Evidence-Based Exercise Recommendations to Improve Mental Wellbeing in Women with Breast Cancer during Active Treatment: A Systematic Review and Meta-Analysis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Data Extraction
2.4. Risk of Bias and Quality Assessment within Studies
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Risk of Bias and Quality Assessment within Studies
3.3. Characteristics of Included Trials
3.4. Participants
3.5. Interventions
3.6. Outcome Measures
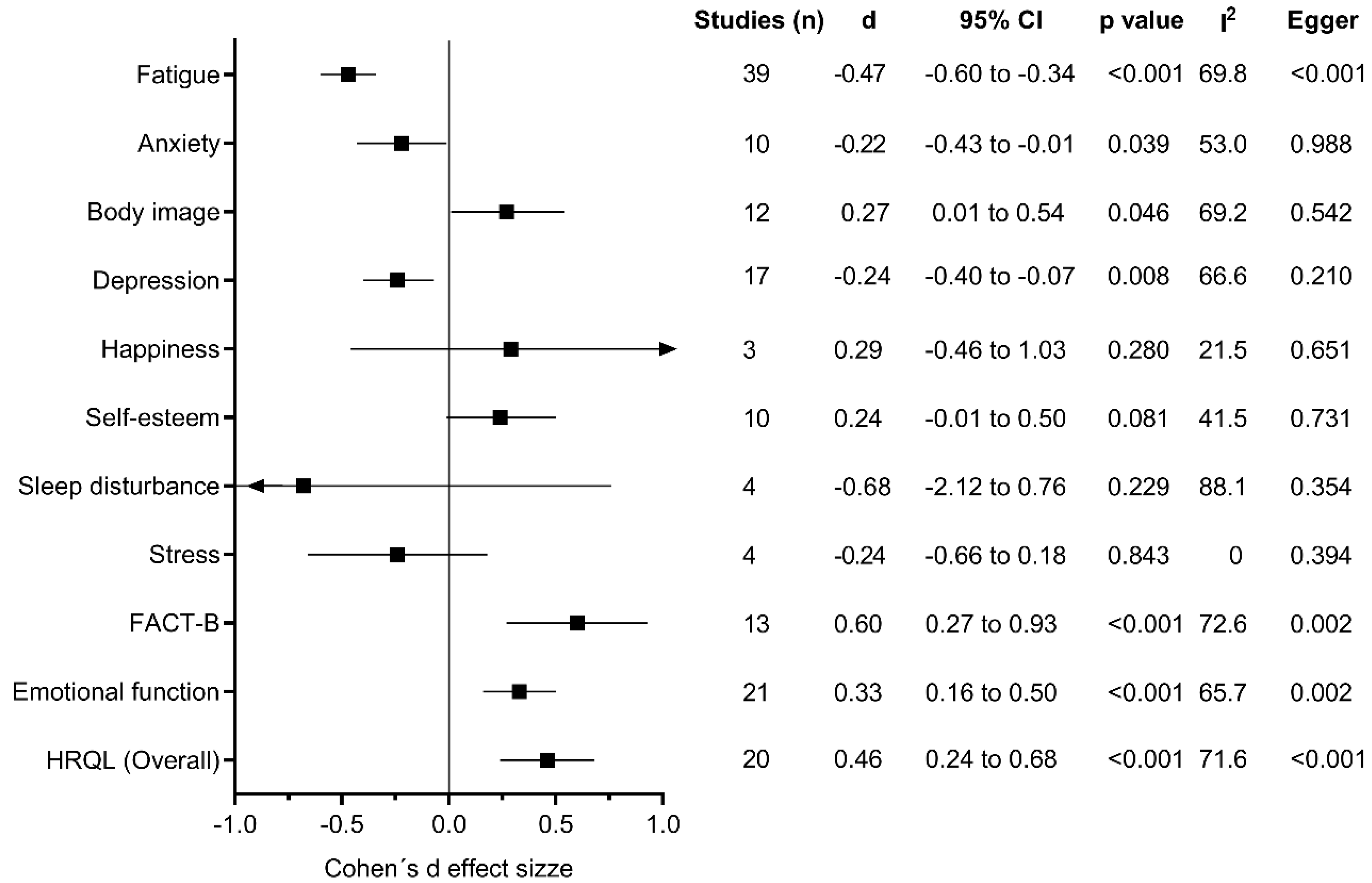
3.7. Summary of Evidence and Heterogeneity for Meta-Analysis
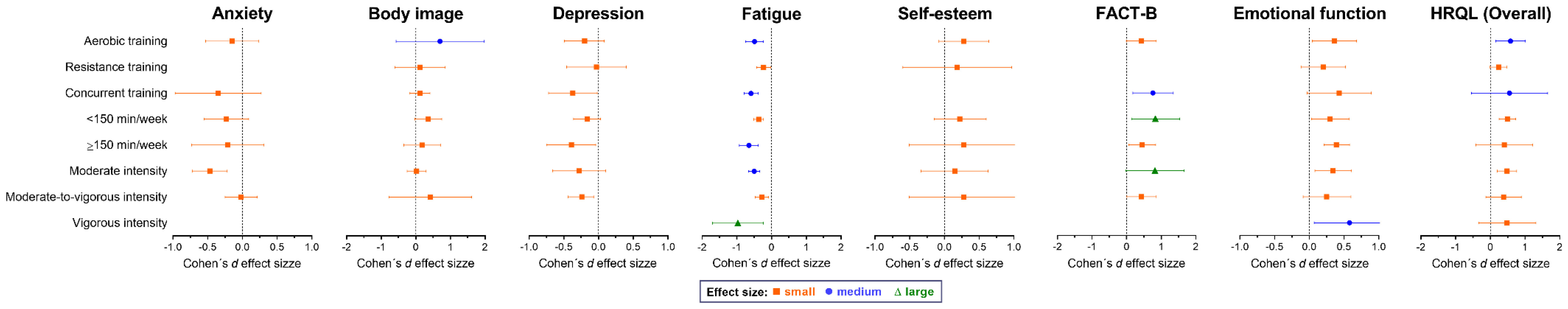
3.8. Subgroup Analysis
3.9. Meta-Regression Analysis
3.10. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Cancer Institute, Surveillance, Epidemiology and ERP (SEER). Cancer Stat Facts: Female Breast Cancer. Available online: http://seer.cancer.gov/statfacts/html/breast.html (accessed on 26 June 2020).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeSantis, C.E.; Ma, J.; Gaudet, M.M.; Newman, L.A.; Mph, K.D.M.; Sauer, A.G.; Jemal, A.; Siegel, R.L. Breast cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Kamińska, M.; Ciszewski, T.; Łopacka-Szatan, K.; Miotla, P.; Starosławska, E. Breast cancer risk factors. Menopause Rev. 2015, 3, 196–202. [Google Scholar] [CrossRef] [Green Version]
- Moo, T.-A.; Sanford, R.; Dang, C.; Morrow, M. Overview of Breast Cancer Therapy. PET Clin. 2018, 13, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.Y.T.; Ho, J.W.C.; Hui, B.P.H.; Lee, A.M.; Macfarlane, D.J.; Leung, S.S.K.; Cerin, E.; Chan, W.Y.Y.; Leung, I.P.F.; Lam, S.H.S.; et al. Physical activity for cancer survivors: Meta-analysis of randomised controlled trials. BMJ 2012, 344, e70. [Google Scholar] [CrossRef] [Green Version]
- Stout, N.L.; Baima, J.; Swisher, A.K.; Winters-Stone, K.M.; Welsh, J. A Systematic Review of Exercise Systematic Reviews in the Cancer Literature (2005–2017). PM&R 2017, 9, S347–S384. [Google Scholar] [CrossRef]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Supervised exercise reduces cancer-related fatigue: A systematic review. J. Physiother. 2015, 61, 3–9. [Google Scholar] [CrossRef] [Green Version]
- Dennett, A.M.; Peiris, C.L.; Shields, N.; Prendergast, L.A.; Taylor, N.F. Moderate-intensity exercise reduces fatigue and improves mobility in cancer survivors: A systematic review and meta-regression. J. Physiother. 2016, 62, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [Green Version]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Midtgaard, J.; Hammer, N.M.; Andersen, C.; Larsen, A.; Bruun, D.-M.; Jarden, M. Cancer survivors’ experience of exercise-based cancer rehabilitation—A meta-synthesis of qualitative research. Acta Oncol. 2015, 54, 609–617. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Choi, M.; Choi, J.; Kang, M.; Jo, A.; Chung, S.H.; Sim, S.H.; Kim, Y.J.; Yang, E.J.; Yu, S.-Y. Supervised Physical Rehabilitation in the Treatment of Patients with Advanced Cancer: A Systematic Review and Meta-analysis. J. Korean Med. Sci. 2020, 35, e242. [Google Scholar] [CrossRef]
- Turner, R.R.; Steed, L.; Quirk, H.; Greasley, R.U.; Saxton, J.M.; Taylor, S.J.; Rosario, D.J.; Thaha, M.A.; Bourke, L. Interventions for promoting habitual exercise in people living with and beyond cancer (Review). Cochrane Database Syst. Rev. 2018, 9, 128. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Prisma Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Robinson, K.; Dickersin, K. Development of a highly sensitive search strategy for the retrieval of reports of controlled trials using PubMed. Int. J. Epidemiol. 2002, 31, 150–153. [Google Scholar] [CrossRef]
- Caspersen, C.J.; Powell, K.E.; Christenson, G.M. Physical activity, exercise, and physical fitness: Definitions and distinctions for health-related research. Public Health Rep. 1985, 10, 126–131. [Google Scholar]
- De Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef] [Green Version]
- Follmann, D.; Elliott, P.; Suh, I.; Cutler, J. Variance imputation for overviews of clinical trials with continuous response. J. Clin. Epidemiol. 1992, 45, 769–773. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates Publishers: New York, NY, USA, 1988. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Riebe, D.; Ehrman, J.K.; Liguori, G.; Magal, M.; American College of Sports Medicine (Eds.) ACSM’s Guidelines for Exercise Testing and Prescription; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2018. [Google Scholar]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid. Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method of Testing and Adjusting for Publication Bias in Meta-Analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.C.; Segal, R.J.; McKenzie, D.C.; Vallerand, J.R.; Morielli, A.R.; Mackey, J.R.; Gelmon, K.; Friedenreich, C.M.; Reid, R.D.; Courneya, K.S. Impact of resistance and aerobic exercise on sarcopenia and dynapenia in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. Breast Cancer Res. Treat. 2016, 158, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Ammitzbøll, G.; Kjær, T.K.; Johansen, C.; Lanng, C.; Andersen, E.W.; Kroman, N.; Zerahn, B.; Hyldegaard, O.; Bidstrup, P.E.; Dalton, S.O. Effect of progressive resistance training on health-related quality of life in the first year after breast cancer surgery—Results from a randomized controlled trial. Acta Oncol. 2019, 58, 665–672. [Google Scholar] [CrossRef] [Green Version]
