Aberrant Dyskerin Expression Is Related to Proliferation and Poor Survival in Endometrial Cancer
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
2.1. In Silico Interrogation of the Cancer Genome Atlas (TCGA) Endometrioid and Serous EC Dataset Demonstrates Dysregulation of DKC1 to Be Associated with Poor Survival
2.2. Study Cohort
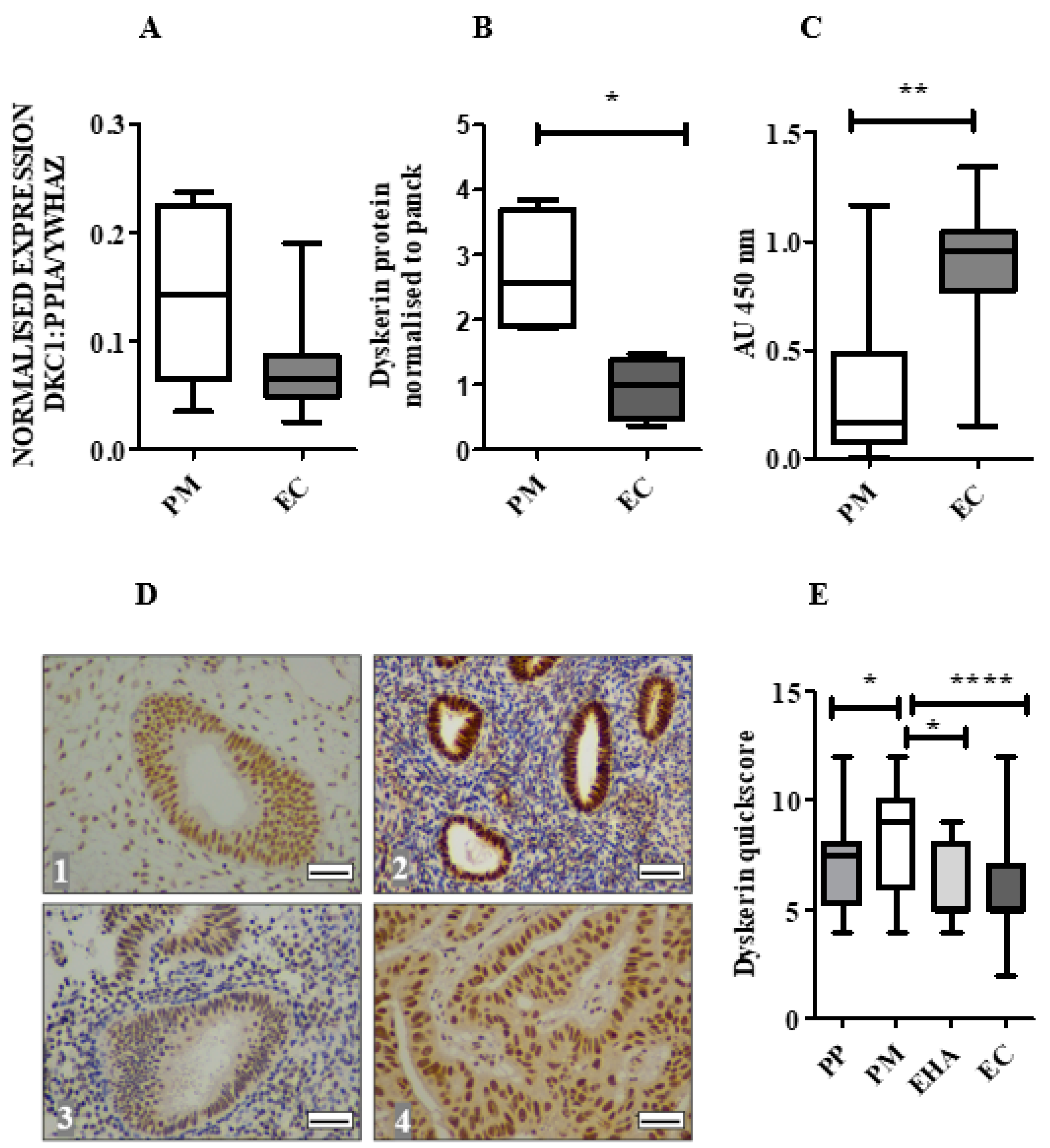
2.3. Dyskerin mRNA Was Lower in ECs Compared with Normal PM Endometrium
2.4. Dyskerin Protein is Significantly Reduced in ECs When Compared with Healthy PM Control Endometrium
2.5. Loss of Dyskerin Was a Feature of Precancerous and Cancerous Endometrial Epithelial Cells
2.6. Endometrial Epithelial Dyskerin Immunoscores Correlate with ERβ Scores and Inversely with the Ki67 Proliferation Index (PI)
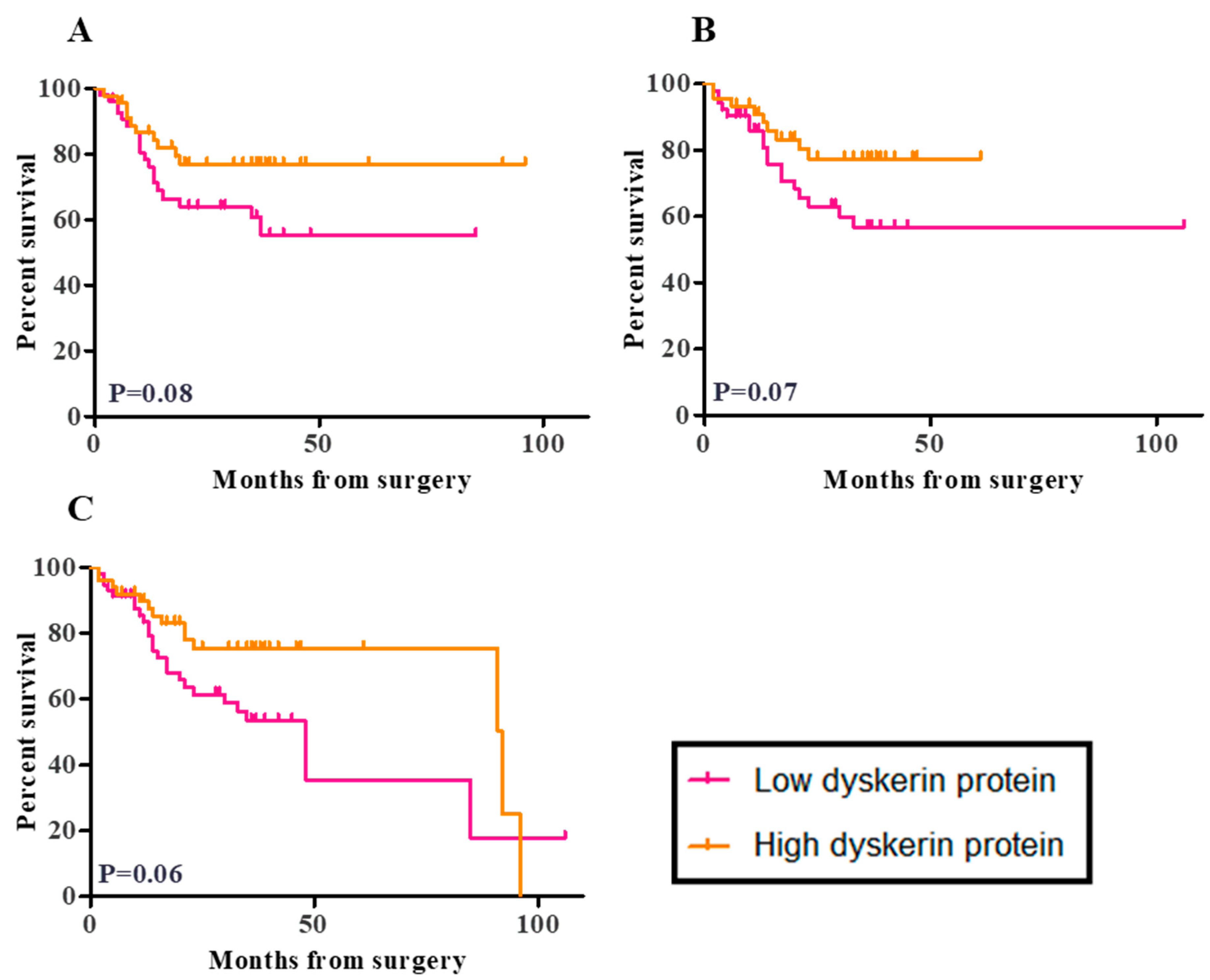
2.7. Survival Analysis
2.8. In Vitro Transient Transfection of ISK Cells with the DKC1 Gene Resulted in Successful Overexpression of Dyskerin Protein
2.9. Transient Overexpression of the DKC1 Gene Reduced ISK Cell Proliferation In Vitro
3. Discussion
4. Materials and Methods
4.1. Study Groups:
4.1.1. TCGA Database Cohort
4.1.2. Local Study Cohort
4.2. Collection of Endometrial Samples
4.3. Immunohistochemistry (IHC)
4.4. Real-Time qPCR
4.5. TRAP Assay
4.6. Cell Culture
4.7. Transient Transfection
4.8. SDS-PAGE and Immunoblotting
4.9. Immunofluorescence
4.10. CFSE Labelling and Flow Cytometry
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Lange, T. Shelterin: The protein complex that shapes and safeguards human telomeres. Genes Dev. 2005, 19, 2100–2110. [Google Scholar] [CrossRef]
