The Calcitriol/Vitamin D Receptor System Regulates Key Immune Signaling Pathways in Chronic Lymphocytic Leukemia
Abstract
Simple Summary
Abstract
1. Introduction
2. Results
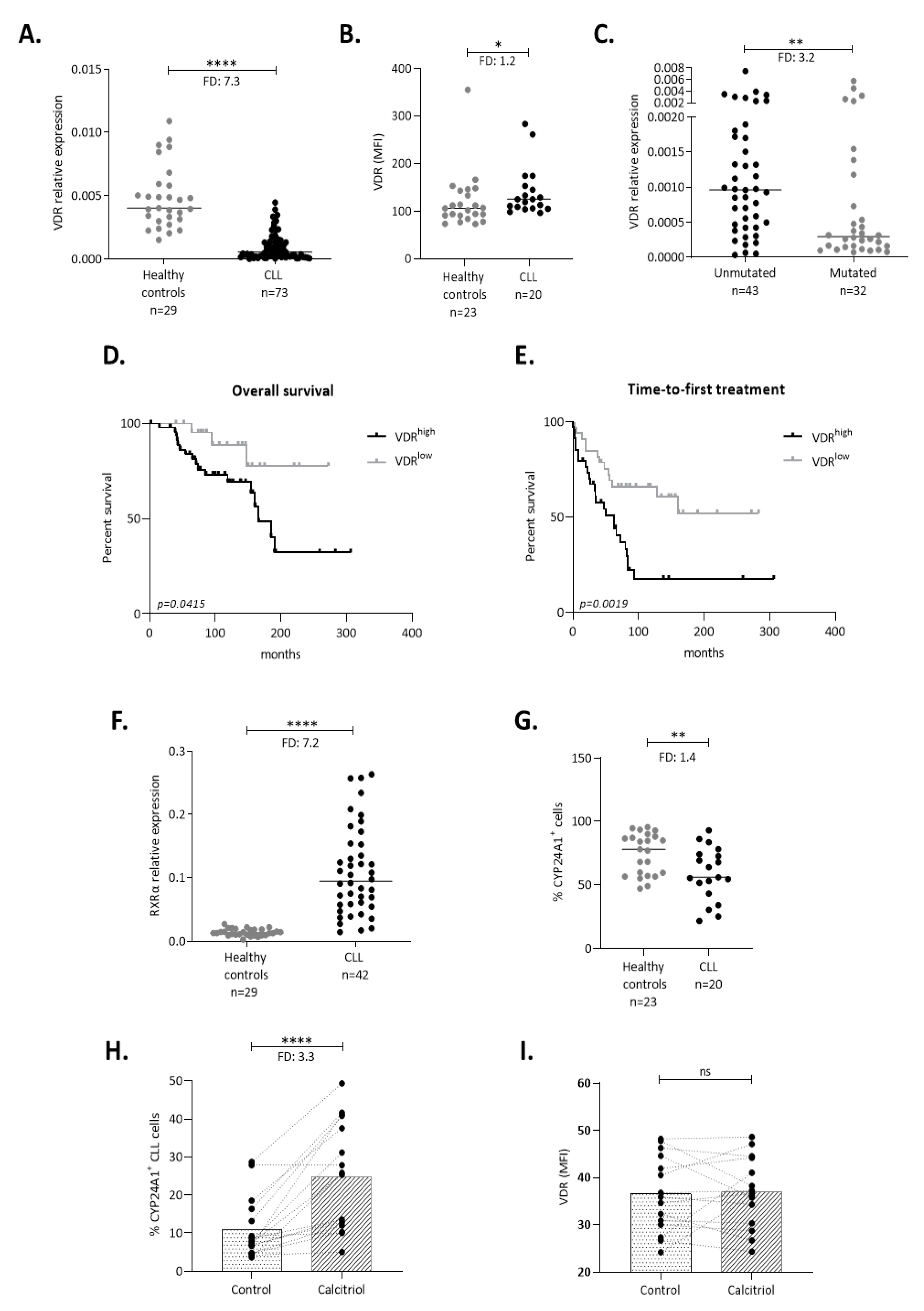
2.1. The VDR Is Expressed and Acts as a Transcription Factor in CLL Cells
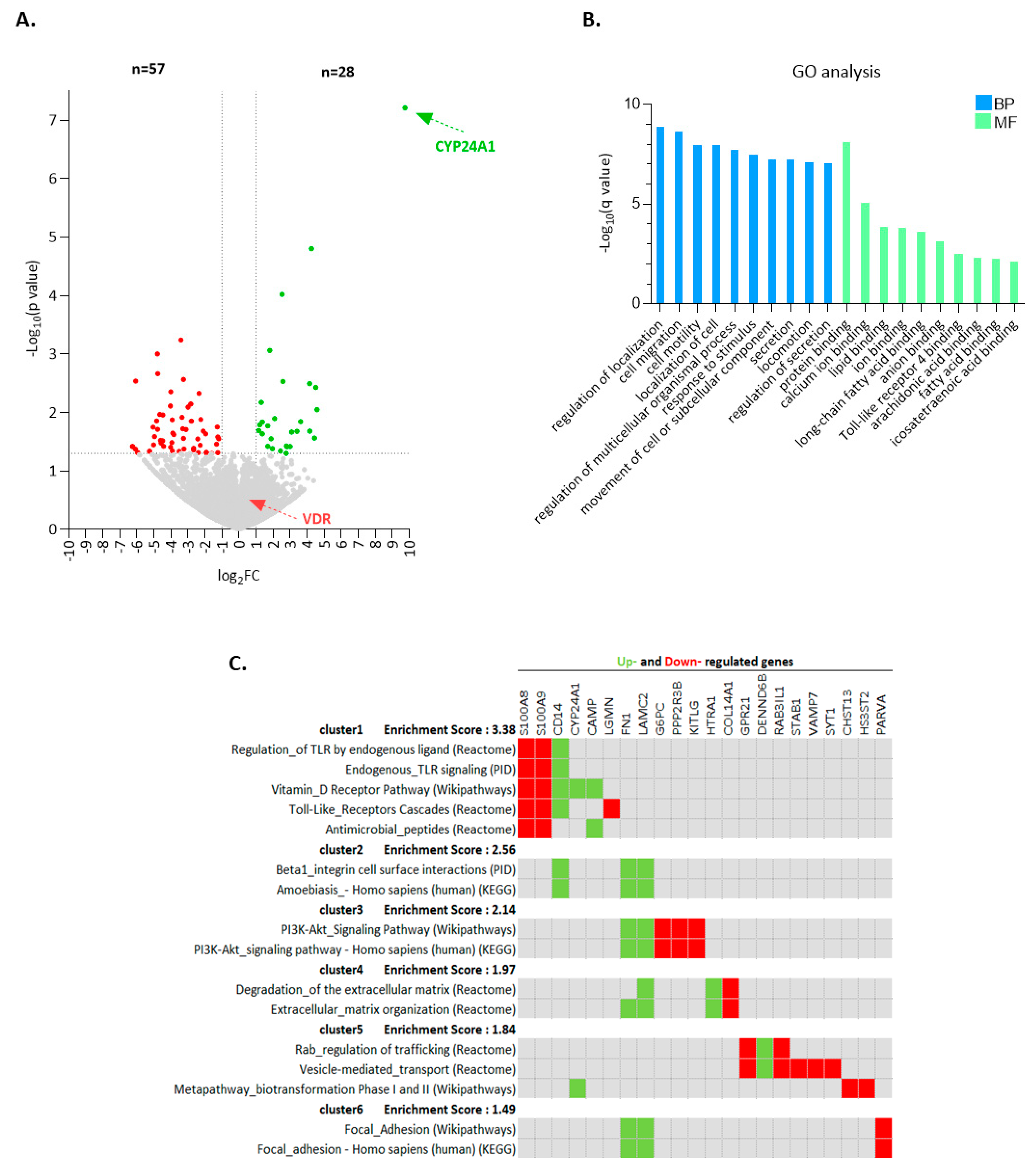
2.2. Calcitriol Induces a Distinctive Transcriptional Program in CLL Cells
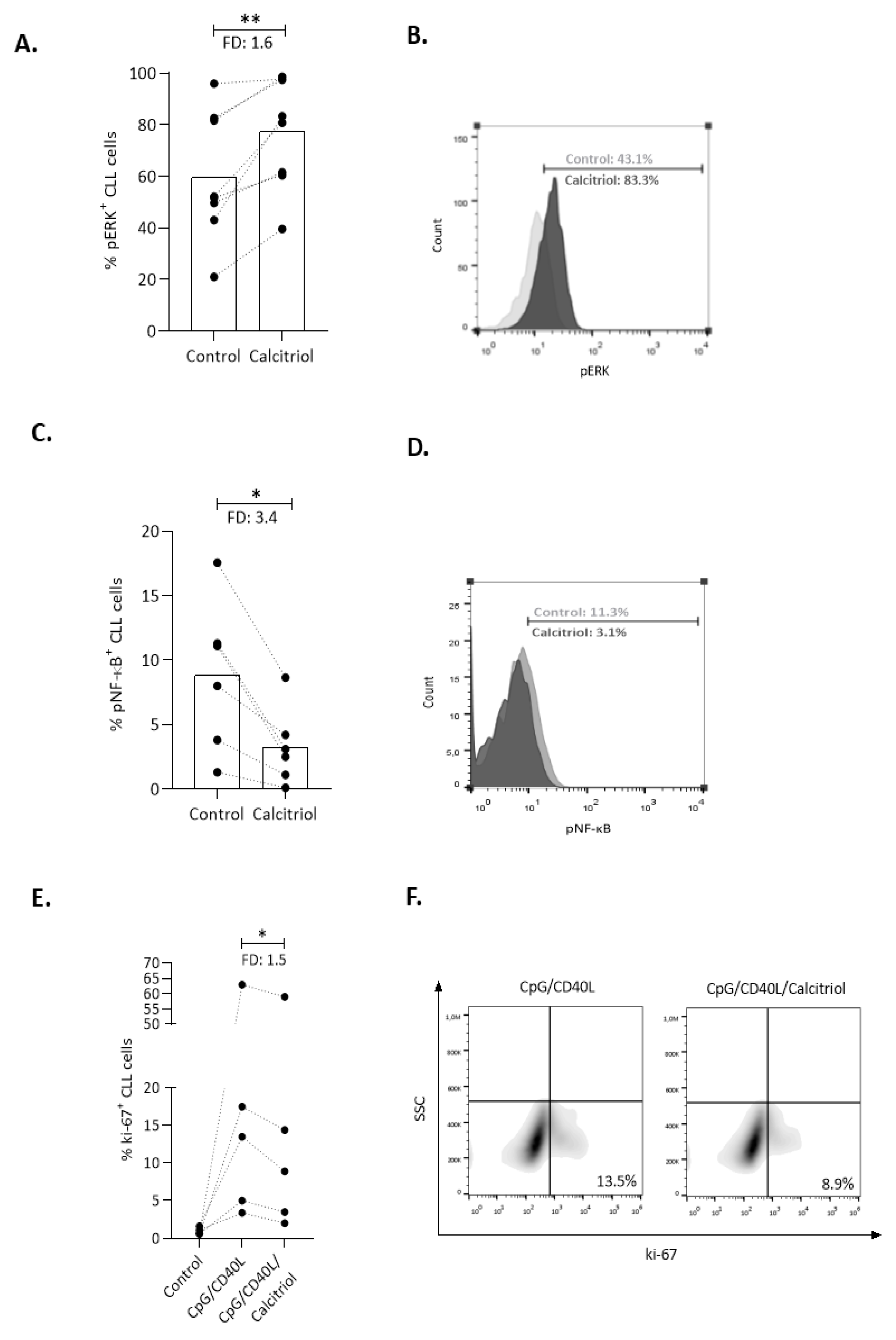
2.3. Calcitriol Affects Key Immune Signaling Cascades in CLL Cells
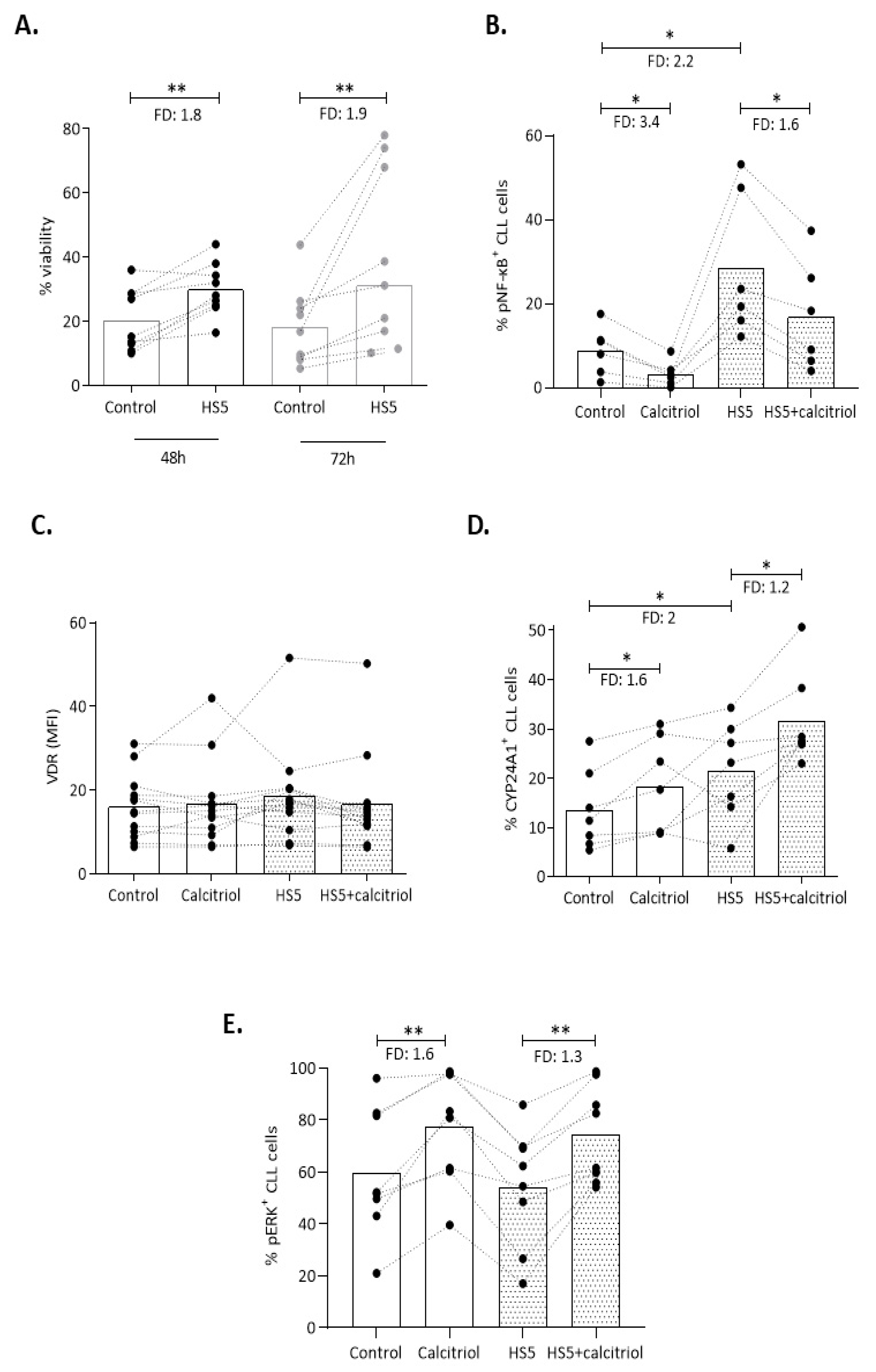
2.4. Mincroenvironmental Signals Affect VDR Signaling, yet Do not Impede the Action of Calcitriol in CLL
2.5. Preserved Calcitriol/VDR Signaling Capacity in CLL Cases under Ibrutinib Therapy
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Cell Isolation and Cell Cultures
4.3. Quantification of VDR and RXRα mRNA Expression
4.4. RNA Sequencing
4.5. Flow Cytometry Studies
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haussler, M.R.; Whitfield, G.K.; Haussler, C.A.; Hsieh, J.C.; Thompson, P.D.; Selznick, S.H.; Dominguez, C.E.; Jurutka, P.W. The Nuclear Vitamin D Receptor: Biological and Molecular Regulatory Properties Revealed. J. Bone Miner. Res. 1998, 13, 325–349. [Google Scholar] [CrossRef] [PubMed]
- Carlberg, C.; Bendik, I.; Wyss, A.; Meier, E.; Sturzenbecker, L.J.; Grippo, J.F.; Hunziker, W. Two Nuclear Signalling Pathways for Vitamin D. Nature 1993, 361, 657–660. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Sims, G.P.; Chen, X.X.; Gu, Y.Y.; Chen, S.; Lipsky, P.E. Modulatory Effects of 1,25-Dihydroxyvitamin D3 on Human B Cell Differentiation. J. Immunol. 2007, 179, 1634–1647. [Google Scholar] [CrossRef] [PubMed]
- Rolf, L.; Muris, A.H.; Hupperts, R.; Damoiseaux, J. Vitamin D Effects on B Cell Function in Autoimmunity: Vitamin D Effects on B Cells. Ann. N. Y. Acad. Sci. 2014, 1317, 84–91. [Google Scholar] [CrossRef]