- Baglia, M.L.; Lin, I.-H.; Cartmel, B.; Sanft, T.; Ligibel, J.; Hershman, D.L.; Harrigan, M.; Ferrucci, L.M.; Li, F.-Y.; Irwin, M.L. Endocrine-related quality of life in a randomized trial of exercise on aromatase inhibitor-induced arthralgias in breast cancer survivors. Cancer 2019, 125, 2262–2271. [Google Scholar] [CrossRef]
- Basen-Engquist, K.M.; Raber, M.; Carmack, C.L.; Arun, B.; Brewster, A.M.; Fingeret, M.; Schembre, S.M.; Harrison, C.; Perkins, H.Y.; Li, Y.; et al. Feasibility and efficacy of a weight gain prevention intervention for breast cancer patients receiving neoadjuvant chemotherapy: A randomized controlled pilot study. Support. Care Cancer 2020, 28, 5821–5832. [Google Scholar] [CrossRef] [PubMed]
- Bloomquist, K.; Adamsen, L.; Hayes, S.C.; Lillelund, C.; Andersen, C.; Christensen, K.B.; Oturai, P.; Ejlertsen, B.; Tuxen, M.K.; Møller, T. Heavy-load resistance exercise during chemotherapy in physically inactive breast cancer survivors at risk for lymphedema: A randomized trial. Acta Oncol. 2019, 58, 1667–1675. [Google Scholar] [CrossRef] [PubMed]
- Cadmus, L.A.; Salovey, P.; Yu, H.; Chung, G.; Kasl, S.; Irwin, M.L. Exercise and quality of life during and after treatment for breast cancer: Results of two randomized controlled trials. Psycho-Oncology 2009, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A.; Mutrie, N.; White, F.; McGuire, F.; Kearney, N. A pilot study of a supervised group exercise programme as a rehabilitation treatment for women with breast cancer receiving adjuvant treatment. Eur. J. Oncol. Nurs. 2005, 9, 56–63. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Rodriguez, L.D.; Fernández-De-Las-Peñas, C.; Del Moral-Avila, R.; Arroyo-Morales, M. A multimodal exercise program and multimedia support reduce cancer-related fatigue in breast cancer survivors: A randomised controlled clinical trial. Eur. J. Integr. Med. 2011, 3, e189–e200. [Google Scholar] [CrossRef]
- Cantarero-Villanueva, I.; Fernández-Lao, C.; Cuesta-Vargas, A.I.; Del Moral-Avila, R.; Fernández-De-Las-Peñas, C.; Arroyo-Morales, M. The Effectiveness of a Deep Water Aquatic Exercise Program in Cancer-Related Fatigue in Breast Cancer Survivors: A Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2013, 94, 221–230. [Google Scholar] [CrossRef]
- Carayol, M.; Ninot, G.; Senesse, P.; Bleuse, J.-P.; Gourgou-Bourgade, S.; Sancho-Garnier, H.; Sari, C.; Romieu, I.; Romieu, G.; Jacot, W. Short- and long-term impact of adapted physical activity and diet counseling during adjuvant breast cancer therapy: The “APAD1” randomized controlled trial. BMC Cancer 2019, 19, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Casla, S.; López-Tarruella, S.; Jerez, Y.; Marquez-Rodas, I.; Galvão, D.A.; Newton, R.; Cubedo, R.; Calvo, I.; Sampedro, J.; Barakat, R.; et al. Supervised physical exercise improves VO2max, quality of life, and health in early stage breast cancer patients: A randomized controlled trial. Breast Cancer Res. Treat. 2015, 153, 371–382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cormie, P.; Pumpa, K.; Galvão, D.A.; Turner, E.; Spry, N.A.; Saunders, C.; Zissiadis, Y.; Newton, R. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: A randomised controlled trial. J. Cancer Surviv. 2013, 7, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Cornette, T.; Vincent, F.; Mandigout, S.; Antonini, M.T.; Leobon, S.; Labrunie, A.; Venat, L.; Lavau-Denes, S.; Tubiana-Mathieu, N. Effects of home-based exercise training on VO2 in breast cancer patients under adjuvant or neoadjuvant chemotherapy (SAPA): A randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2015, 52, 25986222. [Google Scholar]
- Courneya, K.S.; Mackey, J.R.; Bell, G.J.; Jones, L.W.; Field, C.J.; Fairey, A.S. Randomized Controlled Trial of Exercise Training in Postmenopausal Breast Cancer Survivors: Cardiopulmonary and Quality of Life Outcomes. J. Clin. Oncol. 2003, 21, 1660–1668. [Google Scholar] [CrossRef]
- Courneya, K.S.; Segal, R.J.; Mackey, J.R.; Gelmon, K.; Reid, R.D.; Friedenreich, C.M.; Ladha, A.B.; Proulx, C.; Vallance, J.K.; Lane, K.; et al. Effects of Aerobic and Resistance Exercise in Breast Cancer Patients Receiving Adjuvant Chemotherapy: A Multicenter Randomized Controlled Trial. J. Clin. Oncol. 2007, 25, 4396–4404. [Google Scholar] [CrossRef]
- Daley, A.J.; Crank, H.; Saxton, J.M.; Mutrie, N.; Coleman, R.; Roalfe, A. Randomized Trial of Exercise Therapy in Women Treated for Breast Cancer. J. Clin. Oncol. 2007, 25, 1713–1721. [Google Scholar] [CrossRef]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. 2018, 20, 124. [Google Scholar] [CrossRef]
- Dong, X.; Xiangren, Y.; Shuyuan, H.; Dezong, G.; Mengyao, C.; Meng, D. The effects of combined exercise intervention based on Internet and social media software for postoperative patients with breast cancer: Study protocol for a randomized controlled trial. Trials 2018, 19, 477. [Google Scholar] [CrossRef]
- Ergun, M.; Eyigor, S.; Karaca, B.; Kisim, A.; Uslu, R. Effects of exercise on angiogenesis and apoptosis-related molecules, quality of life, fatigue and depression in breast cancer patients. Eur. J. Cancer Care 2013, 22, 626–637. [Google Scholar] [CrossRef]
- Fernández-Lao, C.; Cantarero-Villanueva, I.; Ariza-Garcia, A.; Courtney, C.A.; Fernández-De-Las-Peñas, C.; Arroyo-Morales, M. Water versus land-based multimodal exercise program effects on body composition in breast cancer survivors: A controlled clinical trial. Support. Care Cancer 2012, 21, 521–530. [Google Scholar] [CrossRef]
