Biomarkers for Precision Urothelial Carcinoma Diagnosis: Current Approaches and the Application of Single-Cell Technologies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Current Research Gaps in UC Diagnosis
3. FDA-Approved and Investigational UC Biomarkers
3.1. FDA-Approved IVD Tests for UC Diagnosis
3.1.1. Bladder Tumor Antigen (BTA) Assay
3.1.2. Nuclear Matrix Protein 22 (NMP22)
3.1.3. ImmunoCyt/uCyt+ Assay
3.1.4. UroVysion FISH
3.2. Novel/Investigational Biomarkers for UC Detection
3.2.1. Cell-Based Biomarkers
BLCA-4
MCM5
hTERT
CTCs/UETCs
CK-20
CxBladder
Xpert Bladder
Survivin
UroSEEK
AssureMDX
3.2.2. Soluble Biomarkers
UBC Test
CYFRA 21-1
Apo-A1
IL-8
VEGF
CCL18
HA/HAse
sFAS
3.2.3. Cell-Based/Soluble Biomarker
CD44/CD44 Isoform
miRNA Markers
4. Single-Cell Technologies
Applications of Single-Cell Technologies for UC Diagnosis
5. Discussion and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Richters, A.; Aben, K.K.H.; Kiemeney, L.A. The global burden of urinary bladder cancer: An update. World J. Urol. 2020, 38, 1895–1904. [Google Scholar] [CrossRef] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlay, J. Global Cancer Observatory: Cancer Today. Available online: http://gco.iarc.fr/today (accessed on 1 August 2020).
- Lee, C.S.; Yoon, C.Y.; Witjes, J.A. The past, present and future of cystoscopy: The fusion of cystoscopy and novel imaging technology. BJU Int. 2008, 102, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Risk, M.C.; Soubra, A. Diagnostics techniques in nonmuscle invasive bladder cancer. Indian J. Urol. 2015, 31, 283–288. [Google Scholar] [CrossRef]
- Chakraborty, A.; Dasari, S.; Long, W.; Mohan, C. Urine protein biomarkers for the detection, surveillance, and treatment response prediction of bladder cancer. Am. J. Cancer Res. 2019, 9, 1104–1117. [Google Scholar] [PubMed]
- D’Costa, J.J.; Goldsmith, J.C.; Wilson, J.S.; Bryan, R.T.; Ward, D.G. A Systematic Review of the Diagnostic and Prognostic Value of Urinary Protein Biomarkers in Urothelial Bladder Cancer. Bl. Cancer 2016, 2, 301–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodison, S.; Chang, M.; Dai, Y.; Urquidi, V.; Rosser, C.J. A Multi-Analyte Assay for the Non-Invasive Detection of Bladder Cancer. PLoS ONE 2012, 7, e47469. [Google Scholar] [CrossRef]
- Guo, A.; Wang, X.; Shi, J.; Sun, C.; Wan, Z. Bladder tumour antigen (BTA stat) test compared to the urine cytology in the diagnosis of bladder cancer: A meta-analysis. Can. Urol. Assoc. J. 2014, 8, 347–352. [Google Scholar] [CrossRef] [Green Version]
- Glas, A.S.; Roos, D.; Deutekom, M.; Zwinderman, A.H.; Bossuyt, P.M.; Kurth, K.H. Tumor Markers in the Diagnosis of Primary Bladder Cancer. A Systematic Review. J. Urol. 2003, 169, 1975–1982. [Google Scholar] [CrossRef]
- Wang, Z.; Que, H.; Suo, C.; Han, Z.; Tao, J.; Huang, Z.; Ju, X.; Tan, R.; Gu, M. Evaluation of the NMP22 BladderChek test for detecting bladder cancer: A systematic review and meta-analysis. Oncotarget 2017, 8, 100648–100656. [Google Scholar] [CrossRef]
- He, H.; Han, C.; Hao, L.; Zang, G. ImmunoCyt test compared to cytology in the diagnosis of bladder cancer: A meta-analysis. Oncol. Lett. 2016, 12, 83–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Q.; Wu, Y.; Guo, Z.; Gong, R.; Tang, Y.; Yang, K.; Li, X.; Guo, X.; Niu, Y.; Zhao, Y. Urine BLCA-4 exerts potential role in detecting patients with bladder cancers: A pooled analysis of individual studies. Oncotarget 2015, 6, 37500–37510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, J.D.; Dudderidge, T.J.; Wollenschlaeger, A.; Okoturo, O.; Burling, K.; Tulloch, F.; Halsall, I.; Prevost, T.; Prevost, A.T.; Vasconcelos, J.C.; et al. Bladder Cancer Diagnosis and Identification of Clinically Significant Disease by Combined Urinary Detection of Mcm5 and Nuclear Matrix Protein 22. PLoS ONE 2012, 7, e40305. [Google Scholar] [CrossRef] [Green Version]
- E Khalbuss, W.; Goodison, S. Immunohistochemical detection of hTERT in urothelial lesions: A potential adjunct to urine cytology. CytoJournal 2006, 3, 18. [Google Scholar] [CrossRef]
- Allison, D.B.; Sharma, R.; Cowan, M.L.; Vandenbussche, C.J. Evaluation of Sienna Cancer Diagnostics hTERT Antibody on 500 Consecutive Urinary Tract Specimens. Acta Cytol. 2018, 62, 302–310. [Google Scholar] [CrossRef]
- Zhang, X.; Marjani, S.L.; Hu, Z.; Weissman, S.M.; Pan, X.; Wu, S. Single-Cell Sequencing for Precise Cancer Research: Progress and Prospects. Cancer Res. 2016, 76, 1305–1312. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R.; Arora, V.K.; Aggarwal, S.; Bhatia, A.; Singh, N.; Agrawal, V. Cytokeratin-20 immunocytochemistry in voided urine cytology and its comparison with nuclear matrix protein-22 and urine cytology in the detection of urothelial carcinoma. Diagn. Cytopathol. 2011, 40, 755–759. [Google Scholar] [CrossRef]
