a Bone Disease? The Tissue Disruption-Induced Cell Stochasticity (TiDiS) Theory. Cancers 2020, 12, 2158. [Google Scholar] [CrossRef]
- Terpos, E.; Szydlo, R.; Apperley, J.F.; Hatjiharissi, E.; Politou, M.; Meletis, J.; Viniou, N.; Yataganas, X.; Goldman, J.M.; Rahemtulla, A. Soluble receptor activator of nuclear factor kappaB ligand-osteoprotegerin ratio predicts survival in multiple myeloma: Proposal for a novel prognostic index. Blood 2003, 102, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Abe, M.; Hiasa, M.; Oda, A.; Amou, H.; Kido, S.; Harada, T.; Tanaka, O.; Miki, H.; Nakamura, S.; et al. Tgf-Beta inhibition restores terminal osteoblast differentiation to suppress myeloma growth. PLoS ONE 2010, 5, e9870. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Y.W.; Shaughnessy, J.D., Jr.; Yaccoby, S. Wnt3a signaling within bone inhibits multiple myeloma bone disease and tumor growth. Blood 2008, 112, 374–382. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreaux, J.; Cremer, F.W.; Reme, T.; Raab, M.; Mahtouk, K.; Kaukel, P.; Pantesco, V.; De Vos, J.; Jourdan, E.; Jauch, A.; et al. The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 2005, 106, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kido, S.; Hiasa, M.; Nakano, A.; Oda, A.; Amou, H.; Matsumoto, T. BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: A rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia 2006, 20, 1313–1315. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Acharya, C.; Feng, X.; Wen, K.; Zhong, M.; Zhang, L.; Munshi, N.C.; Qiu, L.; Tai, Y.T.; Anderson, K.C. Osteoclasts promote immune suppressive microenvironment in multiple myeloma: Therapeutic implication. Blood 2016, 128, 1590–1603. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Bellido, T.; Roodman, G.D. Role of osteocytes in multiple myeloma bone disease. Curr. Opin. Support. Palliat. Care 2014, 8, 407–413. [Google Scholar] [CrossRef]
- Moreaux, J.; Hose, D.; Kassambara, A.; Reme, T.; Moine, P.; Requirand, G.; Goldschmidt, H.; Klein, B. Osteoclast-gene expression profiling reveals osteoclast-derived CCR2 chemokines promoting myeloma cell migration. Blood 2011, 117, 1280–1290. [Google Scholar] [CrossRef]
- Politou, M.C.; Heath, D.J.; Rahemtulla, A.; Szydlo, R.; Anagnostopoulos, A.; Dimopoulos, M.A.; Croucher, P.I.; Terpos, E. Serum concentrations of Dickkopf-1 protein are increased in patients with multiple myeloma and reduced after autologous stem cell transplantation. Int. J. Cancer 2006, 119, 1728–1731. [Google Scholar] [CrossRef]
- Heider, U.; Kaiser, M.; Mieth, M.; Lamottke, B.; Rademacher, J.; Jakob, C.; Braendle, E.; Stover, D.; Sezer, O. Serum concentrations of DKK-1 decrease in patients with multiple myeloma responding to anti-myeloma treatment. Eur. J. Haematol. 2009, 82, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, N.; Morandi, F.; Tagliaferri, S.; Lazzaretti, M.; Bonomini, S.; Crugnola, M.; Mancini, C.; Martella, E.; Ferrari, L.; Tabilio, A.; et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood 2007, 110, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Zangari, M.; Yaccoby, S.; Pappas, L.; Cavallo, F.; Kumar, N.S.; Ranganathan, S.; Suva, L.J.; Gruenwald, J.M.; Kern, S.; Zhan, F.; et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica 2011, 96, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Raje, N.; Schoonmaker, J.A.; Liu, J.C.; Hideshima, T.; Wein, M.N.; Jones, D.C.; Vallet, S.; Bouxsein, M.L.; Pozzi, S.; et al. Pharmacologic targeting of a stem/progenitor population in vivo is associated with enhanced bone regeneration in mice. J. Clin. Investig. 2008, 118, 491–504. [Google Scholar] [CrossRef]