- Fillion, L.; Gagnon, P.; Leblond, F.; Gélinas, C.; Savard, J.; Dupuis, R.; Duval, K.; LaRochelle, M. A Brief Intervention for Fatigue Management in Breast Cancer Survivors. Cancer Nurs. 2008, 31, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Galiano-Castillo, N.; Cantarero-Villanueva, I.; Fernández-Lao, C.; Ariza-García, A.; Rodriguez, L.D.; Del-Moral-Ávila, R.; Arroyo-Morales, M. Telehealth system: A randomized controlled trial evaluating the impact of an internet-based exercise intervention on quality of life, pain, muscle strength, and fatigue in breast cancer survivors. Cancer 2016, 122, 3166–3174. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, H.; Akyolcu, N. The Impact of Lifestyle Interventions in Breast Cancer Women after Completion of Primary Therapy: A Randomized Study. J. Breast Health 2017, 13, 94–99. [Google Scholar] [CrossRef]
- Gokal, K.; Wallis, D.; Ahmed, S.; Boiangiu, I.; Kancherla, K.; Munir, F. Effects of a self-managed home-based walking intervention on psychosocial health outcomes for breast cancer patients receiving chemotherapy: A randomised controlled trial. Support. Care Cancer 2016, 24, 1139–1166. [Google Scholar] [CrossRef] [Green Version]
- Hagstrom, A.; Marshall, P.W.M.; Lonsdale, C.; Cheema, B.S.; Singh, M.F.; Green, S.M. Resistance training improves fatigue and quality of life in previously sedentary breast cancer survivors: A randomised controlled trial. Eur. J. Cancer Care 2015, 25, 784–794. [Google Scholar] [CrossRef]
- Harvie, M.; Pegington, M.; McMullan, D.; Bundred, N.; Livingstone, K.; Campbell, A.; Wolstenholme, J.; Lovato, E.; Campbell, H.; Adams, J.; et al. The effectiveness of home versus community-based weight control programmes initiated soon after breast cancer diagnosis: A randomised controlled trial. Br. J. Cancer 2019, 121, 443–454. [Google Scholar] [CrossRef]
- Hayes, S.C.; Rye, S.; Disipio, T.; Yates, P.; Bashford, J.; Pyke, C.; Saunders, C.; Battistutta, D.; Eakin, E. Exercise for health: A randomized, controlled trial evaluating the impact of a pragmatic, translational exercise intervention on the quality of life, function and treatment-related side effects following breast cancer. Breast Cancer Res. Treat. 2012, 137, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Herrero, F.; Juan, A.F.S.; Fleck, S.J.; Balmer, J.; Pérez, M.; Cañete, S.; Earnest, C.P.; Foster, C.; Lucía, A. Combined Aerobic and Resistance Training in Breast Cancer Survivors: A Randomized, Controlled Pilot Trial. Int. J. Sports Med. 2006, 27, 573–580. [Google Scholar] [CrossRef]
- Huang, H.-P.; Wen, F.-H.; Yang, T.-Y.; Lin, Y.-C.; Tsai, J.-C.; Shun, S.-C.; Jane, S.-W.; Chen, M.-L. The effect of a 12-week home-based walking program on reducing fatigue in women with breast cancer undergoing chemotherapy: A randomized controlled study. Int. J. Nurs. Stud. 2019, 99, 103376. [Google Scholar] [CrossRef]
- Hwang, J.H.; Chang, H.J.; Shim, Y.H.; Park, W.H.; Huh, S.J.; Yang, J.-H.; Park, W. Effects of Supervised Exercise Therapy in Patients Receiving Radiotherapy for Breast Cancer. Yonsei Med. J. 2008, 49, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Ligibel, J.A.; Giobbie-Hurder, A.; Shockro, L.; Campbell, N.; Partridge, A.H.; Tolaney, S.; Lin, N.U.; Winer, E.P. Randomized trial of a physical activity intervention in women with metastatic breast cancer. Cancer 2016, 122, 1169–1177. [Google Scholar] [CrossRef]
- Mijwel, S.; Jervaeus, A.; Bolam, K.A.; Norrbom, J.; Bergh, J.; Rundqvist, H.; Wengström, Y. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J. Cancer Surviv. 2019, 13, 244–256. [Google Scholar] [CrossRef] [Green Version]
- Milne, H.M.; Wallman, K.E.; Gordon, S.; Courneya, K.S. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: A randomized controlled trial. Breast Cancer Res. Treat. 2007, 108, 279–288. [Google Scholar] [CrossRef]
- Murtezani, A.; Ibraimi, Z.; Bakalli, A.; Krasniqi, S.; Disha, E.D.; Kurtishi, I. The effect of aerobic exercise on quality of life among breast cancer survivors: A randomized controlled trial. J. Cancer Res. Ther. 2014, 10, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Musanti, R. A Study of Exercise Modality and Physical Self-esteem in Breast Cancer Survivors. Med. Sci. Sports Exerc. 2012, 44, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mutrie, N.; Campbell, A.M.; Whyte, F.; McConnachie, A.; Emslie, C.; Lee, L.; Kearney, N.; Walker, A.; Ritchie, D. Benefits of supervised group exercise programme for women being treated for early stage breast cancer: Pragmatic randomised controlled trial. BMJ 2007, 334, 517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naraphong, W.; Lane, A.; Schafer, J.; Whitmer, K.; Wilson, B.R.A. Exercise intervention for fatigue-related symptoms in Thai women with breast cancer: A pilot study. Nurs. Health Sci. 2014, 17, 33–41. [Google Scholar] [CrossRef]
- Ohira, T.; Schmitz, K.H.; Ahmed, R.L.; Yee, D. Effects of weight training on quality of life in recent breast cancer survivors. Cancer 2006, 106, 2076–2083. [Google Scholar] [CrossRef]
- De Paulo, T.R.; Rossi, F.E.; Viezel, J.; Tosello, G.T.; Seidinger, S.C.; Simões, R.R.; De Freitas, R.; Freitas, I.F. The impact of an exercise program on quality of life in older breast cancer survivors undergoing aromatase inhibitor therapy: A randomized controlled trial. Health Qual. Life Outcomes 2019, 17, 17. [Google Scholar] [CrossRef]