- I Morsi, M.; I Youssef, A.; E E Hassouna, M.; El-Sedafi, A.S.; A Ghazal, A.; Zaher, E.R. Telomerase activity, cytokeratin 20 and cytokeratin 19 in urine cells of bladder cancer patients. J. Egypt. Natl. Cancer Inst. 2006, 18, 82–92. [Google Scholar]
- Golijanin, D.; Shapiro, A.; Pode, D. Immunostaining of cytokeratin 20 in cells from voided urine for detection of bladder cancer. J. Urol. 2000, 164, 1922–1925. [Google Scholar] [CrossRef]
- Mi, Y.; Zhao, Y.; Shi, F.; Zhang, M.; Wang, C.; Liu, X. Diagnostic accuracy of urine cytokeratin 20 for bladder cancer: A meta-analysis. Asia-Pac. J. Clin. Oncol. 2019, 15, e11–e19. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, P.; Sharples, K.; Dalphin, M.; Davidson, P.; Gilling, P.; Cambridge, L.; Harvey, J.; Toro, T.; Giles, N.; Luxmanan, C.; et al. A Multigene Urine Test for the Detection and Stratification of Bladder Cancer in Patients Presenting with Hematuria. J. Urol. 2012, 188, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Van Valenberg, F.J.P.; Hiar, A.M.; Wallace, E.; Bridge, J.A.; Mayne, D.J.; Beqaj, S.; Sexton, W.J.; Lotan, Y.; Weizer, A.Z.; Jansz, G.K.; et al. Prospective Validation of an mRNA-based Urine Test for Surveillance of Patients with Bladder Cancer. Eur. Urol. 2019, 75, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Casella, R.; Khoddami, S.M.; Hernandez, G.; Sulser, T.; Gasser, T.C.; Lerner, S.P. Urine Detection of Survivin is a Sensitive Marker for the Noninvasive Diagnosis of Bladder Cancer. J. Urol. 2004, 171, 626–630. [Google Scholar] [CrossRef]
- Springer, S.U.; Chen, C.-H.; Pena, M.D.C.R.; Li, L.; Douville, C.; Wang, Y.; Cohen, J.D.; Taheri, D.; Silliman, N.; Schaefer, J.; et al. Non-invasive detection of urothelial cancer through the analysis of driver gene mutations and aneuploidy. eLife 2018, 7. [Google Scholar] [CrossRef]
- Van Kessel, K.E.; Beukers, W.; Lurkin, I.; Der Made, A.Z.-V.; Van Der Keur, K.A.; Boormans, J.L.; Dyrskjøt, L.; Márquez, M.; Ørntoft, T.F.; Real, F.X.; et al. Validation of a DNA Methylation-Mutation Urine Assay to Select Patients with Hematuria for Cystoscopy. J. Urol. 2017, 197, 590–595. [Google Scholar] [CrossRef]
- Beukers, W.; Van Der Keur, K.A.; Kandimalla, R.; Vergouwe, Y.; Steyerberg, E.W.; Boormans, J.L.; Jensen, J.B.; Lorente, J.A.; Real, F.X.; Segersten, U.; et al. FGFR3, TERT and OTX1 as a Urinary Biomarker Combination for Surveillance of Patients with Bladder Cancer in a Large Prospective Multicenter Study. J. Urol. 2017, 197, 1410–1418. [Google Scholar] [CrossRef]
- Ecke, T.H.; Weiß, S.; Stephan, C.; Hallmann, S.; Barski, D.; Otto, T.; Gerullis, H. UBC®Rapid Test for detection of carcinoma in situ for bladder cancer. Tumor Biol. 2017, 39, 1010428317701624. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-L.; Chen, J.; Yan, W.; Zang, D.; Qin, Q.; Deng, A. Diagnostic accuracy of cytokeratin-19 fragment (CYFRA 21-1) for bladder cancer: A systematic review and meta-analysis. Tumor Biol. 2015, 36, 3137–3145. [Google Scholar] [CrossRef]
- Li, C.; Li, H.; Zhang, T.; Li, J.; Liu, L.; Chang, J. Discovery of Apo-A1 as a potential bladder cancer biomarker by urine proteomics and analysis. Biochem. Biophys. Res. Commun. 2014, 446, 1047–1052. [Google Scholar] [CrossRef]
- Li, H.; Li, C.; Wu, H.; Zhang, T.; Wang, J.; Wang, S.; Chang, J. Identification of Apo-A1 as a biomarker for early diagnosis of bladder transitional cell carcinoma. Proteome Sci. 2011, 9, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-T.; Chen, C.-L.; Chen, H.-W.; Chung, T.; Wu, C.-C.; Chen, C.-D.; Hsu, C.-W.; Chen, M.-C.; Tsui, K.-H.; Chang, P.-L.; et al. Discovery of Novel Bladder Cancer Biomarkers by Comparative Urine Proteomics Using iTRAQ Technology. J. Proteome Res. 2010, 9, 5803–5815. [Google Scholar] [CrossRef] [PubMed]
- Abogunrin, F.; O’Kane, H.F.; Ruddock, M.W.; Stevenson, M.; Reid, C.N.; O’Sullivan, J.M.; Anderson, N.H.; O’Rourke, D.; Duggan, B.; Lamont, J.V.; et al. The impact of biomarkers in multivariate algorithms for bladder cancer diagnosis in patients with hematuria. Cancer 2011, 118, 2641–2650. [Google Scholar] [CrossRef] [PubMed]
- Rosser, C.; Charles, J.; Dai, Y.; Miyake, M.; Zhang, G.; Goodison, S. Simultaneous multi-analyte urinary protein assay for bladder cancer detection. BMC Biotechnol. 2014, 14, 24. [Google Scholar] [CrossRef] [Green Version]
- Sheryka, E.; Wheeler, M.A.; Hausladen, D.A.; Weiss, R.M. Urinary interleukin-8 levels are elevated in subjects with transitional cell carcinoma. Urol. 2003, 62, 162–166. [Google Scholar] [CrossRef]