- Ryan, J.W.; Anderson, P.H.; Morris, H.A. Pleiotropic Activities of Vitamin D Receptors–Adequate Activation for Multiple Health Outcomes. Clin. Biochem. Rev. 2015, 36, 53–61. [Google Scholar] [PubMed]
- Hii, C.S.; Ferrante, A. The Non-Genomic Actions of Vitamin D. Nutrients 2016, 8. [Google Scholar] [CrossRef]
- DeLuca, H.F. Overview of General Physiologic Features and Functions of Vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef]
- Deeb, K.K.; Trump, D.L.; Johnson, C.S. Vitamin D Signalling Pathways in Cancer: Potential for Anticancer Therapeutics. Nat. Rev. Cancer 2007, 7, 684–700. [Google Scholar] [CrossRef]
- Heine, G.; Anton, K.; Henz, B.M.; Worm, M. 1alpha,25-Dihydroxyvitamin D3 Inhibits Anti-CD40 plus IL-4-Mediated IgE Production in Vitro. Eur. J. Immunol. 2002, 32, 3395–3404. [Google Scholar] [CrossRef]
- Matheu, V.; Bäck, O.; Mondoc, E.; Issazadeh-Navikas, S. Dual Effects of Vitamin D-Induced Alteration of TH1/TH2 Cytokine Expression: Enhancing IgE Production and Decreasing Airway Eosinophilia in Murine Allergic Airway Disease. J. Allergy Clin. Immunol. 2003, 112, 585–592. [Google Scholar] [CrossRef]
- Kelly, J.L.; Salles, G.; Goldman, B.; Fisher, R.I.; Brice, P.; Press, O.; Casasnovas, O.; Maloney, D.G.; Soubeyran, P.; Rimsza, L.; et al. Low Serum Vitamin D Levels Are Associated With Inferior Survival in Follicular Lymphoma: A Prospective Evaluation in SWOG and LYSA Studies. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1482–1490. [Google Scholar] [CrossRef] [PubMed]
- Bittenbring, J.T.; Neumann, F.; Altmann, B.; Achenbach, M.; Reichrath, J.; Ziepert, M.; Geisel, J.; Regitz, E.; Held, G.; Pfreundschuh, M. Vitamin D Deficiency Impairs Rituximab-Mediated Cellular Cytotoxicity and Outcome of Patients With Diffuse Large B-Cell Lymphoma Treated With but Not Without Rituximab. J. Clin. Oncol. 2014, 32, 3242–3248. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Drake, M.T.; Maurer, M.J.; Allmer, C.; Rabe, K.G.; Slager, S.L.; Weiner, G.J.; Call, T.G.; Link, B.K.; Zent, C.S.; et al. Vitamin D Insufficiency and Prognosis in Chronic Lymphocytic Leukemia. Blood 2011, 117, 1492–1498. [Google Scholar] [CrossRef] [PubMed]