- Pinto, B.M.; Frierson, G.M.; Rabin, C.; Trunzo, J.J.; Marcus, B.H. Home-Based Physical Activity Intervention for Breast Cancer Patients. J. Clin. Oncol. 2005, 23, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.D.; Pereira, P.T.V.T.; Diniz, R.R.; de Castro Filha, J.G.L.; Dos Santos, A.M.; Ramallo, B.T.; Filho, F.A.A.; Navarro, F.; Garcia, J.B.S. Effect of exercise on pain and functional capacity in breast cancer patients. Health Qual. Life Outcomes 2018, 16, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.Q.; Vicari, S.; Trammell, R.; Hopkins-Price, P.; Fogleman, A.; Spenner, A.; Rao, K.; Courneya, K.S.; Hoelzer, K.S.; Robbs, R.; et al. Biobehavioral Factors Mediate Exercise Effects on Fatigue in Breast Cancer Survivors. Med. Sci. Sports Exerc. 2014, 46, 1077–1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, L.Q.; Courneya, K.S.; Anton, P.M.; Verhulst, S.; Vicari, S.K.; Robbs, R.S.; McAuley, E. Effects of a multicomponent physical activity behavior change intervention on fatigue, anxiety, and depressive symptomatology in breast cancer survivors: Randomized trial. Psycho-Oncology 2017, 26, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Saarto, T.; Penttinen, H.; Sievänen, H.; Kellokumpu-Lehtinen, P.-L.; Hakamies-Blomqvist, L.; Nikander, R.; Huovinen, R.; Luoto, R.; Kautiainen, H.; Järvenpää, S.; et al. Effectiveness of a 12-month exercise program on physical performance and quality of life of breast cancer survivors. Anticancer Res. 2012, 32, 3875–3884. [Google Scholar] [PubMed]
- Saxton, J.M.; Scott, E.; Daley, A.J.; Woodroofe, M.N.; Mutrie, N.; Crank, H.; Powers, H.J.; Coleman, R.E. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation and immune function after early-stage breast cancer: A randomised controlled trial. Breast Cancer Res. 2014, 16, R39. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, T.; Weisser, B.; Jonat, W.; Baumann, F.T.; Mundhenke, C. Gentle strength training in rehabilitation of breast cancer patients compared to conventional therapy. Anticancer Res. 2012, 32, 3229–3233. [Google Scholar]
- Schmidt, M.E.; Wiskemann, J.; Armbrust, P.; Schneeweiss, A.; Ulrich, C.M.; Steindorf, K. Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: A randomized controlled trial. Int. J. Cancer 2015, 137, 471–480. [Google Scholar] [CrossRef]
- Schmidt, T.; Weisser, B.; Dürkop, J.; Jonat, W.; Van Mackelenbergh, M.; Röcken, C.; Mundhenke, C. Comparing Endurance and Resistance Training with Standard Care during Chemotherapy for Patients with Primary Breast Cancer. Anticancer Res. 2015, 35, 5623–5630. [Google Scholar]
- Schmidt, M.E.; Meynköhn, A.; Habermann, N.; Wiskemann, J.; Oelmann, J.; Hof, H.; Wessels, S.; Klassen, O.; Debus, J.; Potthoff, K.; et al. Resistance Exercise and Inflammation in Breast Cancer Patients Undergoing Adjuvant Radiation Therapy: Mediation Analysis from a Randomized, Controlled Intervention Trial. Int. J. Radiat. Oncol. 2016, 94, 329–337. [Google Scholar] [CrossRef]
- Scott, E.; Daley, A.J.; Doll, H.; Woodroofe, M.N.; Coleman, R.E.; Mutrie, N.; Crank, H.; Powers, H.J.; Saxton, J.M. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long-term prognosis after early-stage breast cancer: A randomized controlled trial. Cancer Causes Control. 2012, 24, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Segal, R.; Evans, W.; Johnson, D.; Smith, J.; Colletta, S.; Gayton, J.; Woodard, S.; Wells, G.; Reid, R. Structured Exercise Improves Physical Functioning in Women With Stages I and II Breast Cancer: Results of a Randomized Controlled Trial. J. Clin. Oncol. 2001, 19, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Shobeiri, F.; Masoumi, S.Z.; Nikravesh, A.; Moghadam, R.H.; Karami, M. The Impact of Aerobic Exercise on Quality of Life in Women with Breast Cancer: A Randomized Controlled Trial. J. Res. Health Sci. 2016, 16, 127–132. [Google Scholar] [PubMed]
- Speck, R.M.; Gross, C.R.; Hormes, J.M.; Ahmed, R.L.; Lytle, L.A.; Hwang, W.-T.; Schmitz, K.H. Changes in the Body Image and Relationship Scale following a one-year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Res. Treat. 2010, 121, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Steindorf, K.; Schmidt, M.E.; Klassen, O.; Ulrich, C.M.; Oelmann, J.; Habermann, N.; Beckhove, P.; Owen, R.; Debus, J.; Wiskemann, J.; et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: Results on cancer-related fatigue and quality of life. Ann. Oncol. 2014, 25, 2237–2243. [Google Scholar] [CrossRef]
- Travier, N.; Velthuis, M.J.; Bisschop, C.N.S.; Buijs, B.V.D.; Monninkhof, E.M.; Backx, F.J.G.; Los, M.; Erdkamp, F.L.G.; Bloemendal, H.J.; Rodenhuis, C.; et al. Effects of an 18-week exercise programme started early during breast cancer treatment: A randomised controlled trial. BMC Med. 2015, 13, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.-J.; Boehmke, M.; Wu, Y.-W.B.; Dickerson, S.S.; Fisher, N. Effects of a 6-Week Walking Program on Taiwanese Women Newly Diagnosed With Early-Stage Breast Cancer. Cancer Nurs. 2011, 34, E1–E13. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Dobek, J.; Bennett, J.A.; Nail, L.M.; Leo, M.C.; Schwartz, A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: A randomized controlled trial. J. Cancer Surviv. 