- Griffith, J.D.; Comeau, L.; Rosenfield, S.; Stansel, R.M.; Bianchi, A.; Moss, H.; De Lange, T. Mammalian Telomeres End in a Large Duplex Loop. Cell 1999, 97, 503–514. [Google Scholar] [CrossRef]
- Van Steensel, B.; Smogorzewska, A.; de Lange, T. TRF2 protects human telomeres from end-to-end fusions. Cell 1998, 92, 401–413. [Google Scholar] [CrossRef]
- Levy, M.Z.; Allsopp, R.C.; Futcher, A.; Greider, C.W.; Harley, C.B. Telomere end-replication problem and cell aging. J. Mol. Biol. 1992, 225, 951–960. [Google Scholar] [CrossRef]
- Von Zglinicki, T.; Saretzki, G.; Döcke, W.; Lotze, C. Mild hyperoxia shortens telomeres and inhibits proliferation of fibroblasts: A model for senescence? Exp. Cell Res. 1995, 220, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J.; di Fagagna, F.D.A. Cellular senescence: When bad things happen to good cells. Nat. Rev. Mol. Cell Biol. 2007, 8, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, E.H.; Greider, C.W.; Henderson, E.; Lee, M.S.; Shampay, J.; Shippen-Lentz, D. Recognition and elongation of telomeres by telomerase. Genome 1989, 31, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.B.; Graham, M.E.; Lovrecz, G.O.; Bache, N.; Robinson, P.J.; Reddel, R.R. Protein Composition of Catalytically Active Human Telomerase from Immortal Cells. Science 2007, 315, 1850–1853. [Google Scholar] [CrossRef]
- Kim, N.W.; Piatyszek, M.A.; Prowse, K.R.; Harley, C.B.; West, M.D.; Ho, P.L.C.; Coviello, G.M.; Wright, W.E.; Weinrich, S.L.; Shay, J.W. Specific association of human telomerase activity with immortal cells and cancer. Science 1994, 266, 2011–2015. [Google Scholar] [CrossRef]