- Urquidi, V.; Chang, M.; Dai, Y.; Kim, J.; Wolfson, E.D.; Goodison, S.; Rosser, C.J. IL-8 as a urinary biomarker for the detection of bladder cancer. BMC Urol. 2012, 12, 12. [Google Scholar] [CrossRef] [Green Version]
- Bian, W.; Xu, Z. Combined assay of CYFRA21-1, telomerase and vascular endothelial growth factor in the detection of bladder transitional cell carcinoma. Int. J. Urol. 2007, 14, 108–111. [Google Scholar] [CrossRef]
- Chen, L.-M.; Chang, M.; Dai, Y.; Chai, K.; Karl, X.; Dyrskjot, L.; Sanchez-Carbayo, M.; Szarvas, T.; Zwarthoff, E.; Lokeshwar, V.; et al. External Validation of a Multiplex Urinary Protein Panel for the Detection of Bladder Cancer in a Multicenter Cohort. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1804–1812. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; He, D.-L.; Ma, Q.; Wan, X.-Y.; Zhu, G.-D.; Li, L.; Luo, Y.; He, H.; Yang, L. Comparison of seven screening methods in the diagnosis of bladder cancer. Chin. Med. J. 2006, 119, 1763–1771. [Google Scholar] [CrossRef]
- Urquidi, V.; Kim, J.; Chang, M.; Dai, Y.; Rosser, C.J.; Goodison, S. CCL18 in a Multiplex Urine-Based Assay for the Detection of Bladder Cancer. PLoS ONE 2012, 7, e37797. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Zhang, Q.; Wang, C.; Shi, F.; Cao, H.; Yu, Y.; Zhang, M.; Liu, X. Hyaluronic acid/Hyaluronidase as biomarkers for bladder cancer: A diagnostic meta-analysis. Neoplasma 2017, 64, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Singh, P.K.; Singh, D.; Dalela, D.; Rath, S.K.; Bhatt, M. Clinical utility of urinary soluble Fas in screening for bladder cancer. Asia-Pac. J. Clin. Oncol. 2014, 12, e215–e221. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Cheng, Y.; Deng, X.; Yang, X.; Li, P.; Wang, Z.; Li, P.; Tao, J.; Zhang, X. Urine microRNAs as biomarkers for bladder cancer: A diagnostic meta-analysis. OncoTargets Ther. 2015, 8, 2089–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, H.-B.; Yu, J.-X.; Yu, J.-X.; Feng, Z.; Zhang, C.; Li, G.-Y.; Zhao, R.-N.; Yang, X.-B. Diagnostic significance of microRNAs as novel biomarkers for bladder cancer: A meta-analysis of ten articles. World J. Surg. Oncol. 2017, 15, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eissa, S.; Zohny, S.F.; Swellam, M.; Mahmoud, M.H.; El-Zayat, T.M.; Salem, A.M. Comparison of CD44 and cytokeratin 20 mRNA in voided urine samples as diagnostic tools for bladder cancer. Clin. Biochem. 2008, 41, 1335–1341. [Google Scholar] [CrossRef]
- Woodman, A.C.; Goodison, S.; Drake, M.; Noble, J.; Tarin, D. Noninvasive diagnosis of bladder carcinoma by enzyme-linked immunosorbent assay detection of CD44 isoforms in exfoliated urothelia. Clin. Cancer Res. 2000, 6, 2381–2392. [Google Scholar]
- Smith, Z.L.; Guzzo, T.J. Urinary markers for bladder cancer. F1000Prime Rep. 2013, 5, 21. [Google Scholar] [CrossRef] [Green Version]
- Yafi, F.A.; Brimo, F.; Steinberg, J.; Aprikian, A.G.; Tanguay, S.; Kassouf, W. Prospective analysis of sensitivity and specificity of urinary cytology and other urinary biomarkers for bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2015, 33, 66.e25–66.e31. [Google Scholar] [CrossRef]
- Chan, K.M.; Gleadle, J.M.; Li, J.Y.; Vasilev, K.; MacGregor, M. Shedding Light on Bladder Cancer Diagnosis in Urine. Diagnostics 2020, 10, 383. [Google Scholar] [CrossRef]
- Kinders, R.; Jones, T.; Root, R.; Bruce, C.; Murchison, H.; Corey, M.; Williams, L.; Enfield, D.; Hass, G.M. Complement factor H or a related protein is a marker for transitional cell cancer of the bladder. Clin. Cancer Res. 1998, 4, 2511–2520. [Google Scholar]
- Konety, B.R. Molecular markers in bladder cancer: A critical appraisal. Urol. Oncol. Semin. Orig. Investig. 2006, 24, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Gonzalgo, M.L.; Darwiche, F.; Parekh, D.J. Biomarkers for non-muscle invasive bladder cancer: Current tests and future promise. Indian J. Urol. 2015, 31, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Leiblich, A. Recent Developments in the Search for Urinary Biomarkers in Bladder Cancer. Curr. Urol. Rep. 2017, 18, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, K.L.; Berry, A.; Konety, B.R. Diagnostic Utility of the ImmunoCyt/uCyt+ Test in Bladder Cancer. Rev. Urol. 2006, 8, 190–197. [Google Scholar]
- Fradet, Y.; Lockhard, C. Performance characteristics of a new monoclonal antibody test for bladder cancer: ImmunoCyt trade mark. Can. J. Urol. 1997, 4, 400–405. [Google Scholar]
- Toma, M.I.; Friedrich, R.E.; Hautmann, S.H.; Erbersdobler, A.; Hellstern, A.; Huland, H. Comparison of the ImmunoCyt test and urinary cytology with other urine tests in the detection and surveillance of bladder cancer. World J. Urol. 2004, 22, 145–149. [Google Scholar] [CrossRef]