2011, 6, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Correa-Bautista, J.E.; Schmidt-Riovalle, J.; Ramírez-Vélez, R. Efectividad del ejercicio físico en la fatiga de pacientes con cáncer durante el tratamiento activo: Revisión sistemática y metaanálisis [Effectiveness of physical exercise on fatigue in cancer patients during active treatment: A systematic review and meta-analysis]. Cad. Saúde Pública 2015, 31, 667–681. (In Spanish) [Google Scholar] [CrossRef] [Green Version]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of supervised exercise on cancer-related fatigue in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2015, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Ramírez-Vélez, R. Effects of Supervised Multimodal Exercise Interventions on Cancer-Related Fatigue: Systematic Review and Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2015, 2015, 328636. [Google Scholar] [CrossRef]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Correa, J.E.; Ramírez-Vélez, R. [Supervised physical activity interventions in the management of cancer-related fatigue: A systematic review]. Nutr. Hosp. 2014, 30, 486–497. [Google Scholar] [PubMed]
- Kidwell, K.M.; Harte, S.E.; Hayes, D.F.; Storniolo, A.M.; Carpenter, J.; Flockhart, D.A.; Stearns, V.; Clauw, D.J.; Williams, D.A.; Henry, N.L. Patient-reported symptoms and discontinuation of adjuvant aromatase inhibitor therapy. Cancer 2014, 120, 2403–2411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giese-Davis, J.; Collie, K.; Rancourt, K.M.; Neri, E.; Kraemer, H.C.; Spiegel, D. Decrease in Depression Symptoms Is Associated With Longer Survival in Patients With Metastatic Breast Cancer: A Secondary Analysis. J. Clin. Oncol. 2011, 29, 413–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, P.; Baumann, F.T.; Oberste, M.; Wright, P.; Garthe, A.; Schenk, A.; Elter, T.; Galvao, D.A.; Bloch, W.; Hübner, S.T.; et al. Effects of Exercise Interventions and Physical Activity Behavior on Cancer Related Cognitive Impairments: A Systematic Review. BioMed Res. Int. 2016, 2016, 1820954. [Google Scholar] [CrossRef] [PubMed]
- Muzzatti, B.; Bomben, F.; Flaiban, C.; Piccinin, M.; Annunziata, M.A. Quality of life and psychological distress during cancer: A prospective observational study involving young breast cancer female patients. BMC Cancer 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bedillion, M.F.; Ansell, E.B.; Thomas, G.A. Cancer treatment effects on cognition and depression: The moderating role of physical activity. Breast 2019, 44, 73–80. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M.-G. Effects of Exercise Interventions on Breast Cancer Patients during Adjuvant Therapy. Cancer Nurs. 2020, 43, 115–125. [Google Scholar] [CrossRef]
- Kruijsen-Jaarsma, M.; Revesz, D.; Bierings, M.B.; Buffart, L.M.; Takken, T. Effects of exercise on immune function in patients with cancer: A systematic review. Exerc. Immunol. Rev. 2013, 19, 120–143. [Google Scholar] [CrossRef]
- Schmidt, T.; Van Mackelenbergh, M.; Wesch, D.; Mundhenke, C. Physical activity influences the immune system of breast cancer patients. J. Cancer Res. Ther. 2017, 13, 392–398. [Google Scholar]
- Meneses-Echávez, J.F.; González-Jiménez, E.; Schmidt-Riovalle, J.; Correa-Bautista, J.E.; Izquierdo, M.; Ramírez-Vélez, R. The insulin-like growth factor system is modulated by exercise in breast cancer survivors: A systematic review and meta-analysis. BMC Cancer 2016, 16, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meneses-Echávez, J.F.; Correa-Bautista, J.E.; González-Jiménez, E.; Río-Valle, J.S.; Elkins, M.R.; Lobelo, F.; Ramírez-Vélez, R. The Effect of Exercise Training on Mediators of Inflammation in Breast Cancer Survivors: A Systematic Review with Meta-analysis. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1009–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saanijoki, T.; Tuominen, L.; Tuulari, J.J.; Nummenmaa, L.; Arponen, E.; Kalliokoski, K.; Hirvonen, J. Opioid Release after High-Intensity Interval Training in Healthy Human Subjects. Neuropsychopharmacology 2018, 43, 246–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Design |
| Randomized controlled trials, low risk bias (A score of 5 out of 10). |
| Population |
| Breast cancer survivors (>18 years old) without restriction to stage of disease or undergoing (neo-)adjuvant cancer treatment (hormonal therapy, chemotherapy, radiotherapy, mastectomy, or combination). |
| Intervention |
| Exercise programs: |
| —Aerobic or endurance exercise |
| Strengthening or resistance training |
| Stretching |
| Combined exercise training (i.e., aerobic plus resistance training and/or stretching training) |
| Comparisons |
| Supervised exercise or home exercise versus women receiving standard of care |
| Supervised versus or home exercise women who were on a waiting list or on attention control (where reported) |
| Outcome Measures |
| Mental wellbeing or mental health: |
| Quality of life (overall, FACT-B, emotional functions) |
| Fatigue |
| Self-esteem |
| Depression |
| Anxiety |
| Sleep |
| Body image |
| Study (Year) [Reference] Acronym | BC Stage Treatment Timing | Groups Sample Size | Age | WHO PA Guidelines Supervised or Home-Based | F Ses/ Wk | I | T Min/ Ses | T | D | Outcome Measures | PRO Measure Instrument | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adams (2016) [25] START | I–III a (During CT) | N = 200 AE = 64 RE = 66 UC = 70 | 48.8 (25–78) | 🗹 AE 🗹 RE |   | 3 | Moderate- vigorous 60–80% VO2 peak 2 × 8–12 reps (9 exercises) 60–70% 1RM | 60′ |   | 17 (9–24) | QoL, Fatigue | FACT-Na, TOI-An subscale, Fatigue subscale |
| Ammitzboll (2019) [26] LYCA | I–III (CT, RT, HT; First year after BC surgery) | N = 158 RE = 82 UC = 76 | 53 (33–73) 52 (30–74) | 🗹 RE |   | 3 | Low-moderate 3 × 8–12 reps (6 exercises) | 50–55′ |  | 52 | HRQoL, Fatigue | EORTC QLQ C30, FACIT, EORTC C30, subscales |