- Liu, K.; Schoonmaker, M.M.; Levine, B.L.; June, C.H.; Hodes, R.J.; Weng, N.-P. Constitutive and regulated expression of telomerase reverse transcriptase (hTERT) in human lymphocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 5147–5152. [Google Scholar] [CrossRef]
- Yasumoto, S.; Kunimura, C.; Kikuchi, K.; Tahara, H.; Ohji, H.; Yamamoto, H.; Ide, T.; Utakoji, T. Telomerase activity in normal human epithelial cells. Oncogene 1996, 13, 433–439. [Google Scholar] [PubMed]
- Hiyama, E.; Hiyama, K. Telomere and telomerase in stem cells. Br. J. Cancer 2007, 96, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- Valentijn, A.J.; Saretzki, G.; Tempest, N.; Critchley, H.O.D.; Hapangama, D.K. Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Hum. Reprod. 2015, 30, 2816–2828. [Google Scholar] [CrossRef] [PubMed]
- Khattar, E.; Kumar, P.; Liu, C.Y.; Akıncılar, S.C.; Raju, A.; Lakshmanan, M.; Maury, J.J.P.; Qiang, Y.; Li, S.; Tan, E.Y.; et al. Telomerase reverse transcriptase promotes cancer cell proliferation by augmenting tRNA expression. J. Clin. Investig. 2016, 126, 4045–4060. [Google Scholar] [CrossRef] [PubMed]
- Mistry, M.; Parkin, D.M.; Ahmad, A.S.; Sasieni, P. Cancer incidence in the United Kingdom: Projections to the year 2030. Br. J. Cancer 2011, 105, 1795–1803. [Google Scholar] [CrossRef]
- CRUK. Uterine Cancer Statistics. Available online: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/uterine-cancer (accessed on 4 January 2020).