- Kamat, A.M.; Hegarty, P.K.; Gee, J.R.; Clark, P.E.; Svatek, R.S.; Hegarty, N.; Shariat, S.F.; Xylinas, E.; Schmitz-Dräger, B.J.; Lotan, Y.; et al. ICUD-EAU International Consultation on Bladder Cancer 2012: Screening, Diagnosis, and Molecular Markers. Eur. Urol. 2013, 63, 4–15. [Google Scholar] [CrossRef]
- Forsburg, S.L. Eukaryotic MCM Proteins: Beyond Replication Initiation. Microbiol. Mol. Biol. Rev. 2004, 68, 109–131. [Google Scholar] [CrossRef] [Green Version]
- Williams, G.H.; Stoeber, K. Cell cycle markers in clinical oncology. Curr. Opin. Cell Biol. 2007, 19, 672–679. [Google Scholar] [CrossRef]
- Stoeber, K.; Swinn, R.; Prevost, A.T.; De Clive-Lowe, P.; Halsall, I.; Dilworth, S.M.; Marr, J.; Turner, W.H.; Bullock, N.; Doble, A.; et al. Diagnosis of Genito-Urinary Tract Cancer by Detection of Minichromosome Maintenance 5 Protein in Urine Sediments. J. Natl. Cancer Inst. 2002, 94, 1071–1079. [Google Scholar] [CrossRef]
- Zhang, Q.; Kim, N.-K.; Feigon, J. Architecture of human telomerase RNA. Proc. Natl. Acad. Sci. USA 2011, 108, 20325–20332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantel, K.; Alix-Panabières, C.; Riethdorf, S. Cancer micrometastases. Nat. Rev. Clin. Oncol. 2009, 6, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Gao, P.; Sun, J.; Chen, X.; Song, Y.; Zhao, J.; Xu, H.; Wang, Z.-N. Clinicopathological and prognostic significance of circulating tumor cells in patients with gastric cancer: A meta-analysis. Int. J. Cancer 2014, 136, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Q.; Gong, L.; Zhang, T.; Ye, J.; Chai, L.; Ni, C.; Ni, C. Prognostic value of circulating tumor cells in metastatic breast cancer: A systemic review and meta-analysis. Clin. Transl. Oncol. 2015, 18, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, N.N.; Aigner, M.; Thorlund, K.; Mollberg, N.; Motschall, E.; Jensen, K.; Diener, M.K.; Büchler, M.W.; Koch, M.; Weitz, J. Meta-analysis Shows That Detection of Circulating Tumor Cells Indicates Poor Prognosis in Patients With Colorectal Cancer. Gastroenterology 2010, 138, 1714–1726.e13. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, G.; Cheng, B.; Chen, F.; Wang, Z.; Chen, Y.; Wang, Y.; Xiong, B. Circulating Tumor Cells (CTCs) Detected by RT-PCR and Its Prognostic Role in Gastric Cancer: A Meta-Analysis of Published Literature. PLoS ONE 2014, 9, e99259. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, W.; Deng, Q.; Tang, S.; Wang, P.; Xu, P.; Wang, J.; Yu, M. The prognostic and diagnostic value of circulating tumor cells in bladder cancer and upper tract urothelial carcinoma: A meta-analysis of 30 published studies. Oncotarget 2017, 8, 59527–59538. [Google Scholar] [CrossRef] [Green Version]
- Rink, M.; Schwarzenbach, H.; Vetterlein, M.W.; Riethdorf, S.; Soave, A. The current role of circulating biomarkers in non-muscle invasive bladder cancer. Transl. Androl. Urol. 2019, 8, 61–75. [Google Scholar] [CrossRef]
- Bhatia, A.; Dey, P.; Kumar, Y.; Gautam, U.; Kakkar, N.; Srinivasan, R.; Nijhawan, R. Expression of cytokeratin 20 in urine cytology smears: A potential marker for the detection of urothelial carcinoma. Cytopathology. 2007, 18, 84–86. [Google Scholar] [CrossRef]
- Lin, S.; Hirschowitz, S.L.; Williams, C.; Shintako, P.; Said, J.; Rao, J.Y. Cytokeratin 20 as an immunocytochemical marker for detection of urothelial carcinoma in atypical cytology: Preliminary retrospective study on archived urine slides. Cancer Detect. Prev. 2001, 25, 202–209. [Google Scholar]
- Mai, K.T.; Teo, I.; Robertson, S.J.; Marginean, C.; Islam, S.; Yazdi, H.M. Immunostaining as a Diagnostic Aid in Cytopathologic Study of Upper Urinary Tract Urothelial Carcinoma. Acta Cytol. 2009, 53, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Bin Lim, S.; Lim, C.T.; Lim, W.T. Single-Cell Analysis of Circulating Tumor Cells: Why Heterogeneity Matters. Cancers 2019, 11, 1595. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.; Gallagher, L.; Rohan, S. Survivin: Role in Diagnosis, Prognosis, and Treatment of Bladder Cancer. Adv. Anat. Pathol. 2006, 13, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Ashfaq, R.; Karakiewicz, P.I.; Saeedi, O.; Sagalowsky, A.I.; Lotan, Y. Survivin expression is associated with bladder cancer presence, stage, progression, and mortality. Cancer 2007, 109, 1106–1113. [Google Scholar] [CrossRef]
- Pena, M.D.C.R.; Springer, S.U.; Taheri, D.; Li, L.; Tregnago, A.C.; Eich, M.-L.; Eltoum, I.-E.A.; Vandenbussche, C.J.; Papadopoulos, N.; Kinzler, K.W.; et al. Performance of novel non-invasive urine assay UroSEEK in cohorts of equivocal urine cytology. Virchows Archiv 2020, 476, 423–429. [Google Scholar] [CrossRef]