| Baglia (2019) [27] HOPE | I–III (CT/RT) | N = 121 Exc = 61 UC = 60 | 62.0 (7.0) 60.5 (7.0) | 🗹 AE + RE |   | 3-5 (AE) 2 (RE) | 50–80% HRmax | 150′ (AE) |   | 12 | QoL, Fatigue | FACT, FACT-G, FACT-B, SF-36, FACIT-Fatigue |
| Basen- Engquist (2020) [28] | II–III (CT/ RT—during CT treatment) | N = 37 Exp = 19 UC = 18 | 49.6 (13.3) 49.2 (9.2) | 🗹 RE + Flexibility exercises |  | 3 | Moderate | 60′ |   | 13 | QoL, Body Image | SF-36, BIS |
| Bloomquist (2019) [29] | I–III (CT—during treatment) | N = 153 Exc = 75 Con = 78 | 51.5 (9.6) 52.0 (9.3) | 🗹 HIGH RE + AE 🗵 Con = AE |    | 3 | 70–90% 1 RM (RE) 85–90% HRmax (HIIT) Low-intensity (target = 10,000 steps) | 90–120′ |    | 12 | QoL | EORTC QLQ- BR23 |
| Cadmus (2009) [30] IMPACT | 0–III a (CH/RT/HT—during treatment) | N = 50 Exc = 25 UC = 25 | 54.5 (8.2) 54.0 (10.9) | 🗹 AE |  | 5 | Moderate-vigorous (60–80% HRmax) | 30′ |  | 24 | Happiness depression anxiety, stress, self-esteem, QoL | HM CES-D STAI, 10-PSS Rosenberg S-ES, FACT-B SF-36 |
| Cadmus (2009) [31] YES | 0–III a (CH/RT/HT—Post-treatment, 6-moth follow-up) | N = 75 Exc = 37 UC = 38 | 56.5 (9.5) 55.1 (7.7) | 🗹 AE |  | 5 | Modrate-vigorous (60–80% HRmax) | 30′ |  | 24 | Happines, Depression, Anxiety, Stress, Self-esteem, QoL | HM, CES-D, STAI-YI, 10-PSS, Rosenberg S-ES, FACT-B SF-36 |
| Campbell (2005) [31] | I–II (CT/RT—during treatment) | N =22 Exc = 12 UC = 10 | 48 (10) 47 (5) | 🗵 AE + RE |  | 2 | 60–75% HRmax | 20′ |   | 12 | Fatigue QoL | FACT-G FACT-B SWLS, PFS |
| Cantarero-Villanueva (2011) [32] CUIDATE | I–III A (CT/RT—Post-treatment) | N = 67 Exc = 32 UC = 35 | 49 (9) 48 (9) | 🗵 Multimodal intervention |  | 3 | Low-intensity | 90′ | - | 8 | Fatigue | PFS |
| Cantarero-Villanueva (2013) [33] | I–III A (CT/RT—Post-treatment) | N = 61 Exc = 32 UC= 29 | 49 (7) 47 (8) | 🗹 AE + RE (deep pool) |  | 3 | 60–75% HRmax | 60′ |   | 8 | Fatigue Mood state | PFS Profile of Mood States (Spanish version) |
| Carayol (2019) [34] APAD1 | I–III c (CT/RT—during treatment) | N = 143 Exc = 72 UC = 71 | 51.2 (10.9) 52.1 (9.3) | 🗵 RE + AE + nutritional therapeutic education sessions |  | 2 (AE) 1 (RE) | Moderate (AE) 50–75% HRmax | 30–45′ |   | 26′ | Fatigue QoL Anxiety Depression | MFI, EORTC QLQ-C30 HADS |
| Casla (2015) [35] | I–III (CT/RT—post-treatment) | N = 94 Exc = 47 UC = 47 | 45.91 (8.21) 51.87 (8.21) | 🗹 AE + RE |  | 3 | Progresive 55–85% HRR (AE) 10–20 Borg Scale (RE) | 60′ |   | 12 | QoL | SF-36 |
| Cormie (2013) [36] | I–III (CT/RT/HT—timing not reported) | N = 62 Exc HL = 22 Exc LL = 21 UC= 19 | 56.1 (8.1) 57.0 (10.0) 58.6 (6.7) | 🗹 RE HL = High-load 🗹 RE LL = Low-load |   | 2 | Moderate-high 12–16 Borg scale [RPE] RE HL (10–6 reps, 75–85% 1RM); RE LL (20–15 reps, 55–65% 1RM) | 60′ |   | 12 | QoL | QLQ-BR23 SF-36 |
| Cornette (2016) [37] SAPA | I–III b (CT/RT—during treatment) | N = 42 Exc = 20 UC = 22 | 52 (37–73) 49 (37–68) | 🗵 AE (APA) + RE |  | 2 (AE) 1 (RE) | 60 cycling revolutions/min or HR RE (2 sets of 8–12 reps of 5 exercises) | 25-45′ |   | 27 | QoL, Fatigue, Anxiety and Depression | MFI-20, EORTC QLQ-C30, HADS |
| Courneya (2003) [38] | I–III a (CH/RT/HT—post-treatment) | N = 52 Exc = 24 UC = 28 | 59 (5) 58 (6) | 🗵 AE |  | 3 | 70–75% VO2 max | 35′ |  | 15 | QoL, Well-being, Happiness, Self-Esteem, Fatigue | FACT-B, FACT-G, HM, Rosenberg S-ES, FACT-F |
| Courneya (2007) [39] | I–III a (CT—during adjuvant CT) | N = 242 Exc AE = 78 Exc RE = 82 UC = 82 | 49.2 (25–78) 49.0 (30–75) 49.5 (25–76) 49.0 (26–78) | 🗵 AE RE |  | 3 | 60–80% VO2 max 60–70% 1RM | 15–45′ |   | 17 (9–24) | Fatigue, Self-Esteem, Depression, Anxiety | FACT-A, Rosenberg S-ES, CES-D, Spielberger SAI |
| Daley (2007) [40] | Stage not reported (CT/RT/HT—post-treatment) | N = 108 Exc = 34 Exc PE = 36 UC = 38 | 51.6 (8.8) 50.6 (8.7) 51.1 (8.6) | 🗹 AE |  | 3 | Moderate- intensity (65–85% HRmax and 12–13 RPE) Placebo Exercise (light-intensity 40% HRmax) | 50′ |   | 8 | QoL, Fatigue, Depression | FACT-G, FACTB, PFS, BDI-II |
| Dieli (2018) [41] | 0–III (CT/RT— post-treatment) | N = 91 Exc = 46 UC = 45) | 53.5 (10.4) | 🗹 AE + RE |  | 3 | Moderate-vigorous (65–85% HRmax)  | 50′ (AE) |   | 16 | QoL, Fatigue, Depression | FACT-B, SF-36, BFI, CES-D |
| Dong (2019) [42] CEIBISMS | I–III (CT/RT—post-treatment) | N = 50 Exc = 26 UC = 24 | 48.00 (5.54) 51.63 (7.49) | 🗹 (Internet and social media app) AE + RE |  | 4 (AE) 3 (RE) | not reported (RPE) | 30′ |   | 12 | QoL | SF-36 |
| Ergun (2013) [43] | I–III A (CT/RT/mastectomy, axillary dissection and sentinel lymph node biopsy—post-treatment) | N = 60 Exp (1) = 20 Exp (2) = 20 UC = 20 | 49.65 (8.25) 55.05 (6.85) 55.30 (10.37) | 🗹 AE + RE + Brisk walk 🗵 Brisk walk |   | 3 + 3 3 | Moderate | 45′ + 30′ 30′ |    | 12 | QoL, Fatigue, Depression | EORTC QLQ-C30, BFI, BDI |
| Fernández-Lao (2013) [44] | I–III a (CT/RT/HT post-CT treatment) | N = 98 Exc L = 31 Exc W = 33 UC = 34 | 49 (8) 48 (7) 48 (8) | Multimodal (AE + RE) 🗹 Exp L = Land 🗹 Exp W = Water |  | 3 | 60% HRmax | 60′ |   | 8 | QoL, Body image | EORTC QLQ-BR23 |
| Fillion (2008) [45] | 0–III (RT + CT/HT—post-treatment) | N = 87 Exc = 44 UC = 43 | 53.09 (9.65) 51.84 (10.25) | 🗹 AE + Psycho-educative and fatigue management sessions |  | 3–5 | 65–75% HRmax | 20–30′ |  | 10 | Fatigue, Energy level, QoL, Emotional distress | MFI, Vigor-POMS, SF-12, POMS anxiety + depression |