- Kyo, S.; Kanaya, T.; Ishikawa, H.; Ueno, H.; Inoue, M. Telomerase activity in gynecological tumors. Clin. Cancer Res. 1996, 2, 2023–2028. [Google Scholar]
- Ebina, Y.; Yamada, H.; Fujino, T.; Furuta, I.; Sakuragi, N.; Yamamoto, R.; Katoh, M.; Oshimura, M.; Fujimoto, S. Telomerase activity correlates with histo-pathological factors in uterine endometrial carcinoma. Int. J. Cancer 1999, 84, 529–532. [Google Scholar] [CrossRef]
- Marrone, A.; Mason, P.J. Dyskeratosis congenita. Cell Mol. Life Sci. 2003, 60, 507–517. [Google Scholar] [CrossRef]
- Montanaro, L.; Calienni, M.; Ceccarelli, C.; Santini, N.; Taffurelli, M.; Pileri, S.; Treré, D.; Derenzini, M. Relationship between Dyskerin Expression and Telomerase Activity in Human Breast Cancer. Cell. Oncol. 2008, 30, 483–490. [Google Scholar]
- Montanaro, L.; Brigotti, M.; Clohessy, J.; Barbieri, S.; Ceccarelli, C.; Santini, D.; Taffurelli, M.; Calienni, M.; Teruya-Feldstein, J.; Trerè, D.; et al. Dyskerin expression influences the level of ribosomal RNA pseudo-uridylation and telomerase RNA component in human breast cancer. J. Pathol. 2006, 210, 10–18. [Google Scholar] [CrossRef]
- Ruggero, D.; Grisendi, S.; Piazza, F.; Rego, E.; Mari, F.; Rao, P.H.; Cordon-Cardo, C.; Pandolfi, P.P. Dyskeratosis Congenita and Cancer in Mice Deficient in Ribosomal RNA Modification. Science 2003, 299, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Alawi, F.; Lin, P. Dyskerin is required for tumor cell growth through mechanisms that are independent of its role in te-lomerase and only partially related to its function in precursor rRNA processing. Mol. Carcinog. 2011, 50, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Alnafakh, R.A.A.; Adishesh, M.; Button, L.; Saretzki, G.; Hapangama, D.K. Telomerase and Telomeres in Endometrial Cancer. Front. Oncol. 2019, 9, 344. [Google Scholar] [CrossRef] [PubMed]
- Sieron, P.; Hader, C.; Hatina, J.; Engers, R.; Wlazlinski, A.; Müller, M.; Schulz, W.A. DKC1 overexpression associated with prostate cancer progression. Br. J. Cancer 2009, 101, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- ElSharawy, K.A.; Mohammed, O.J.; Aleskandarany, M.A.; Hyder, A.; El-Gammal, H.L.; Abou-Dobara, M.I.; Green, A.R.; Dalton, L.W.; Rakha, E.A. The nucleolar-related protein Dyskerin pseudouridine synthase 1 (DKC1) predicts poor prognosis in breast cancer. Br. J. Cancer 2020, 123, 1543–1552. [Google Scholar] [CrossRef]
- Bellodi, C.; Krasnykh, O.; Haynes, N.; Theodoropoulou, M.; Peng, G.; Montanaro, L.; Ruggero, D. Loss of function of the tumor suppressor DKC1 perturbs p27 translation control and contributes to pituitary tumorigenesis. Cancer Res. 2010, 70, 6026–6035. [Google Scholar] [CrossRef] [PubMed]
- Parry, E.M.; Alder, J.K.; Lee, S.S.; Phillips, J.A.; Loyd, J.E.; Duggal, P.; Armanios, M. Decreased dyskerin levels as a mechanism of telomere shortening in X-linked dyskeratosis congenita. J. Med. Genet. 2011, 48, 327–333. [Google Scholar] [CrossRef]
- Alter, B.P.; Giri, N.; Savage, S.A.; Rosenberg, P.S. Cancer in dyskeratosis congenita. Blood 2009, 113, 6549–6557. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Stern, D.F.; Zhao, H. Transcriptional Profiles from Paired Normal Samples Offer Complementary Information on Cancer Patient Survival—Evidence from TCGA Pan-Cancer Data. Sci. Rep. 2016, 6, 20567. [Google Scholar] [CrossRef]