- Qiang, Y.W.; Hu, B.; Chen, Y.; Zhong, Y.; Shi, B.; Barlogie, B.; Shaughnessy, J.D., Jr. Bortezomib induces osteoblast differentiation via Wnt-independent activation of beta-catenin/TCF signaling. Blood 2009, 113, 4319–4330. [Google Scholar] [CrossRef]
- Lawson, M.A.; McDonald, M.M.; Kovacic, N.; Hua Khoo, W.; Terry, R.L.; Down, J.; Kaplan, W.; Paton-Hough, J.; Fellows, C.; Pettitt, J.A.; et al. Osteoclasts control reactivation of dormant myeloma cells by remodelling the endosteal niche. Nat. Commun. 2015, 6, 8983. [Google Scholar] [CrossRef]
- Caraux, A.; Vincent, L.; Bouhya, S.; Quittet, P.; Moreaux, J.; Requirand, G.; Veyrune, J.L.; Olivier, G.; Cartron, G.; Rossi, J.F.; et al. Residual malignant and normal plasma cells shortly after high dose melphalan and stem cell transplantation. Highlight of a putative therapeutic window in Multiple Myeloma? Oncotarget 2012, 3, 1335–1347. [Google Scholar] [CrossRef]
- Costa, F.; Toscani, D.; Chillemi, A.; Quarona, V.; Bolzoni, M.; Marchica, V.; Vescovini, R.; Mancini, C.; Martella, E.; Campanini, N.; et al. Expression of CD38 in myeloma bone niche: A rational basis for the use of anti-CD38 immunotherapy to inhibit osteoclast formation. Oncotarget 2017, 8, 56598–56611. [Google Scholar] [CrossRef]
- Boutsikas, G.; Terpos, E.; Papatheodorou, A.; Tsirkinidis, P.; Tsirigotis, P.; Meletiou, A.; Lalou, E.; Telonis, V.; Zannou, A.; Kanellopoulos, A.; et al. Study of bone metabolism and angiogenesis in patients undergoing high-dose chemotherapy/autologous hematopoietic stem cell transplantation. Eur. J. Haematol. 2018, 100, 131–139. [Google Scholar] [CrossRef]
- Fulciniti, M.; Tassone, P.; Hideshima, T.; Vallet, S.; Nanjappa, P.; Ettenberg, S.A.; Shen, Z.; Patel, N.; Tai, Y.T.; Chauhan, D.; et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood 2009, 114, 371–379. [Google Scholar] [CrossRef]
- Maura, F.; Bolli, N.; Angelopoulos, N.; Dawson, K.J.; Leongamornlert, D.; Martincorena, I.; Mitchell, T.J.; Fullam, A.; Gonzalez, S.; Szalat, R.; et al. Genomic landscape and chronological reconstruction of driver events in multiple myeloma. Nat. Commun. 2019, 10, 3835. [Google Scholar] [CrossRef] [PubMed]
- Sottoriva, A.; Kang, H.; Ma, Z.; Graham, T.A.; Salomon, M.P.; Zhao, J.; Marjoram, P.; Siegmund, K.; Press, M.F.; Shibata, D.; et al. A Big Bang model of human colorectal tumor growth. Nat. Genet. 2015, 47, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Kikuchi, J. Molecular basis of clonal evolution in multiple myeloma. Int. J. Hematol. 2020, 111, 496–511. [Google Scholar] [CrossRef] [PubMed]
- Lohr, J.G.; Stojanov, P.; Carter, S.L.; Cruz-Gordillo, P.; Lawrence, M.S.; Auclair, D.; Sougnez, C.; Knoechel, B.; Gould, J.; Saksena, G.; et al. Widespread genetic heterogeneity in multiple myeloma: Implications for targeted therapy. Cancer Cell 2014, 25, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Pawlyn, C.; Morgan, G.J. Evolutionary biology of high-risk multiple myeloma. Nat. Rev. Cancer 2017, 17, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.B.; Shi, C.X.; Tembe, W.; Christoforides, A.; Kurdoglu, A.; Sinari, S.; Middha, S.; Asmann, Y.; Schmidt, J.; Braggio, E.; et al. Whole-genome sequencing of multiple myeloma from diagnosis to plasma cell leukemia reveals genomic initiating events, evolution, and clonal tides. Blood 2012, 120, 1060–1066. [Google Scholar] [CrossRef]
- Todoerti, K.; Agnelli, L.; Fabris, S.; Lionetti, M.; Tuana, G.; Mosca, L.; Lombardi, L.; Grieco, V.; Bianchino, G.; D’Auria, F.; et al. Transcriptional characterization of a prospective series of primary plasma cell leukemia revealed signatures associated with tumor progression and poorer outcome. Clin. Cancer Res. 2013, 19, 3247–3258. [Google Scholar] [CrossRef]