- Hallek, M. Chronic Lymphocytic Leukemia: 2015 Update on Diagnosis, Risk Stratification, and Treatment. Am. J. Hematol. 2015, 90, 446–460. [Google Scholar] [CrossRef] [PubMed]
- Rozovski, U.; Keating, M.J.; Estrov, Z. Targeting Inflammatory Pathways in Chronic Lymphocytic Leukemia. Crit. Rev. Oncol. Hematol. 2013, 88. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Digiesi, G.; Antenucci, A.; Levato, L.; Mirabelli, R.; Molica, M.; Gentile, M.; Giannarelli, D.; Sperduti, I.; Morabito, F.; et al. Vitamin D Insufficiency Predicts Time to First Treatment (TFT) in Early Chronic Lymphocytic Leukemia (CLL). Leuk. Res. 2012, 36, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Sfeir, J.G.; Drake, M.T.; LaPlant, B.R.; Maurer, M.J.; Link, B.K.; Berndt, T.J.; Shanafelt, T.D.; Cerhan, J.R.; Habermann, T.M.; Feldman, A.L.; et al. Validation of a Vitamin D Replacement Strategy in Vitamin D-Insufficient Patients with Lymphoma or Chronic Lymphocytic Leukemia. Blood Cancer J. 2017, 7, e526. [Google Scholar] [CrossRef]
- Kubeczko, M.; Nowara, E.; Spychałowicz, W.; Wdowiak, K.; Bednarek, A.; Karwasiecka, D.; Chudek, J.; Wojnar, J. Efficacy and Safety of Vitamin D Supplementation in Patients with Chronic Lymphocytic Leukemia. Postepy Hig. Med. Doswiadczalnej Online 2016, 70, 534–541. [Google Scholar] [CrossRef]
- Pepper, C. The Vitamin D3 Analog EB1089 Induces Apoptosis via a P53-Independent Mechanism Involving P38 MAP Kinase Activation and Suppression of ERK Activity in B-Cell Chronic Lymphocytic Leukemia Cells in Vitro. Blood 2003, 101, 2454–2459. [Google Scholar] [CrossRef]
- Kubeczko, M.; Nowara, E.; Karwasiecka, D.; Siewior, G.; Czajka-Francuz, P.; Chudek, J.; Wojnar, J.C.C. Motif Ligand 11 Reduction in CLL Patients Serum after Vitamin D Supplementation. Hematology 2016, 21, 343–350. [Google Scholar] [CrossRef]