- Sundar, S.; Balega, J.; Crosbie, E.; Drake, A.; Edmondson, R.; Fotopoulou, C.; Gallos, I.; Ganesan, R.; Gupta, J.; Johnson, N.; et al. BGCS uterine cancer guidelines: Recommendations for practice. Eur. J. Obs. Gynecol. Reprod. Biol. 2017, 213, 71–97. [Google Scholar] [CrossRef]
- Wang, S.-J.; Sakamoto, T.; Yasuda, S.-I.; Fukasawa, I.; Ota, Y.; Hayashi, M.; Okura, T.; Zheng, J.-H.; Inaba, N. The Relationship between Telomere Length and Telomerase Activity in Gynecologic Cancers. Gynecol. Oncol. 2002, 84, 81–84. [Google Scholar] [CrossRef]
- Sbarrato, T.; Horvilleur, E.; Pöyry, T.; Hill, K.; Chaplin, L.C.; Spriggs, R.V.; Stoneley, M.; Wilson, L.; Jayne, S.; Vulliamy, T.; et al. A ribosome-related signature in peripheral blood CLL B cells is linked to reduced survival following treat-ment. Cell Death Dis. 2016, 7, e2249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, B.; Zhang, J.; Huang, C.; Liu, H. Dyskerin Overexpression in Human Hepatocellular Carcinoma Is Associated with Advanced Clinical Stage and Poor Patient Prognosis. PLoS ONE 2012, 7, e43147. [Google Scholar] [CrossRef] [PubMed]
- Turano, M.; Angrisani, A.; De Rosa, M.; Izzo, P.; Furia, M. Real-time PCR quantification of human DKC1 expression in colorectal cancer. Acta Oncol. 2008, 47, 1598–1599. [Google Scholar] [CrossRef]
- Chen, C.-H.; Chen, R.-J. Prevalence of Telomerase Activity in Human Cancer. J. Med. Assoc. 2011, 110, 275–289. [Google Scholar] [CrossRef]
- Tanaka, M.; Kyo, S.; Takakura, M.; Kanaya, T.; Sagawa, T.; Yamashita, K.; Okada, Y.; Hiyama, E.; Inoue, M. Expression of telomerase activity in human endometrium is localized to epithelial glandular cells and regu-lated in a menstrual phase-dependent manner correlated with cell proliferation. Am. J. Pathol. 1998, 153, 1985–1991. [Google Scholar] [CrossRef]
- Kamal, A.M.; Bulmer, J.N.; DeCruze, S.B.; Stringfellow, H.F.; Martin-Hirsch, P.; Hapangama, D.K. Androgen receptors are acquired by healthy postmenopausal endometrial epithelium and their subsequent loss in endometrial cancer is associated with poor survival. Br. J. Cancer 2016, 114, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Cabellos, J.S.; Pérez-Machado, G.; Seco-Cervera, M.; Berenguer-Pascual, E.; García-Giménez, J.L.; Pallardó, F.V. Acute telomerase components depletion triggers oxidative stress as an early event previous to telomeric shortening. Redox Biol. 2018, 14, 398–408. [Google Scholar] [CrossRef]
- Angrisani, A.; Vicidomini, R.; Turano, M.; Furia, M. Human dyskerin: Beyond telomeres. Biol. Chem. 2014, 395, 593–610. [Google Scholar] [CrossRef]
- Dos Santos, P.C.; Panero, J.; Stanganelli, C.; Nagore, V.P.; Stella, F.; Bezares, R.; Slavutsky, I. Dysregulation of H/ACA ribonucleoprotein components in chronic lymphocytic leukemia. PLoS ONE 2017, 12, e0179883. [Google Scholar] [CrossRef]
- Penzo, M.; Rocchi, L.; Brugiere, S.; Carnicelli, D.; Onofrillo, C.; Couté, Y.; Brigotti, M.; Montanaro, L. Human ribosomes from cells with reduced dyskerin levels are intrinsically altered in translation. FASEB J. 2015, 29, 3472–3482. [Google Scholar] [CrossRef] [PubMed]