- Cifola, I.; Lionetti, M.; Pinatel, E.; Todoerti, K.; Mangano, E.; Pietrelli, A.; Fabris, S.; Mosca, L.; Simeon, V.; Petrucci, M.T.; et al. Whole-exome sequencing of primary plasma cell leukemia discloses heterogeneous mutational patterns. Oncotarget 2015, 6, 17543–17558. [Google Scholar] [CrossRef]
- Ohta, T.; Kimura, M. A model of mutation appropriate to estimate the number of electrophoretically detectable alleles in a finite population*. Genet. Res. 2007, 89, 367–370. [Google Scholar] [CrossRef]
- Furukawa, Y.; Kikuchi, J. Molecular pathogenesis of multiple myeloma. Int. J. Clin. Oncol. 2015, 20, 413–422. [Google Scholar] [CrossRef]
- Johnson, D.C.; Lenive, O.; Mitchell, J.; Jackson, G.; Owen, R.; Drayson, M.; Cook, G.; Jones, J.R.; Pawlyn, C.; Davies, F.E.; et al. Neutral tumor evolution in myeloma is associated with poor prognosis. Blood 2017, 130, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Usmani, S.Z.; Heuck, C.; Mitchell, A.; Szymonifka, J.; Nair, B.; Hoering, A.; Alsayed, Y.; Waheed, S.; Haider, S.; Restrepo, A.; et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica 2012, 97, 1761–1767. [Google Scholar] [CrossRef] [PubMed]
- Vrábel, D.; Pour, L.; Ševčíková, S. The impact of NF-κB signaling on pathogenesis and current treatment strategies in multiple myeloma. Blood Rev. 2019, 34, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Lodé, L.; Eveillard, M.; Trichet, V.; Soussi, T.; Wuillème, S.; Richebourg, S.; Magrangeas, F.; Ifrah, N.; Campion, L.; Traullé, C.; et al. Mutations in TP53 are exclusively associated with del(17p) in multiple myeloma. Haematologica 2010, 95, 1973–1976. [Google Scholar] [CrossRef]
- Weinhold, N.; Ashby, C.; Rasche, L.; Chavan, S.S.; Stein, C.; Stephens, O.W.; Tytarenko, R.; Bauer, M.A.; Meissner, T.; Deshpande, S.; et al. Clonal selection and double-hit events involving tumor suppressor genes underlie relapse in myeloma. Blood 2016, 128, 1735–1744. [Google Scholar] [CrossRef]
- Jovanović, K.K.; Escure, G.; Demonchy, J.; Willaume, A.; Van de Wyngaert, Z.; Farhat, M.; Chauvet, P.; Facon, T.; Quesnel, B.; Manier, S. Deregulation and Targeting of TP53 Pathway in Multiple Myeloma. Front. Oncol. 2019, 8, 665. [Google Scholar] [CrossRef]
- Walker, B.A.; Mavrommatis, K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.E.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; et al. Identification of novel mutational drivers reveals oncogene dependencies in multiple myeloma. Blood 2018, 132, 587–597. [Google Scholar] [CrossRef]
- Thanendrarajan, S.; Tian, E.; Qu, P.; Mathur, P.; Schinke, C.; van Rhee, F.; Zangari, M.; Rasche, L.; Weinhold, N.; Alapat, D.; et al. The level of deletion 17p and bi-allelic inactivation of TP53 has a significant impact on clinical outcome in multiple myeloma. Haematologica 2017, 102, e364–e367. [Google Scholar] [CrossRef]
- Walerych, D.; Lisek, K.; Sommaggio, R.; Piazza, S.; Ciani, Y.; Dalla, E.; Rajkowska, K.; Gaweda-Walerych, K.; Ingallina, E.; Tonelli, C.; et al. Proteasome machinery is instrumental in a common gain-of-function program of the p53 missense mutants in cancer. Nat. Cell Biol. 2016, 18, 897–909. [Google Scholar] [CrossRef]
- Chin, M.; Sive, J.I.; Allen, C.; Roddie, C.; Chavda, S.J.; Smith, D.; Blombery, P.; Jones, K.; Ryland, G.L.; Popat, R.; et al. Prevalence and timing of TP53 mutations in del(17p) myeloma and effect on survival. Blood Cancer J. 2017, 7, e610. [Google Scholar] [CrossRef]