- Bruns, H.; Böttcher, M.; Qorraj, M.; Fabri, M.; Jitschin, S.; Dindorf, J.; Busch, L.; Jitschin, R.; Mackensen, A.; Mougiakakos, D. CLL-Cell-Mediated MDSC Induction by Exosomal MiR-155 Transfer Is Disrupted by Vitamin D. Leukemia 2017, 31, 985–988. [Google Scholar] [CrossRef] [PubMed]
- Arvaniti, E.; Ntoufa, S.; Papakonstantinou, N.; Touloumenidou, T.; Laoutaris, N.; Anagnostopoulos, A.; Lamnissou, K.; Caligaris-Cappio, F.; Stamatopoulos, K.; Ghia, P.; et al. Toll-like Receptor Signaling Pathway in Chronic Lymphocytic Leukemia: Distinct Gene Expression Profiles of Potential Pathogenic Significance in Specific Subsets of Patients. Haematologica 2011, 96, 1644–1652. [Google Scholar] [CrossRef]
- Palacios, F.; Abreu, C.; Prieto, D.; Morande, P.; Ruiz, S.; Fernández-Calero, T.; Naya, H.; Libisch, G.; Robello, C.; Landoni, A.I.; et al. Activation of the PI3K/AKT Pathway by MicroRNA-22 Results in CLL B-Cell Proliferation. Leukemia 2015, 29, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Prieto, D.; Sotelo, N.; Seija, N.; Sernbo, S.; Abreu, C.; Durán, R.; Gil, M.; Sicco, E.; Irigoin, V.; Oliver, C.; et al. S100-A9 Protein in Exosomes from Chronic Lymphocytic Leukemia Cells Promotes NF-ΚB Activity during Disease Progression. Blood 2017, 130, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.F.; Pontier, A.; Gurbuxani, S.; Sipkins, D.A. Stem Cell Factor Expression in B Cell Malignancies Is Influenced by the Niche. Leuk. Lymphoma 2013, 54, 2274–2280. [Google Scholar] [CrossRef] [PubMed]
- Purroy, N.; Abrisqueta, P.; Carabia, J.; Carpio, C.; Palacio, C.; Bosch, F.; Crespo, M. Co-Culture of Primary CLL Cells with Bone Marrow Mesenchymal Cells, CD40 Ligand and CpG ODN Promotes Proliferation of Chemoresistant CLL Cells Phenotypically Comparable to Those Proliferating in Vivo. Oncotarget 2015, 6, 7632–7643. [Google Scholar] [CrossRef] [PubMed]