- Van Kessel, K.E.M.; Van Neste, L.; Lurkin, I.; Zwarthoff, E.C.; Van Criekinge, W. Evaluation of an Epigenetic Profile for the Detection of Bladder Cancer in Patients with Hematuria. J. Urol. 2016, 195, 601–607. [Google Scholar] [CrossRef]
- Southgate, J.; Harnden, P.; Trejdosiewicz, L.K. Cytokeratin expression patterns in normal and malignant urothelium: A review of the biological and diagnostic implications. Histol. Histopathol. 1999, 14, 657–664. [Google Scholar]
- Pichler, R.; Tulchiner, G.; Fritz, J.; Schaefer, G.; Horninger, W.; Heidegger, I. Urinary UBC Rapid and NMP22 Test for Bladder Cancer Surveillance in Comparison to Urinary Cytology: Results from a Prospective Single-Center Study. Int. J. Med Sci. 2017, 14, 811–819. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.; Park, Y.; Cho, Y.; Kim, Y.R.; Kim, H.-S. Diagnostic values of urine CYFRA21-1, NMP22, UBC, and FDP for the detection of bladder cancer. Clin. Chim. Acta 2012, 414, 93–100. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Chen, H.-W.; Domański, D.; Smith, D.S.; Liang, K.-H.; Wu, C.-C.; Chen, C.-L.; Chung, T.; Chen, M.-C.; Chang, Y.-S.; et al. Multiplexed quantification of 63 proteins in human urine by multiple reaction monitoring-based mass spectrometry for discovery of potential bladder cancer biomarkers. J. Proteom. 2012, 75, 3529–3545. [Google Scholar] [CrossRef]
- Koçak, H.; Oner-Iyidogan, Y.; Koçak, T.; Oner, P. Determination of diagnostic and prognostic values of urinary interleukin-8, tumor necrosis factor-α, and leukocyte arylsulfatase-A activity in patients with bladder cancer. Clin. Biochem. 2004, 37, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Sağnak, A.L.; Ersoy, H.; Ozok, U.; Senturk, B.; Erçil, H.; Bahar, G.; Öztürk, E. Predictive Value of Urinary Interleukin-8 Cutoff Point for Recurrences After Transurethral Resection Plus Induction Bacillus Calmette-Guérin Treatment in Non–Muscle-Invasive Bladder Tumors. Clin. Genitourin. Cancer 2009, 7, E16–E23. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.C.; Volpert, O.V.; Ivanovich, M.; Bouck, N.P. Molecular mediators of angiogenesis in bladder cancer. Cancer Res. 1998, 58, 1298–1304. [Google Scholar] [PubMed]
- Kumari, N.; Agrawal, U.; Mishra, A.K.; Kumar, A.; Vasudeva, P.; Mohanty, N.K.; Saxena, S. Predictive role of serum and urinary cytokines in invasion and recurrence of bladder cancer. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morera, D.S.; Hennig, M.S.; Talukder, A.; Lokeshwar, S.D.; Wang, J.; Garcia-Roig, M.; Ortiz, N.; Yates, T.J.; E Lopez, L.; Kallifatidis, G.; et al. Hyaluronic acid family in bladder cancer: Potential prognostic biomarkers and therapeutic targets. Br. J. Cancer 2017, 117, 1507–1517. [Google Scholar] [CrossRef]
- Hautmann, S.H.; Lokeshwar, V.B.; Schroeder, G.L.; Civantos, F.; Duncan, R.C.; Gnann, R.; Friedrich, M.G.; Soloway, M.S. Elevated Tissue Expression of Hyaluronic Acid and Hyaluronidase Validates the ha-Haase urine test for bladder cancer. J. Urol. 2001, 165, 2068–2074. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Obek, C.; Soloway, M.S.; Block, N.L. Tumor-associated hyaluronic acid: A new sensitive and specific urine marker for bladder cancer. Cancer Res. 1997, 57, 773–777. [Google Scholar]
- Svatek, R.S.; Herman, M.P.; Lotan, Y.; Casella, R.; Hsieh, J.-T.; I Sagalowsky, A.; Shariat, S.F. Soluble Fas—A promising novel urinary marker for the detection of recurrent superficial bladder cancer. Cancer 2006, 106, 1701–1707. [Google Scholar] [CrossRef]
- Desai, S.; Lim, S.D.; Jimenez, R.E.; Chun, T.; Keane, T.E.; McKenney, J.K.; Zavala-Pompa, A.; Cohen, C.; Young, R.H.; Amin, M.B. Relationship of Cytokeratin 20 and CD44 Protein Expression with WHO/ISUP Grade in pTa and pT1 Papillary Urothelial Neoplasia. Mod. Pathol. 2000, 13, 1315–1323. [Google Scholar] [CrossRef]
- Yu, Q.; Stamenkovic, I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999, 13, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Liu, X.; Wu, Y.; Wu, Q.; Wang, Q.; Yang, Z.; Li, L. MicroRNAs in biofluids are novel tools for bladder cancer screening. Oncotarget 2017, 8, 32370–32379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pardini, B.; Cordero, F.; Naccarati, A.; Viberti, C.; Birolo, G.; Oderda, M.; Di Gaetano, C.; Arigoni, M.; Martina, F.; Calogero, R.A.; et al. microRNA profiles in urine by next-generation sequencing can stratify bladder cancer subtypes. Oncotarget 2018, 9, 20658–20669. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Cui, Z.; Liu, Y.; Bai, Y.; Lan, F. MicroRNAs as Biomarkers for the Diagnostics of Bladder Cancer: A Meta-Analysis. Clin. Lab. 2015, 59, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Kim, J.K.; Svensson, V.; Marioni, J.C.; Teichmann, S.A. The Technology and Biology of Single-Cell RNA Sequencing. Mol. Cell 2015, 58, 610–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winterhoff, B.J.; Maile, M.; Mitra, A.K.; Sebe, A.; Bazzaro, M.; Geller, M.A.; Abrahante, J.E.; Klein, M.; Hellweg, R.; Mullany, S.A.; et al. Single cell sequencing reveals heterogeneity within ovarian cancer epithelium and cancer associated stromal cells. Gynecol. Oncol. 2017, 144, 598–606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, S.J.; Punt, C.J.A.; Iannotti, N.; Saidman, B.H.; Sabbath, K.D.; Gabrail, N.Y.; Picus, J.; Morse, M.; Mitchell, E.; Miller, M.C.; et al. Relationship of Circulating Tumor Cells to Tumor Response, Progression-Free Survival, and Overall Survival in Patients With Metastatic Colorectal Cancer. J. Clin. Oncol. 2008, 26, 3213–3221. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [Green Version]
- De Bono, J.S.; Scher, H.I.; Montgomery, R.B.; Parker, C.; Miller, M.C.; Tissing, H.; Doyle, G.; Terstappen, L.W.; Pienta, K.J.; Raghavan, D. Circulating Tumor Cells Predict Survival Benefit from Treatment in Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2008, 14, 6302–6309. [Google Scholar] [CrossRef] [Green Version]
- Riethdorf, S.; Fritsche, H.; Müller, V.; Rau, T.; Schindlbeck, C.; Rack, B.; Janni, W.; Coith, C.; Beck, K.; Jänicke, F.; et al. Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Metastatic Breast Cancer: A Validation Study of the CellSearch System. Clin. Cancer Res. 2007, 13, 920–928. [Google Scholar] [CrossRef] [Green Version]
- Riethdorf, S.; Soave, A.; Rink, M. The current status and clinical value of circulating tumor cells and circulating cell-free tumor DNA in bladder cancer. Transl. Androl. Urol. 2017, 6, 1090–1110. [Google Scholar] [CrossRef]
- Chen, A.; Fu, G.; Xu, Z.; Sun, Y.; Chen, X.; Cheng, K.S.; Neoh, K.H.; Tang, Z.; Chen, S.; Liu, M.; et al. Detection of Urothelial Bladder Carcinoma via Microfluidic Immunoassay and Single-Cell DNA Copy-Number Alteration Analysis of Captured Urinary-Exfoliated Tumor Cells. Cancer Res. 2018, 78, 4073–4085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.-G.; Kong, M.-Q.; Zhou, S.; Sheng, Y.-F.; Wang, P.; Yu, T.; Inci, F.; Kuo, W.P.; Li, L.-J.; Demirci, U.; et al. An integrated double-filtration microfluidic device for isolation, enrichment and quantification of urinary extracellular vesicles for detection of bladder cancer. Sci. Rep. 2017, 7, srep46224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, S.; Yu, J.; Zhao, Y.; Li, H.; Zheng, F.; Liu, N.; Li, D.; Sun, X. A Microfluidic Detection System for Bladder Cancer Tumor Cells. Micromachines 2019, 10, 871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eissa, S.; Habib, H.; Ali, E.; Kotb, Y. Evaluation of urinary miRNA-96 as a potential biomarker for bladder cancer diagnosis. Med. Oncol. 2014, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Mengual, L.; Lozano, J.J.; Ingelmo-Torres, M.; Gazquez, C.; Ribal, M.J.; Alcaraz, A. Using microRNA profiling in urine samples to develop a non-invasive test for bladder cancer. Int. J. Cancer 2013, 133, 2631–2641. [Google Scholar] [CrossRef]
- Urquidi, V.; Netherton, M.; Gomes-Giacoia, E.; Serie, D.J.; Eckel-Passow, J.; Rosser, C.J.; Goodison, S. A microRNA biomarker panel for the non-invasive detection of bladder cancer. Oncotarget 2016, 7, 86290–86299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.-Z.; Lau, K.-M.; Chan, E.S.Y.; Wang, G.; Szeto, C.-C.; Wong, K.; Choy, R.K.W.; Ng, C.-F. Cell-Free Urinary MicroRNA-99a and MicroRNA-125b Are Diagnostic Markers for the Non-Invasive Screening of Bladder Cancer. PLoS ONE 2014, 9, e100793. [Google Scholar] [CrossRef]
- Feber, A.; Dhami, P.; Dong, L.; De Winter, P.; Tan, W.S.; Martínez-Fernández, M.; Paul, D.S.; Hynes-Allen, A.; Rezaee, S.; Gurung, P.; et al. UroMark—a urinary biomarker assay for the detection of bladder cancer. Clin. Epigenetics 2017, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Su, S.-F.; Abreu, A.L.D.C.; Chihara, Y.; Tsai, Y.; Andreu-Vieyra, C.; Daneshmand, S.; Skinner, E.C.; Jones, P.A.; Siegmund, K.; Liang, G. A Panel of Three Markers Hyper- and Hypomethylated in Urine Sediments Accurately Predicts Bladder Cancer Recurrence. Clin. Cancer Res. 2014, 20, 1978–1989. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, Y.; Ye, R.; Zhang, D.; Li, Q.; An, D.; Fang, L.; Lin, Y.; Hou, Y.; Xu, A.; et al. An epigenetic biomarker combination of PCDH17 and POU4F2 detects bladder cancer accurately by methylation analyses of urine sediment DNA in Han Chinese. Oncotarget 2016, 7, 2754–2764. [Google Scholar] [CrossRef]
 Created with Biorender.com.
Created with Biorender.com.
 Created with Biorender.com.