- Thakurta, A.; Ortiz, M.; Blecua, P.; Towfic, F.; Corre, J.; Serbina, N.V.; Flynt, E.; Yu, Z.; Yang, Z.; Palumbo, A.; et al. High subclonal fraction of 17p deletion is associated with poor prognosis in multiple myeloma. Blood 2019, 133, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Lakshman, A.; Painuly, U.; Rajkumar, S.V.; Ketterling, R.P.; Kapoor, P.; Greipp, P.T.; Dispenzieri, A.; Gertz, M.A.; Buadi, F.K.; Lacy, M.Q.; et al. Impact of acquired del(17p) in multiple myeloma. Blood Adv. 2019, 3, 1930–1938. [Google Scholar] [CrossRef] [PubMed]
- Lancman, G.; Tremblay, D.; Barley, K.; Barlogie, B.; Cho, H.J.; Jagannath, S.; Madduri, D.; Moshier, E.; Parekh, S.; Chari, A. The effect of novel therapies in high-molecular-risk multiple myeloma. Clin. Adv. Hematol. Oncol. 2017, 15, 870–879. [Google Scholar] [PubMed]
- Rasche, L.; Chavan, S.S.; Stephens, O.W.; Patel, P.H.; Tytarenko, R.; Ashby, C.; Bauer, M.; Stein, C.; Deshpande, S.; Wardell, C.; et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat. Commun. 2017, 8, 268. [Google Scholar] [CrossRef]
- Zhou, X.; Dierks, A.; Kertels, O.; Samnick, S.; Kircher, M.; Buck, A.K.; Haertle, L.; Knorz, S.; Böckle, D.; Scheller, L.; et al. The Link between Cytogenetics/Genomics and Imaging Patterns of Relapse and Progression in Patients with Relapsed/Refractory Multiple Myeloma: A Pilot Study Utilizing 18F-FDG PET/CT. Cancers 2020, 12, E2399. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Murison, A.; Boyle, E.M.; Begum, D.B.; Dahir, N.M.; Proszek, P.Z.; Melchor, L.; Pawlyn, C.; Kaiser, M.F.; et al. APOBEC family mutational signatures are associated with poor prognosis translocations in multiple myeloma. Nat. Commun. 2015, 6, 6997. [Google Scholar] [CrossRef]
- Pawlyn, C.; Loehr, A.; Ashby, C.; Tytarenko, R.; Deshpande, S.; Sun, J.; Fedorchak, K.; Mughal, T.; Davies, F.E.; Walker, B.A.; et al. Loss of heterozygosity as a marker of homologous repair deficiency in multiple myeloma: A role for PARP inhibition? Leukemia 2018, 32, 1561–1566. [Google Scholar] [CrossRef]
- Weißbach, S.; Langer, C.; Puppe, B.; Nedeva, T.; Bach, E.; Kull, M.; Bargou, R.; Einsele, H.; Rosenwald, A.; Knop, S.; et al. The molecular spectrum and clinical impact of DIS3 mutations in multiple myeloma. Br. J. Haematol. 2015, 169, 57–70. [Google Scholar] [CrossRef]
- Bolli, N.; Avet-Loiseau, H.; Wedge, D.C.; Van Loo, P.; Alexandrov, L.B.; Martincorena, I.; Dawson, K.J.; Iorio, F.; Nik-Zainal, S.; Bignell, G.R.; et al. Heterogeneity of genomic evolution and mutational profiles in multiple myeloma. Nat. Commun. 2014, 5, 2997. [Google Scholar] [CrossRef]
- An, G.; Yan, Y.; Xu, Y.; Mao, X.; Liu, J.; Fan, H.; Wang, Q.; Du, C.; Li, Z.; Yi, S.; et al. Monitoring the cytogenetic architecture of minimal residual plasma cells indicates therapy-induced clonal selection in multiple myeloma. Leukemia 2020, 34, 578–588. [Google Scholar] [CrossRef]
- Jones, J.R.; Weinhold, N.; Ashby, C.; Walker, B.A.; Wardell, C.; Pawlyn, C.; Rasche, L.; Melchor, L.; Cairns, D.A.; Gregory, W.M.; et al. Clonal evolution in myeloma: The impact of maintenance lenalidomide and depth of response on the genetics and sub-clonal structure of relapsed disease in uniformly treated newly diagnosed patients. Haematologica 2019, 104, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Rasche, L.; Alapat, D.; Kumar, M.; Gershner, G.; McDonald, J.; Wardell, C.P.; Samant, R.; Van Hemert, R.; Epstein, J.; Williams, A.F.; et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia 2019, 33, 1713–1722. [Google Scholar] [CrossRef] [PubMed]