- Trimarco, V.; Ave, E.; Facco, M.; Chiodin, G.; Frezzato, F.; Martini, V.; Gattazzo, C.; Lessi, F.; Giorgi, C.A.; Visentin, A.; et al. Cross-Talk between Chronic Lymphocytic Leukemia (CLL) Tumor B Cells and Mesenchymal Stromal Cells (MSCs): Implications for Neoplastic Cell Survival. Oncotarget 2015, 6, 42130–42149. [Google Scholar] [CrossRef] [PubMed]
- Lwin, T.; Hazlehurst, L.; Li, Z.; Dessureault, S.; Sotomayor, E.; Moscinski, L.; Dalton, W.; Tao, J. Bone Marrow Stromal Cells Prevent Apoptosis of Lymphoma Cells by Upregulation of Anti-Apoptotic Proteins Associated with Activation of NF-JB (RelB/P52) in Non-Hodgkin’s Lymphoma Cells. Leukemia 2007, 21, 121–1531. [Google Scholar] [CrossRef]
- Byrd, J.C.; Furman, R.R.; Coutre, S.E.; Flinn, I.W.; Burger, J.A.; Blum, K.A.; Grant, B.; Sharman, J.P.; Coleman, M.; Wierda, W.G.; et al. Targeting BTK with Ibrutinib in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2013, 369, 32–42. [Google Scholar] [CrossRef]
- Farinello, D.; Wozińska, M.; Lenti, E.; Genovese, L.; Bianchessi, S.; Migliori, E.; Sacchetti, N.; di Lillo, A.; Bertilaccio, M.T.S.; de Lalla, C.; et al. A Retinoic Acid-Dependent Stroma-Leukemia Crosstalk Promotes Chronic Lymphocytic Leukemia Progression. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Song, X.; Bishop, J.E.; Okamura, W.H.; Norman, A.W. Stimulation of Phosphorylation of Mitogen-Activated Protein Kinase by 1alpha,25-Dihydroxyvitamin D3 in Promyelocytic NB4 Leukemia Cells: A Structure-Function Study. Endocrinol. 1998, 139, 457–465. [Google Scholar] [CrossRef][Green Version]
- Nanduri, R.; Mahajan, S.; Bhagyaraj, E.; Sethi, K.; Kalra, R.; Chandra, V.; Gupta, P. The Active Form of Vitamin D Transcriptionally Represses Smad7 Signaling and Activates Extracellular Signal-Regulated Kinase (ERK) to Inhibit the Differentiation of a Inflammatory T Helper Cell Subset and Suppress Experimental Autoimmune Encephalomyelitis. J. Biol. Chem. 2015, 290, 12222–12236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Sun, T.; Huang, Y.; Wang, Y.; Deb, D.K.; Yoon, D.; Kong, J.; Thadhani, R.; Li, Y.C. 1,25-Dihydroxyvitamin D Promotes Negative Feedback Regulation of TLR Signaling via Targeting MicroRNA-155–SOCS1 in Macrophages. J. Immunol. 2013, 190, 3687–3695. [Google Scholar] [CrossRef] [PubMed]