| Galiano-Castillo (2016) [46] e-CUIDATE | 0–III (CT/RT—adjuvant therapy/except HT) | N = 81 Exc = 40 UC = 41 | 47.4 (9.6) 49.2 (7.9) | 🗹 AE + RE Internet-based |  | 3 | Moderate | 90′ |   | 8 | QoL, Fatigue, Body image | EORTC QLQ-C30, R-PFS |
| Ghavami and Akyolcu (2017) [47] | I–III (CT/RT— Post-treatment) | N = 80 Exc (N = 40) UC = 40 | 48.75 (9.49) 49.23 (9.46) | 🗹 AE Life-style intervention (dietary energy-restriction) |  | 3–5 | 70–85% HRR | 45–60′ |  | 24 | Fatigue, Quality of sleep, QoL | CFS, PSQI, EORTC QLQ-C30, QLQ-BR23 |
| Gokal (2016) [48] | I–III (CT—during CT treatment) | N = 50 Exc = 25 UC = 25 | 52 (11.7) 52 (8.9) | 🗹 AE |  | 5 | Moderate (brisk walking) | 10–30′ |  | 12 | Anxiety, Depression, Fatigue, Self-esteem, Mood | HADS, FACT-F Rosenberg S-ES, POMS-SF |
| Hagstrom (2016) [49] | I–III a (CT/RT/HT—post- CT/ treatment) | N = 39 Exc = 20 UC =19 | 51.2 (8.5) 52.7 (9.4) | 🗹 RE |  | 3 | 8RM (VPA) (3 sets of 8–10 reps of 6 full body exercises) | 60′ |  | 16 | QoL, Fatigue | FACIT-F, FACT-G |
| Harvie (2019) [50] | Early stage (CT/RT/HT —during CT treatment | N = 409 Exc C = 137 Exc H =134 UC =138 | 54.0 (9.2) 54.6 (11.2) 55.3 (10.5) | 🗹 Exp C = Community Exp H = Home (phone and mail programme) |   | 5 (AE) 2 (RE) | Moderate (50–80% HRmax) | 30′ (AE) + 10′ (RE) |   | 52 | Fatigue | FACT-TOI |
| Hayes (2013) [51] Exercise for Health | 0–III (CT/RT/HT—Pre, during and/or post- intervention) | N = 194 Exc FtF = 67 Exc Tel = 67 UC = 60 | 51.2 (8.8) 52.2 (8.6) 53.9 (7.7) | 🗹 Exp FtF = (Face-to-face) AE + RE 🗹 Exp Tel = (Telephone) AE + RE |   | ≥4 (AE) ≥2 (RE) | Low-moderate to moderate-high | ≥45′ |   | 24 | QoL, Fatigue, Anxiety, Depression | FACT-B + 4, FACIT-F |
| Herrero (2006) [52] | I–II of ductal brest carcinoma (CT/RT— Post-treatment) | N = 16 Exc = 8 UC = 8 | 50 (5) 51(10) | 🗹 AE + RE |  | 3 | 70–80% HRmax (AE) 12–15 to 8–10RM (RE) | 90′ |   | 8 | QoL | EORTC QLQ-C30, |
| Huang (2019) [53] | I-III (CT/ RT/ HT – scheduled for adjuvant CT | N= 159 Exc= 81 UC= 78 | 48.32 (7.90) 48.31 (8.65) | 🗹 AE |  | 3–5 | 30–70% HRR | 12–25′ to 35–40′ |  | 12 | Anxiety and Depression | BFI, HADS-A, HADS-D, PSQI |
| Hwang (2008) [54] | Early stage (RT—waiting list for RT) | N = 40 Exc = 17 UC = 23 | 46.3 (7.5) 46.3 (9.5) | 🗹 AE + RE Con = self-shoulder stretching exercise |  | 3 | 50–70% HRmax | 50′ |   | 5 | QoL, Fatigue | WHOQOL-BREF, BFI |
| Ligibel (2016) [55] | Metastatic BC (endocrine therapy vs. CT/biologic therapy—during treatment) | N = 98 Exc = 47 UC = 51 | 49.3 (9.6) 50.7 (9.4) | 🗹 AE |   | Not reported | Moderate | 150′ /wk |  | 16 | QOL, Fatigue | EORTC QLQ C-30, HADS, FACIT-F |
| Mijwel (2019) [56] OptiTrain | I–III a (CT—during adjuvant CT treatment) | N = 206 Exc AE = 72 Exc RE = 74 UC =60 | 54.4 (10.3) 52.7 (10.3) 52.6 (10.2) | 🗵 AE-HIIT 🗵 RE-HIIT |  | 2 | Moderate (AE) High, 70–80% 1RM (RE) | 60′ |   | 16 | Fatigue, QoL, Distress | PFS, EORTC-QLQ-C30, MSAS |
| Milne (2008) [57] | Early stage (CT/RT—Post-treatment w or w/o HT | N = 58 Exc = 29 UC = 29 | 55.2 (8.4) 55.1 (8.0) | 🗵 AE + RE + stretching |  | 3 | (about) 75% HRmax | 30′ |    | 12 | QoL, Fatigue, Anxiety | FACT-B, SCFS, SPAS-7 |
| Murtezani (2014) [58] | Early stage (CT/RT—Post-treatment w or w/o HT) | N = 62 Exp = 30 UC = 32 | 53 (11) 51 (11) | 🗵 AE |  | 3 | 50–75% HRR | 25–45′ |  | 10 | QoL | FACT‑B, FACT-G |
| Musanti (2012) [59] | I–III b (CT/RT— Post-treatment w or w/o HT) | N = 42 Exp AE = 10 Exp RE = 9 Exp AR = 11 Con F = 12 | 51 (5.5) 52 (8.9) 48 (6.7) 52 (7.9) | 🗵 AE 🗹 RE 🗹 AE + RE |  | 3 3 4–5 (AE) 2 (RE) | 40–65% to 85% HRmax 3–5 to 7–8 RPE (0–10 scale) | 15–30′ |     | 12 | Fatigue, Anxiety, Depression, Self-esteem | PFS, HADS, Rosenberg S-ES |
| Mutrie (2007) [60] | 0–III (CT/RT/combined—during and/or post-treatment) | N = 174 Exp = 82 UC = 92 | 51.3 (10.3) 51.8 (8.7) | 🗵 AE + RE |  | 2 | 50–75% HRmax | 45′ |   | 12 | QoL, Depression | FACT-G, FACT-B, FACT-F, PANAS, SPAQ, BDI |
| Naraphong (2015) [61] | I–III a (during CT treatment) | N = 23 Exp = 11 UC = 12 | 46.36 (9.37) 47.17 (6.87) | 🗹 AE |  | 3–5 | Low-moderate (40–60% HRmax) weekly increase of 5% of average past week steps | 30–40′ |  | 10 | Fatigue, Sleep disturbance, Symptom distress | PFS-R, GSD, POMS-BF, MSAS |
| Ohira (2006) [62] WTBS | I–III, DCIS (CT/RT/HT | N = 79 Exp = 39 UC = 40 | 53.3 (8.7) 52.8 (7.6) | 🗹 RE |  | 2 | 10 max/reps | 60′ |  | 24 | QoL, Depression | CARES-SF, CES-D |
| Paulo (2019) [63] | I–III (CT/RT—undergoing aromatase inhibitor therapy) | N = 36 Exp = 18 UC = 18 | 63.2 (7.1) 66.6 (9.6) | 🗹 AE + RE UC = Stretching |   | 3 2 | 60–85% HRmax (AE) 15 reps or 65% of max. reps to 8 reps or 80% of max. reps (RE) | 30′ (AE) 40′ (RE) 45′ |    | 36′ | QoL | SF36, EORTC QLQ-C30, EORTC QLQ-BR23 |
| Pinto (2005) [64] | 0–II (CT/RT—post CT treatment) | N = 82 Exp = 39 UC = 43 | 53.42 (9.08) 52.86 (10.38) | 🗹 AE |  | 2–5 | 55–65% HRmax | 10–30′ |  | 12 | Anxiety and depression, Fatigue | POMS, LASF |
| Reis (2018) [65] | I–IV (CT/RT—during CT/RT treatment) | N = 28 Exp =14 UC =14 | 47.64 (7.60) 45.79 (8.1) | 🗹 AE + RE + Flexibility |   | 3 (AE + RE) 2 (Flex) | 50–60%/80–90% target HR (AE) 3 sets of 12 reps of 12 maximum repetition (RE) | 30′ (AE) 30′ (RE) 15′ |    | 12 | Fatigue | PFS-R |
| Rogers (2015) [66] | I–II BC (CT/RT/HT- DCIS) | N = 44 Exp = 20 UC = 24 | 57.2 ± 5.5 (45–69) 55.2 ± 9.1 (32–67) | 🗹 AE + RE |   | 4 (AE) 2 (RE) | Moderate (48–52% HRR) | 9–40′ |   | 10 | Fatigue, Sleep dysfunction, Anxiety, Depression | FSI, fatigue interference, PROMIS |