- Chaidos, A.; Barnes, C.P.; Cowan, G.; May, P.C.; Melo, V.; Hatjiharissi, E.; Papaioannou, M.; Harrington, H.; Doolittle, H.; Terpos, E.; et al. Clinical drug resistance linked to interconvertible phenotypic and functional states of tumor-propagating cells in multiple myeloma. Blood 2013, 121, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Pourabdollah, M.; Bahmanyar, M.; Atenafu, E.G.; Reece, D.; Hou, J.; Chang, H. High IKZF1/3 protein expression is a favorable prognostic factor for survival of relapsed/refractory multiple myeloma patients treated with lenalidomide. J. Hematol. Oncol. 2016, 9, 123. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, M.; Cren, M.; Schafer, P.; Robert, N.; Duperray, C.; Vincent, L.; Ceballos, P.; Cartron, G.; Rossi, J.F.; Moreaux, J.; et al. Differential effects of lenalidomide during plasma cell differentiation. Oncotarget. 2016, 7, 28096–28111. [Google Scholar] [CrossRef]
- Hosen, N.; Matsuoka, Y.; Kishida, S.; Nakata, J.; Mizutani, Y.; Hasegawa, K.; Mugitani, A.; Ichihara, H.; Aoyama, Y.; Nishida, S.; et al. CD138-negative clonogenic cells are plasma cells but not B cells in some multiple myeloma patients. Leukemia. 2012, 26, 2135–2141. [Google Scholar] [CrossRef]
- Jakubikova, J.; Adamia, S.; Kost-Alimova, M.; Klippel, S.; Cervi, D.; Daley, J.F.; Cholujova, D.; Kong, S.Y.; Leiba, M.; Blotta, S.; et al. Lenalidomide targets clonogenic side population in multiple myeloma: Pathophysiologic and clinical implications. Blood 2011, 117, 4409–4419. [Google Scholar] [CrossRef]
- Binder, M.; Rajkumar, S.V.; Lacy, M.Q.; Gertz, M.A.; Buadi, F.K.; Dispenzieri, A.; Hwa, Y.L.; Fonder, A.; Hobbs, M.; Hayman, S.R.; et al. Peripheral blood biomarkers of early immune reconstitution in newly diagnosed multiple myeloma. Am. J. Hematol. 2019, 94, 306–311. [Google Scholar] [CrossRef]
- Jimenez-Zepeda, V.H.; Reece, D.E.; Trudel, S.; Chen, C.; Franke, N.; Winter, A.; Tiedemann, R.; Kukreti, V. Absolute lymphocyte count as predictor of overall survival for patients with multiple myeloma treated with single autologous stem cell transplant. Leuk. Lymphoma 2015, 56, 2668–2673. [Google Scholar] [CrossRef]
- Arteche-López, A.; Kreutzman, A.; Alegre, A.; Sanz Martín, P.; Aguado, B.; González-Pardo, M.; Espiño, M.; Villar, L.M.; García Belmonte, D.; de la Cámara, R.; et al. Multiple myeloma patients in long-term complete response after autologous stem cell transplantation express a particular immune signature with potential prognostic implication. Bone Marrow Transplant. 2017, 52, 832–838. [Google Scholar] [CrossRef]
- Bryant, C.; Suen, H.; Brown, R.; Yang, S.; Favaloro, J.; Aklilu, E.; Gibson, J.; Ho, P.J.; Iland, H.; Fromm, P.; et al. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J. 2013, 3, e148. [Google Scholar] [CrossRef]
- Paiva, B.; Cedena, M.T.; Puig, N.; Arana, P.; Vidriales, M.B.; Cordon, L.; Flores-Montero, J.; Gutierrez, N.C.; Martín-Ramos, M.L.; Martinez-Lopez, J.; et al. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood 2016, 127, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- De Larrea, C.F.; Cibeira, M.T.; Elena, M.; Arostegui, J.I.; Rosiñol, L.; Rovira, M.; Filella, X.; Yagüe, J.; Bladé, J. Abnormal serum free light chain ratio in patients with multiple myeloma in complete remission has strong association with the presence of oligoclonal bands: Implications for stringent complete remission definition. Blood 2009, 114, 4954–4956. [Google Scholar] [CrossRef] [PubMed]
- Zent, C.S.; Wilson, C.S.; Tricot, G.; Jagannath, S.; Siegel, D.; Desikan, K.R.; Munshi, N.; Bracy, D.; Barlogie, B.; Butch, A.W. Oligoclonal protein bands and Ig isotype switching in multiple myeloma treated with high-dose therapy and hematopoietic cell transplantation. Blood 1998, 91, 3518–3523. [Google Scholar] [CrossRef] [PubMed]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef]