Created with Biorender.com.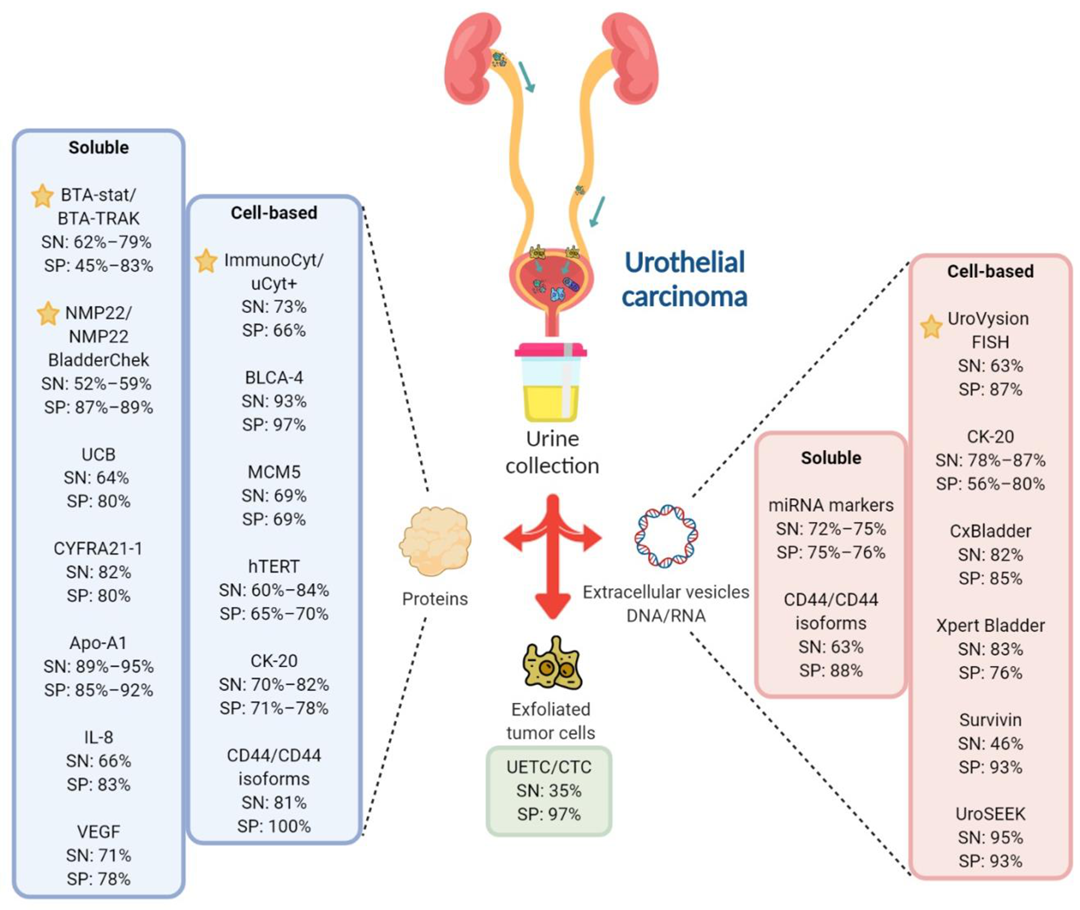

| Test | Biomarker | Type | Sample Material | Method | SN (%) | SP (%) | P (n) | C (n) | Remark | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| BTA-stat | Human complement factor H-related protein | Soluble | Protein | Dipstick immunoassay or POC test | 64–69 * | 73–77 * | 3175 | – | High false positive rates | [9] (meta- analysis) |
| BTA-Trak | Soluble | Protein | ELISA | 79 | 83 | 64 | 63 | [8] | ||
| 62–71 * | 45–81 * | 829 | – | [10] (meta- analysis) | ||||||
| NMP22/ NMP22 BladderChek | NMP22 | Soluble | Protein | ELISA or POC test | 52–59 * | 87–89 * | 5291 | – | Better at detecting high-grade UC; false positives in hematuria or inflammatory bladder conditions | [11] (meta- analysis) |
| Immuno-cyt/ uCyt+ | High-MW form of glycosylated CEA and mucin-like antigen | Cellular | Protein | Immunocyto chemistry | 73 * | 66 * | 1602 | – | Unaffected by hematuria or inflammatory conditions; superior sensitivity to detect early pathological stage than cytology; test results highly dependent on specimen stability and handling | [12] (meta- analysis) |
| UroVysion FISH | Aneuploidy for chromosomes 3, 7, and 17, and loss of 9p21 locus | Cellular | DNA | FISH | 63 * | 87 * | 3445 | – | Complex assay that requires skilled cytopathologist; low sensitivity in the detection of low-grade UC; high rate of false positives; lack of consensus on the criteria to evaluate abnormal cells | [13] (meta- analysis) |
| Biomarker/ Test | Description | Type | Sample Material | Method | SN (%) | SP (%) | P (n) | C (n) | Remarks | References |
|---|---|---|---|---|---|---|---|---|---|---|
| BLCA-4 | Nuclear transcription factor | Cellular | Protein | ELISA | 93 * | 97 * | 1119 (total participants) | High sensitivity and specificity for UC detection; further validation required | [14] (meta- analysis) | |
| MCM5 ^ | MCM family of proteins that assemble into hexameric complexes with DNA helicase activity; vital for DNA synthesis | Cellular | Protein | Immuno- fluorometric assay | 69 | 69 | 210 | 1354 | Mix of low and high-grade patients; higher sensitivity but similar specificity compared with cytology | [15] |
| hTERT ^ | Catalytic subunit of telomerase, a ribonucleoprotein that synthesizes telomeres at the ends of chromosomes, thus ensuring genomic stability | Cellular | Protein | Immunocytochemistry | 84.8 | 65.2 | 101 | – | Higher sensitivity than cytology, regardless of tumor grade and stage; lower specificity than cytology; may be used as an adjunct to cytology to identify patients with increased risk of high-grade UC | [16] |
| 60.6 | 70.4 | 500 | – | [17] | ||||||
| CTCs ^ | Malignant epithelial cells that are shed from the primary tumor into bodily fluids | Cellular | Protein | Immuno magnetic enrichment (CellSearch) | 35 * | 97 * | 2161 | – | The only FDA-approved CTC test; Possibility of staining with different antibodies which allows for the identification of new CTC biomarkers | [18] (meta- analysis) |
| CK-20 | Cytokeratins are components of cytoplasmic intermediate filaments found in epithelial cells; CK-20 is expressed in urothelial carcinoma but not normal urothelial cells | Cellular | Protein | Immuno- staining | 70 | 71 | 42 | 17 | Higher sensitivity than urine cytology as a UC screening test, especially for low-grade low-stage tumor | [19] |
| 80 | 78 | 50 | 20 | [20] | ||||||
| 82 | 77 | 174 | – | [21] | ||||||
| RNA (mRNA) | RT-PCR | 78–87 | 56–80 | 3473 | – | Poor performance for low-grade tumors | [22] (pooled analysis) | |||
| CxBladder | mRNA expression of genes (IGF, HOXA, MDK, CDC, and IL8R) | Cellular | RNA (mRNA) | RT-qPCR | 82 | 85 | 66 | 419 | Can distinguish between low-grade Ta tumors and other detected UC with high sensitivity and specificity | [23] |
| Xpert Bladder ^ | mRNA expression of genes (CRH, IGF2, UPK1B, ANXA10, and ABL1) | Cellular | RNA (mRNA) | RT-qPCR | 83 | 76 | 239 | 508 | Mainly high-grade patients | [24] |
| Survivin | Inhibitor of apoptosis gene | Cellular | DNA | Bio-dot test | 64 | 93 | 117 | 92 | High sensitivity for detecting low-stage and low-grade UC; more accurate than cytology and NMP22 test; requires further validation | [25] |
| UroSEEK | Mutations in FGFR3, TP53, ERBB2, CDKN2A, KRAS, HRAS, MET, PIK3CA, MLL, and VHL and TERTp alterations | Cellular | DNA | Massively parallel sequencing- based assay (NGS/ Sanger sequencing) | 95 | 93 | 570 | – | Higher performance than urine cytology in low-grade tumors | [26] |