- Montanaro, L.; Calienni, M.; Bertoni, S.; Rocchi, L.; Sansone, P.; Storci, G.; Santini, D.; Ceccarelli, C.; Taffurelli, M.; Carnicelli, D.; et al. Novel Dyskerin-Mediated Mechanism of p53 Inactivation through Defective mRNA Translation. Cancer Res. 2010, 70, 4767–4777. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, J.; González, A.; Manguán-García, C.; Pintado-Berninches, L.; Perona, R. p53 pathway activation by telomere attrition in X-DC primary fibroblasts occurs in the absence of ribosome biogenesis failure and as a consequence of DNA damage. Clin. Transl. Oncol. 2013, 16, 529–538. [Google Scholar] [CrossRef]
- Bellodi, C.; Kopmar, N.; Ruggero, D. Deregulation of oncogene-induced senescence and p53 translational control in X-linked dyskeratosis congenita. EMBO J. 2010, 29, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Hapangama, D.K.; Kamal, A.; Bulmer, J. Estrogen receptor β: The guardian of the endometrium. Hum. Reprod. Updat. 2015, 21, 174–193. [Google Scholar] [CrossRef] [PubMed]
- Kupershmidt, I.; Su, Q.J.; Grewal, A.; Sundaresh, S.; Halperin, I.; Flynn, J.; Shekar, M.; Wang, H.; Park, J.; Cui, W.; et al. Ontology-Based Meta-Analysis of Global Collections of High-Throughput Public Data. PLoS ONE 2010, 5, e13066. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2009, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Zaino, R.J.; Kurman, R.J.; Diana, K.L.; Paul Morrow, C. The utility of the revised International Federation of Gynecology and Obstetrics histologic grading of endometrial adenocarcinoma using a defined nuclear grading system. A Gynecologic Oncology Group study. Cancer 1995, 75, 81–86. [Google Scholar] [CrossRef]
- Vossa, M.A.; Ganesan, R.; Ludeman, L.; McCarthy, K.; Gornall, R.; Schaller, G.; Wei, W.; Sundar, S. Should grade 3 endometrioid endometrial carcinoma be considered a type 2 cancer-a clinical and patholog-ical evaluation. Gynecol. Oncol. 2012, 124, 15–20. [Google Scholar] [CrossRef]
- Noyes, R.W.; Hertig, A.T.; Rock, J. Dating the endometrial biopsy. Am. J. Obs. Gynecol. 1975, 122, 262–263. [Google Scholar] [CrossRef]
- MacLean, A.; Kamal, A.M.; Adishesh, M.; Alnafakh, R.; Tempest, N.; Hapangama, D.K. Human Uterine Biopsy: Research Value and Common Pitfalls. Int. J. Reprod. Med. 2020, 2020, 9275360. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Drury, J.A.; Valentijn, A.J.; Vasieva, O.; Hapangama, D.K. In silico, in vitro and in vivo analysis identifies a potential role for steroid hormone regulation of FOXD3 in endometriosis-associated genes. Hum. Reprod. 2016, 31, 345–354. [Google Scholar] [PubMed]
- Jacob, F.; Guertler, R.; Naim, S.; Nixdorf, S.; Fedier, A.; Hacker, N.F.; Heinzelmann-Schwarz, V. Careful selection of reference genes is required for reliable performance of RT-qPCR in human normal and cancer cell lines. PLoS ONE 2013, 8, e59180. [Google Scholar] [CrossRef] [PubMed]
- Marullo, M.; Zuccato, C.; Mariotti, C.; Lahiri, N.; Tabrizi, S.J.; Di Donato, S.; Cattaneo, E. Expressed Alu repeats as a novel, reliable tool for normalization of real-time quantitative RT-PCR data. Genome Biol. 2010, 11, R9. [Google Scholar] [CrossRef]
- Romani, C.; Calza, S.; Todeschini, P.; Tassi, R.A.; Zanotti, L.; Bandiera, E.; Sartori, E.; Pecorelli, S.; Ravaggi, A.; Santin, A.D.; et al. Identification of optimal reference genes for gene expression normalization in a wide cohort of endometri-oid endometrial carcinoma tissues. PLoS ONE 2014, 9, e113781. [Google Scholar] [CrossRef]