- Giri, S.; Grimshaw, A.; Bal, S.; Godby, K.; Kharel, P.; Djulbegovic, B.; Dimopoulos, M.A.; Facon, T.; Usmani, S.Z.; Mateos, M.V.; et al. Evaluation of Daratumumab for the Treatment of Multiple Myeloma in Patients With High-risk Cytogenetic Factors: A Systematic Review and Meta-analysis. JAMA Oncol. 2020, e204338. [Google Scholar] [CrossRef]
- Reimold, A.M.; Iwakoshi, N.N.; Manis, J.; Vallabhajosyula, P.; Szomolanyi-Tsuda, E.; Gravallese, E.M.; Friend, D.; Grusby, M.J.; Alt, F.; Glimcher, L.H. Plasma cell differentiation requires the transcription factor XBP-1. Nature 2001, 412, 300–307. [Google Scholar] [CrossRef]
- Gambella, M.; Rocci, A.; Passera, R.; Gay, F.; Omedè, P.; Crippa, C.; Corradini, P.; Romano, A.; Rossi, D.; Ladetto, M.; et al. High XBP1 expression is a marker of better outcome in multiple myeloma patients treated with bortezomib. Haematologica 2014, 99, e14–e16. [Google Scholar] [CrossRef]
- Ling, S.C.; Lau, E.K.; Al-Shabeeb, A.; Nikolic, A.; Catalano, A.; Iland, H.; Horvath, N.; Ho, P.J.; Harrison, S.; Fleming, S.; et al. Response of myeloma to the proteasome inhibitor bortezomib is correlated with the unfolded protein response regulator XBP-1. Haematologica 2012, 97, 64–72. [Google Scholar] [CrossRef]
- Shi, J.; Tricot, G.J.; Garg, T.K.; Malaviarachchi, P.A.; Szmania, S.M.; Kellum, R.E.; Storrie, B.; Mulder, A.; Shaughnessy, J.D., Jr.; Barlogie, B.; et al. Bortezomib down-regulates the cell-surface expression of HLA class I and enhances natural killer cell-mediated lysis of myeloma. Blood 2008, 111, 1309–1317. [Google Scholar] [CrossRef]
- Hayashi, T.; Hideshima, T.; Akiyama, M.; Podar, K.; Yasui, H.; Raje, N.; Kumar, S.; Chauhan, D.; Treon, S.P.; Richardson, P.G.; et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: Clinical application. Br. J. Haematol. 2005, 128, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Galustian, C.; Meyer, B.; Labarthe, M.C.; Dredge, K.; Klaschka, D.; Henry, J.; Todryk, S.; Chen, R.; Muller, G.; Stirling, D.; et al. The anti-cancer agents lenalidomide and pomalidomide inhibit the proliferation and function of T regulatory cells. Cancer Immunol. Immunother. 2009, 58, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, M.; Janji, B.; Berchem, G. Activation of NK cells and disruption of PD-L1/PD-1 axis: Two different ways for lenalidomide to block myeloma progression. Oncotarget 2017, 8, 24031–24044. [Google Scholar] [CrossRef] [PubMed]
- Benson, D.M., Jr.; Bakan, C.E.; Mishra, A.; Hofmeister, C.C.; Efebera, Y.; Becknell, B.; Baiocchi, R.A.; Zhang, J.; Yu, J.; Smith, M.K.; et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: A therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010, 116, 2286–2294. [Google Scholar] [CrossRef]
- Krönke, J.; Kuchenbauer, F.; Kull, M.; Teleanu, V.; Bullinger, L.; Bunjes, D.; Greiner, A.; Kolmus, S.; Köpff, S.; Schreder, M.; et al. IKZF1 expression is a prognostic marker in newly diagnosed standard-risk multiple myeloma treated with lenalidomide and intensive chemotherapy: A study of the German Myeloma Study Group (DSMM). Leukemia 2017, 31, 1363–1367. [Google Scholar] [CrossRef]
- Seckinger, A.; Hillengass, J.; Emde, M.; Beck, S.; Kimmich, C.; Dittrich, T.; Hundemer, M.; Jauch, A.; Hegenbart, U.; Raab, M.S.; et al. CD38 as Immunotherapeutic Target in Light Chain Amyloidosis and Multiple Myeloma-Association With Molecular Entities, Risk, Survival, and Mechanisms of Upfront Resistance. Front. Immunol. 2018, 9, 1676. [Google Scholar] [CrossRef]
- Ogiya, D.; Liu, J.; Ohguchi, H.; Kurata, K.; Samur, M.K.; Tai, Y.T.; Adamia, S.; Ando, K.; Hideshima, T.; Anderson, K.C. JAK-STAT pathway regulates CD38 on myeloma cells in the bone marrow microenvironment: Therapeutic implications. Blood 2020, 136, 2334–2345. [Google Scholar] [CrossRef]