| Rogers (2017) [67] BEAT Cancer | I–III A (CT/RT/HT-DCIS) | N = 222 Exp = 110 UC = 112 | 54.4 (8.5) | 🗹 AE |   | ≥3 | Moderate (40–59% HRR)  | ≥60′ |  | 10 | Fatigue, Anxiety, Depression | FSI, HAD |
| Saarto (2012) [68] | Early stage (CT/RT) | N = 500 Exp = 263 UC = 237 | 52.3 (36–68) 52.4 (35–68) | 🗹 AE |   | 1 2–3 | 5–7 METs | 60′ |  | 48 | QoL, Fatigue, Depression, Body image | EORTC QLQ-C30, BR-23, FACIT-F, RBDI, WHQ |
| Saxton (2014) [69] | Early stage (post- treatment) | N = 85 Exp (N = 44) UC =41 | 55.8 (10.0) 55.3 (8.8) | 🗹 AE + RE (+ education about hypocaloric diet intake) |  | 3 | 65–85% HRmax | 40–45′ |   | 24 | Depression, Stress | BDI-II, PSS |
| Schmidt (2012) [70] | I–III (CT/RT—Post-treatment) | N = 33 Exp =15 UC =18 | 58 (8.41) 55 (10.59) | 🗵 RE |  | 1 | 50% h 1RM | 60′ |  | 26 | QoL | EORTC QLQ C30, BR23 |
| Schmidt (2015) [71] BEATE | Tumor stage 1–4 (CH, HT etc.—during CT treatment | N = 95 Exp = 49 UC = 46 | 52.2 (9.9) 53.3 (10.2) | 🗵 RE |  | 2 | 60–80% 1RM | 60′ |  | 12 | Fatigue, QoL, Depression | FAQ, EORTC QLQ C30 + BR23, CES-D |
| Schmidt (2015b) [72] | Primary moderate- or high-risk BC (CT—during CT treatment) | N = 67 Exp AE = 20 Exp RE = 21 UC = 26 | 56 (10.15) 53 (12.55) 54 (11.19) | 🗵AE 🗹 RE |  | 2 | 11–14 Borg scale (AE) 50% h 1RM (RE) | 60′ |   | 12 | QoL Fatigue, Body image | EORTC QLQ C30 + BR23, MFI20 |
| Schmidt (2016) [73] BEST | 0–III (RT—During RT) | N = 103 Exc = 54 UC = 49 | 57.1 (8.9) 57.3 (8.8) | 🗹 RE |  | 2 | 3 sets of 8–2 reps of 8 machine-based exercises | 60′ |  | 12 | Fatigue, QoL | FAQ, QLQ-C30, CES-D |
| Scott (2013) [74] | I–III (CT/RT/HT—Post-treatment) | N = 90 Exc = 47 UC = 43 | 55.6 (10.2) 55.9 (8.9) |
Lifestyle intervention 🗵 AE + RE + hypocaloric healthy eating program |  | 3 | 65–85% HRmax | 30′ (AE) 10–15′ (RE) |   | 24 | QoL | FACT-G, FACT-B |
| Segal (2001) [75] | I–II (CH/RT HT—during treatment) | N = 123 Exc S = 42 Exc SD = 40 UC = 41 | 51.0 (8.7) 51.4 (8.7) 50.3 (8.7) | 🗹 AE (S) Supervised 🗹 AE (SD) Self-directed |   | 5 3 | 50–60% VO2 max | 60′ |   | 26 | QoL | MOS SF-36, FACT-G, FACT-B |
| Shobeiri (2016) [76] | I–II (CT/RT—Post-treatment w or w/o HT) | N = 60 Exc = 30 UC = 30 | 42.70 (9.6) 43.50 (8.60) | 🗵 AE |  | 2 | 50–75% HRR | 25–45′ |  | 10 | Fatigue, QoL, Body image | EORTC QLQ-C30, EORTC QLQ-BR23 |
| Speack (2010) [77] PAL | 0–III, DCIS (CT/RT— Post-treatment) W or w/o lymphedema | N = 234 Exc = 59 Exc wL = 54 UC = 63 UC wL = 58 | 55 (7) 58 (9) 57 (8) 58 (9) | 🗹 RE 🗹 RE wL * wL = with lymphedema |  | 2 | not reported | 90′ |   | 52 | Body image, QoL | BIRS, SF-36 |
| Steindorf (2014) [78] BEST | 0–III (CT, RT, HT, trastuzumab therapy—during RT) | N = 155 Exc = 77 UC = 78 | 55.2 (9.5) 56.4 (8.7) | 🗹 RE |  | 2 | 60–80% 1RM | 60′ |  | 12 | Fatigue, QoL, Depression, Body image | FAQ, EORTC QLQ-C30, EORTC QLQ-C23, CES-D, |
| Travier (2015) [79] PACT | M0 (CT—During; RT after intervention) | N =164 Exc = 87 UC = 77 | 49.7 (8.2) 49.5 (7.9) | 🗹 AE + RE |  | 2 | Moderate- vigorous 65–75% to 45% 1RM (RE) HR ventilatory threshold (AE) | 60′ |  | 18 | Fatigue, QoL, Anxiety, Depression | MFI, FQL, SF-36, QLQ-C30, HADS |
| Wang (2011) [80] | I–II (CT/RT—before CT treatment) | N = 72 Exc = 35 UC = 37 | 48.40 (10.15) 52.3 (8.84) | 🗹 AE |  | 3–5 | 40–60% HRmax | ≥30′ |  | 6 | QoL, Fatigue, Sleep disturbances, | FACT-G, FACIT-F, PSQI |
| Winters (2012) [81] POWIR | I–III a (CT/RT—Post-treatment) | N = 106 Exc = 52 UC = 54 | 62.3 (6.7) 62.6 (6.7) | 🗹 RE + impact training (POWIR) UC = Stretching placebo program. (Flexibility) |  | 2 | 60–80% 1RM | 60′ |   | 52 | Fatigue | SCF, SF-36 |
 Supervised intervention;
Supervised intervention;  Home-based intervention;
Home-based intervention;  Aerobic exercise;
Aerobic exercise;  Resistance exercise/strength training;
Resistance exercise/strength training;  Flexibility/Stretching exercises;
Flexibility/Stretching exercises;  Progressive;
Progressive;  Aerobic exercise (control).
Aerobic exercise (control).Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez-Vélez, R.; Zambom-Ferraresi, F.; García-Hermoso, A.; Kievisiene, J.; Rauckiene-Michealsson, A.; Agostinis-Sobrinho, C. Evidence-Based Exercise Recommendations to Improve Mental Wellbeing in Women with Breast Cancer during Active Treatment: A Systematic Review and Meta-Analysis. Cancers 2021, 13, 264. https://doi.org/10.3390/cancers13020264
Ramírez-Vélez R, Zambom-Ferraresi F, García-Hermoso A, Kievisiene J, Rauckiene-Michealsson A, Agostinis-Sobrinho C. Evidence-Based Exercise Recommendations to Improve Mental Wellbeing in Women with Breast Cancer during Active Treatment: A Systematic Review and Meta-Analysis. Cancers. 2021; 13(2):264. https://doi.org/10.3390/cancers13020264
Chicago/Turabian StyleRamírez-Vélez, Robinson, Fabiola Zambom-Ferraresi, Antonio García-Hermoso, Justina Kievisiene, Alona Rauckiene-Michealsson, and César Agostinis-Sobrinho. 2021. "Evidence-Based Exercise Recommendations to Improve Mental Wellbeing in Women with Breast Cancer during Active Treatment: A Systematic Review and Meta-Analysis" Cancers 13, no. 2: 264. https://doi.org/10.3390/cancers13020264
APA StyleRamírez-Vélez, R., Zambom-Ferraresi, F., García-Hermoso, A., Kievisiene, J., Rauckiene-Michealsson, A., & Agostinis-Sobrinho, C. (2021). Evidence-Based Exercise Recommendations to Improve Mental Wellbeing in Women with Breast Cancer during Active Treatment: A Systematic Review and Meta-Analysis. Cancers, 13(2), 264. https://doi.org/10.3390/cancers13020264