- Sadek, K.H.; Cagampang, F.R.; Bruce, K.D.; Shreeve, N.; Macklon, N.; Cheong, Y. Variation in stability of housekeeping genes in endometrium of healthy and polycystic ovarian syndrome women. Hum. Reprod. 2011, 27, 251–256. [Google Scholar] [CrossRef]
- Parkes, C.; Kamal, A.; Valentijn, A.J.; Alnafakh, R.; Gross, S.R.; Barraclough, R.; Moss, D.; Kirwan, J.; Hapangama, D.K. Assessing Estrogen-Induced Proliferative Response in an Endometrial Cancer Cell Line Using a Universally Applicable Methodological Guide. Int. J. Gynecol. Cancer 2018, 28, 122–133. [Google Scholar] [CrossRef]
- Alnafakh, R.; Choi, F.; Bradfield, A.; Adishesh, M.; Saretzki, G.; Hapangama, D.K. Endometriosis Is Associated with a Significant Increase in hTERC and Altered Telomere/Telomerase Associated Genes in the Eutopic Endometrium, an Ex-Vivo and In Silico Study. Biomedicines 2020, 8, 588. [Google Scholar] [CrossRef]




| Study Groups | No | % | * Age (Years) | ** BMI (kg /m2) |
|---|---|---|---|---|
| 1. Healthy (total) | 51 | |||
| • Proliferative phase | 16 | 40 (30–57) | 27 (18–41) | |
| • Postmenopausal | 35 | 63 (40–85) | 26 (20–40) | |
| 2. Endometrial hyperplasia | 15 | 55 (48–72) | 36 (24–57) | |
| 3. Endometrial cancer (total) | 109 | 68 (37–96) | 30 (20–54) | |
| LGEC | 53 | 48.6 | 64 (37–89) | 32 (21–54) |
| • Endometrioid Grade 1 | 34 | 31.2 | 64 (46–89) | 33 (21–53) |
| • Endometrioid Grade 2 | 19 | 17.4 | 60 (37–78) | 30 (22–54) |
| HGEC | 56 | 51.4 | 73 (48–96) | 30 (20–43) |
| • Endometrioid Grade 3 | 12 | 11 | 68 (54–96) | 28 (24–43) |
| • Serous | 12 | 11 | 76 (64–87) | 29 (23–39) |
| • Clear cell | 10 | 9.1 | 74 (48–82) | 30 (27–39) |
| • Carcinosarcoma | 19 | 17.4 | 78 (60–89) | 26 (20–37) |
| • Dedifferentiated | 1 | 0.9 | 79 | 32 |
| • Mixed cell adenocarcinoma | 2 | 1.8 | 63 & 66 | |
| • Metastatic EC | 34 | 68 (27–96) | 28 (21–43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alnafakh, R.; Saretzki, G.; Midgley, A.; Flynn, J.; Kamal, A.M.; Dobson, L.; Natarajan, P.; Stringfellow, H.; Martin-Hirsch, P.; DeCruze, S.B.; et al. Aberrant Dyskerin Expression Is Related to Proliferation and Poor Survival in Endometrial Cancer. Cancers 2021, 13, 273. https://doi.org/10.3390/cancers13020273
Alnafakh R, Saretzki G, Midgley A, Flynn J, Kamal AM, Dobson L, Natarajan P, Stringfellow H, Martin-Hirsch P, DeCruze SB, et al. Aberrant Dyskerin Expression Is Related to Proliferation and Poor Survival in Endometrial Cancer. Cancers. 2021; 13(2):273. https://doi.org/10.3390/cancers13020273
Chicago/Turabian StyleAlnafakh, Rafah, Gabriele Saretzki, Angela Midgley, James Flynn, Areege M. Kamal, Lucy Dobson, Purushothaman Natarajan, Helen Stringfellow, Pierre Martin-Hirsch, Shandya B. DeCruze, and et al. 2021. "Aberrant Dyskerin Expression Is Related to Proliferation and Poor Survival in Endometrial Cancer" Cancers 13, no. 2: 273. https://doi.org/10.3390/cancers13020273
APA StyleAlnafakh, R., Saretzki, G., Midgley, A., Flynn, J., Kamal, A. M., Dobson, L., Natarajan, P., Stringfellow, H., Martin-Hirsch, P., DeCruze, S. B., Coupland, S. E., & Hapangama, D. K. (2021). Aberrant Dyskerin Expression Is Related to Proliferation and Poor Survival in Endometrial Cancer. Cancers, 13(2), 273. https://doi.org/10.3390/cancers13020273