- Krejcik, J.; Casneuf, T.; Nijhof, I.S.; Verbist, B.; Bald, J.; Plesner, T.; Syed, K.; Liu, K.; van de Donk, N.W.; Weiss, B.M.; et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016, 128, 384–394. [Google Scholar] [CrossRef]
- Dosani, T.; Carlsten, M.; Maric, I.; Landgren, O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of myeloma [corrected] and their uses in immunotherapies. Blood Cancer J. 2015, 5, e306. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; San-Miguel, J.; Belch, A.; White, D.; Benboubker, L.; Cook, G.; Leiba, M.; Morton, J.; Ho, P.J.; Kim, K.; et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of POLLUX. Haematologica 2018, 103, 2088–2096. [Google Scholar] [CrossRef] [PubMed]
- Bahlis, N.J.; Dimopoulos, M.A.; White, D.J.; Benboubker, L.; Cook, G.; Leiba, M.; Ho, P.J.; Kim, K.; Takezako, N.; Moreau, P.; et al. Daratumumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended follow-up of POLLUX, a randomized, open-label, phase 3 study. Leukemia 2020, 34, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Calandra, G.; McCarty, J.; McGuirk, J.; Tricot, G.; Crocker, S.A.; Badel, K.; Grove, B.; Dye, A.; Bridger, G. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin’s lymphoma, Hodgkin’s disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: Compassionate use data. Bone Marrow Transplant. 2008, 41, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Gay, F.; Cerrato, C.; Scalabrini, D.R.; Galli, M.; Belotti, A.; Zamagni, E.; Ledda, A.; Grasso, M.; Emanuele Angelucci, E.; Liberati, A.M.; et al. Carfilzomib-Lenalidomide-Dexamethasone (KRd) Induction-Autologous Transplant (ASCT)-Krd Consolidation Vs KRd 12 Cycles Vs Carfilzomib-Cyclophosphamide-Dexamethasone (KCd) Induction-ASCT-KCd Consolidation: Analysis of the Randomized Forte Trial in Newly Diagnosed Multiple Myeloma (NDMM). Blood 2018, 132, 121. [Google Scholar]
- Oliva, S.; Genuardi, E.; Belotti, A.; Frascione, P.M.M.; Galli, M.; Capra, A.; Offidani, M.; Vozella, F.; Zambello, R.; Auclair, D.; et al. Multiparameter flow cytometry (MFC) and next generation sequencing (NGS) for minimal residual disease (MRD) evaluation: Results of the FORTE trial in newly diagnosed multiple myeloma (MM). J. Clin. Oncol. 2020, 38, 8533. [Google Scholar] [CrossRef]
- Radbruch, A.; Muehlinghaus, G.; Luger, E.O.; Inamine, A.; Smith, K.G.; Dörner, T.; Hiepe, F. Competence and competition: The challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006, 6, 741–750. [Google Scholar] [CrossRef]
- Paiva, B.; Pérez-Andrés, M.; Vídriales, M.B.; Almeida, J.; de las Heras, N.; Mateos, M.V.; López-Corral, L.; Gutiérrez, N.C.; Blanco, J.; Oriol, A.; et al. Competition between clonal plasma cells and normal cells for potentially overlapping bone marrow niches is associated with a progressively altered cellular distribution in MGUS vs myeloma. Leukemia 2011, 25, 697–706. [Google Scholar] [CrossRef]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, L.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Ross, F.M.; Ibrahim, A.H.; Vilain-Holmes, A.; Winfield, M.O.; Chiecchio, L.; Protheroe, R.K.; Strike, P.; Gunasekera, J.L.; Jones, A.; Harrison, C.J.; et al. Age has a profound effect on the incidence and significance of chromosome abnormalities in myeloma. Leukemia 2005, 19, 1634–1642. [Google Scholar] [CrossRef]
- Pratt, G.; Goodyear, O.; Moss, P. Immunodeficiency and immunotherapy in multiple myeloma. Br. J. Haematol. 2007, 138, 563–579. [Google Scholar] [CrossRef]