| AssureMDX | Mutation analysis in FGFR3, TERT, and HRAS genes and methylation analysis in OTX1, ONECUT2, and TWIST1 genes | Cellular | DNA | PCR | 93 | 86 | 97 | 103 | Mix of high and low-grade patients tested | [27] |
| 57–83 | 59 | 977 | – | Patients with primary NMIBC | [28] | |||||
| UBC ^ | Soluble fragments of cytoskeletal proteins 8 and 18 | Soluble | Protein | ELISA or POC assay | 64.4 * | 80.3 * | 753 | 1072 | Increased sensitivity when used in combination with cytology; allows separation of high vs. low-grade UC | [7,29] |
| CYFRA 21-1 | Soluble fragments of cytoskeletal protein cytokeratin 19 | Soluble | Protein | Immunoradio-metric assay or ELISA | 82 * | 80 * | 1262 | 1233 | High sensitivity for detection of high-grade and CIS tumors, poor sensitivity for early detection; generates false positive in inflammatory bladder conditions | [30] (Meta- analysis) |
| Apo-A1 | Major high-density lipoprotein | Soluble | Protein | ELISA | 89 | 85 | 223 | 153 | Apolipoproteins are abundant in plasma; hence, urinary concentrations are affected by hematuria | [31] |
| 91.6 | 85.7 | 40 | 24 | [32] | ||||||
| 95 | 92 | 86 | 62 | [33] | ||||||
| IL-8 | Leukocyte chemoattractant and angiogenic factor associated with inflammation and carcinogenesis | Soluble | Protein | ELISA | 66.4 * | 83.1 * | 225 | 273 | Urinary concentrations elevated in urothelial cell carcinoma | [34,35,36,37] |
| VEGF | Tumor angiogenesis factor | Soluble | Protein | ELISA | 71.4 * | 78.1 * | 509 | 389 | Secreted in urine by UC cells | [8,34,35,38,39,40] |
| CCL18 | Cytokine involved in immunoregulatory and inflammatory processes; promotes cancer cells invasiveness | Soluble | Protein | ELISA | 88 | 86 | 64 | 63 | 55 high-grade, 9 low-grade | [41] |
| Hyaluronidase/hyaluronic acid | Glycosidase that mainly degrades hyaluronic acid/glycosaminoglycan known to promote tumor metastasis and help avoid immune surveillance | Soluble | Protein | ELISA-like assay/zymography | 90.8 * | 82.5 * | 981 participants | May permit early detection; high sensitivity and specificity for detection of both primary and recurrent tumors; further validation in larger, multi-center trials are required | [42] (meta- analysis) | |
| sFAS | Anti-apoptotic protein released by UC cells | Soluble | Protein | ELISA | 88 | 89.1 | 117 | 74 | Better sensitivity in detecting low-grade UC than cytology | [43] |
| miRNA markers | Short non-coding RNAs that regulate gene expression by acting at the post-transcriptional level | Soluble or cellular | RNA (miRNA) | RT-PCR/NGS | 75 * | 75 * | 719 | 494 | Multi-miRNA assays have higher diagnostic sensitivity than single miRNA assays | [44] (meta- analysis) |
| 72 * | 76 * | 1556 | 1347 | [45] (meta- analysis) | ||||||
| CD44/CD44 isoforms | Ubiquitously expressed transmembrane glycoprotein involved in cell–cell interactions, cell adhesion and migration | Soluble | RNA (mRNA) | RT-PCR | 63.1 | 88.9 | 136 | 20 | 111 histological diagnosed UC, 25 benign urological disorders | [46] |
| Cellular | Protein | ELISA | 81 | 100 | 65 | 53 | Presence of hematuria can interfere with the assay | [47] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; He, G.; Goh, S.; Low, A.W.X.; Tay, K.J.; Lim, T.K.H.; Yeong, J.; Khor, L.Y.; Lim, T.S. Biomarkers for Precision Urothelial Carcinoma Diagnosis: Current Approaches and the Application of Single-Cell Technologies. Cancers 2021, 13, 260. https://doi.org/10.3390/cancers13020260
Hong M, He G, Goh S, Low AWX, Tay KJ, Lim TKH, Yeong J, Khor LY, Lim TS. Biomarkers for Precision Urothelial Carcinoma Diagnosis: Current Approaches and the Application of Single-Cell Technologies. Cancers. 2021; 13(2):260. https://doi.org/10.3390/cancers13020260
Chicago/Turabian StyleHong, Michelle, George He, Siting Goh, Alvin Wei Xiang Low, Kae Jack Tay, Tony Kiat Hon Lim, Joe Yeong, Li Yan Khor, and Tong Seng Lim. 2021. "Biomarkers for Precision Urothelial Carcinoma Diagnosis: Current Approaches and the Application of Single-Cell Technologies" Cancers 13, no. 2: 260. https://doi.org/10.3390/cancers13020260
APA StyleHong, M., He, G., Goh, S., Low, A. W. X., Tay, K. J., Lim, T. K. H., Yeong, J., Khor, L. Y., & Lim, T. S. (2021). Biomarkers for Precision Urothelial Carcinoma Diagnosis: Current Approaches and the Application of Single-Cell Technologies. Cancers, 13(2), 260. https://doi.org/10.3390/cancers13020260