- Stuelten, C.H.; Parent, C.A.; Montell, D.J. Cell Motility in Cancer Invasion and Metastasis: Insights from Simple Model Organisms. Nat. Rev. Cancer 2018, 18, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.; Madden, S.F.; Synnott, N.C.; Klinger, R.; O’Connor, D.; O’Donovan, N.; Gallagher, W.; Crown, J.; Duffy, M.J. Vitamin D Receptor as a Target for Breast Cancer Therapy. Endocr. Relat. Cancer 2017, 24, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.F.; Gao, S.H.; Wang, P.; Zhang, H.M.; Liu, L.Z.; Ye, M.X.; Zhou, G.M.; Zhang, Z.L.; Li, B.Y. 1α,25(OH)2D3 Suppresses the Migration of Ovarian Cancer SKOV-3 Cells through the Inhibition of Epithelial–Mesenchymal Transition. Int. J. Mol. Sci. 2016, 17. [Google Scholar] [CrossRef]
- Chen, S.; Zhu, J.; Zuo, S.; Ma, J.; Zhang, J.; Chen, G.; Wang, X.; Pan, Y.; Liu, Y.; Wang, P. 1,25(OH)2D3 Attenuates TGF-Β1/Β2-Induced Increased Migration and Invasion via Inhibiting Epithelial-Mesenchymal Transition in Colon Cancer Cells. Biochem. Biophys. Res. Commun. 2015, 468, 130–135. [Google Scholar] [CrossRef]
- Barton, G.M.; Medzhitov, R. Toll-like Receptor Signaling Pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef]
- Bai, D.; Ueno, L.; Vogt, P.K. Akt-Mediated Regulation of NFκB and the Essentialness of NFκB for the Oncogenicity of PI3K and Akt. Int. J. Cancer J. Int. Cancer 2009, 125, 2863–2870. [Google Scholar] [CrossRef]
- Huang, P.; Han, J.; Hui, L. MAPK Signaling in Inflammation-Associated Cancer Development. Protein Cell 2010, 1, 218–226. [Google Scholar] [CrossRef]
- Mansouri, L.; Papakonstantinou, N.; Ntoufa, S.; Stamatopoulos, K.; Rosenquist, R. NF-ΚB Activation in Chronic Lymphocytic Leukemia: A Point of Convergence of External Triggers and Intrinsic Lesions. Semin. Cancer Biol. 2016, 39, 40–48. [Google Scholar] [CrossRef]