- San Miguel, J.F.; González, M.; Gascón, A.; Moro, M.J.; Hernández, J.M.; Ortega, F.; Jiménez, R.; Guerras, L.; Romero, M.; Casanova, F.; et al. Lymphoid subsets and prognostic factors in multiple myeloma. Cooperative Group for the Study of Monoclonal Gammopathies. Br. J. Haematol. 1992, 80, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Suen, H.; Brown, R.; Yang, S.; Weatherburn, C.; Ho, P.J.; Woodland, N.; Nassif, N.; Barbaro, P.; Bryant, C.; Hart, D.; et al. Multiple myeloma causes clonal T-cell immunosenescence: Identification of potential novel targets for promoting tumour immunity and implications for checkpoint blockade. Leukemia 2016, 30, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, M.V.; Krasovsky, J.; Osman, K.; Geller, M.D. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J. Exp. Med. 2003, 198, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Sive, J.; Ambrose, J.; Roddie, C.; Counsell, N.; Lach, A.; Abbasian, M.; Popat, R.; Cavenagh, J.D.; Oakervee, H.; et al. RNA-seq of newly diagnosed patients in the PADIMAC study leads to a bortezomib/lenalidomide decision signature. Blood 2018, 132, 2154–2165. [Google Scholar] [CrossRef]
- Ubels, J.; Sonneveld, P.; van Beers, E.H.; Broijl, A.; van Vliet, M.H.; de Ridder, J. Predicting treatment benefit in multiple myeloma through simulation of alternative treatment effects. Nat. Commun. 2018, 9, 2943. [Google Scholar] [CrossRef]
- Korst, C.L.B.M.; van de Donk, N.W.C.J. Should all newly diagnosed MM patients receive CD38 antibody–based treatment? Hematol. Am. Soc. Hematol. Educ. Program. 2020, 259–263. [Google Scholar] [CrossRef]
- Liwing, J.; Uttervall, K.; Lund, J.; Aldrin, A.; Blimark, C.; Carlson, K.; Enestig, J.; Flogegård, M.; Forsberg, K.; Gruber, A.; et al. Improved survival in myeloma patients: Starting to close in on the gap between elderly patients and a matched normal population. Br. J. Haematol. 2014, 164, 684–693. [Google Scholar] [CrossRef]
- Beksac, M.; Seval, G.C.; Kanellias, N.; Coriu, D.; Rosiñol, L.; Ozet, G.; Goranova-Marinova, V.; Unal, A.; Bila, J.; Ozsan, H.; et al. A real world multicenter retrospective study on extramedullary disease from Balkan Myeloma Study Group and Barcelona University: Analysis of parameters that improve outcome. Haematologica 2020, 105, 201–208. [Google Scholar] [CrossRef]
- Park, S.; Lee, S.J.; Jung, C.W.; Jang, J.H.; Kim, S.J.; Kim, W.S.; Kim, K. DCEP for relapsed or refractory multiple myeloma after therapy with novel agents. Ann. Hematol. 2014, 93, 99–105. [Google Scholar] [CrossRef]
- Abdallah, A.O.; Sigle, M.; Mohyuddin, G.R.; Coggins, E.; Remker, C.; Shune, L.; Mahmoudjafari, Z.; McGuirk, J.; Ganguly, S. Outcomes of VD-PACE With Immunomodulatory Agent as a Salvage Therapy for Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020. [Google Scholar] [CrossRef]
- Raje, N.; Berdeja, J.; Lin, Y.; Siegel, D.; Jagannath, S.; Madduri, D.; Liedtke, M.; Rosenblatt, J.; Maus, M.V.; Turka, A.; et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 380, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Topp, M.S.; Duell, J.; Zugmaier, G.; Attal, M.; Moreau, P.; Langer, C.; Krönke, J.; Facon, T.; Salnikov, A.V.; Lesley, R.; et al. Anti-B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J. Clin. Oncol. 2020, 38, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 38, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Bringhen, S.; Voorhees, P.; Plesner, T.; Mellqvist, U.H.; Reeves, B.; Paba-Prada, C.; Zubair, H.; Byrne, C.; Chauhan, D.; et al. Melflufen plus dexamethasone in relapsed and refractory multiple myeloma (O-12-M1): A multicentre, international, open-label, phase 1-2 study. Lancet Haematol. 2020, 7, e395–e407. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).