- Vogl, T.; Wolf, M.; Petersen, B.; Ehrhardt, C.; van Zoelen, M.; Foell, D.; Roth, J. Human S100A8 and S100A9 Activate Phagocytes via Toll-like Receptor 4 Independent of RAGE. Cell Commun. Signal. 2009, 7, A91. [Google Scholar] [CrossRef]
- Glorieux, G.; Hsu, C.; De Smet, R.; Dhondt, A.; van Emmelo, J.; Waterloos, M.A.; Lameire, N.; Plum, J.; Vanholder, R. The Anti-Proliferative Effect of Calcitriol on HL-60 Cells Is Neutralized by Uraemic Biological Fluids. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2001, 16, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Szymczak, I.; Pawliczak, R. The Active Metabolite of Vitamin D3 as a Potential Immunomodulator. Scand. J. Immunol. 2016, 83, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Studzinski, G.P. AKT Pathway Is Activated by 1, 25-Dihydroxyvitamin D3 and Participates in Its Anti-Apoptotic Effect and Cell Cycle Control in Differentiating HL60 Cells. Cell Cycle Georget. Tex 2006, 5, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Borchmann, S.; Cirillo, M.; Goergen, H.; Meder, L.; Sasse, S.; Kreissl, S.; Bröckelmann, P.J.; von Tresckow, B.; Fuchs, M.; Ullrich, R.T.; et al. Pretreatment Vitamin D Deficiency Is Associated With Impaired Progression-Free and Overall Survival in Hodgkin Lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 3528–3537. [Google Scholar] [CrossRef]
- William, B.M.; Brillhart, K.; Afable, M.; Bakalarz, K.; Cooper, B.; Lazarus, H.M.; Gerson, S.L.; Creger, R.; Nagabhushanam, K.; Grote, J.; et al. A Phase II Study of Curcumin and Vitamin D in Previously Untreated Patients with Early Stage Chronic Lymphocytic Leukemia (CLL) or Small Lymphocytic Lymphoma (SLL). Blood 2018, 132, 1875-1875. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. IwCLL Guidelines for Diagnosis, Indications for Treatment, Response Assessment, and Supportive Management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-Level Expression Analysis of RNA-Seq Experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-Enabled Heat Mapping for All. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-H.; Zhao, L.-F.; Wang, H.-F.; Wen, Y.-T.; Jiang, K.-K.; Mao, X.-M.; Zhou, Z.-Y.; Yao, K.-T.; Geng, Q.-S.; Guo, D.; et al. GenCLiP 3: Mining Human Genes’ Functions and Regulatory Networks from PubMed Based on Co-Occurrences and Natural Language Processing. Bioinformatics 2020, 1973–1975. [Google Scholar] [CrossRef] [PubMed]
- Raudvere, U.; Kolberg, L.; Kuzmin, I.; Arak, T.; Adler, P.; Peterson, H.; Vilo, J. G:Profiler: A Web Server for Functional Enrichment Analysis and Conversions of Gene Lists (2019 Update). Nucleic Acids Res. 2019, 47, W191–W198. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerousi, M.; Psomopoulos, F.; Kotta, K.; Tsagiopoulou, M.; Stavroyianni, N.; Anagnostopoulos, A.; Anastasiadis, A.; Gkanidou, M.; Kotsianidis, I.; Ntoufa, S.; et al. The Calcitriol/Vitamin D Receptor System Regulates Key Immune Signaling Pathways in Chronic Lymphocytic Leukemia. Cancers 2021, 13, 285. https://doi.org/10.3390/cancers13020285
Gerousi M, Psomopoulos F, Kotta K, Tsagiopoulou M, Stavroyianni N, Anagnostopoulos A, Anastasiadis A, Gkanidou M, Kotsianidis I, Ntoufa S, et al. The Calcitriol/Vitamin D Receptor System Regulates Key Immune Signaling Pathways in Chronic Lymphocytic Leukemia. Cancers. 2021; 13(2):285. https://doi.org/10.3390/cancers13020285
Chicago/Turabian StyleGerousi, Marina, Fotis Psomopoulos, Konstantia Kotta, Maria Tsagiopoulou, Niki Stavroyianni, Achilles Anagnostopoulos, Athanasios Anastasiadis, Maria Gkanidou, Ioannis Kotsianidis, Stavroula Ntoufa, and et al. 2021. "The Calcitriol/Vitamin D Receptor System Regulates Key Immune Signaling Pathways in Chronic Lymphocytic Leukemia" Cancers 13, no. 2: 285. https://doi.org/10.3390/cancers13020285
APA StyleGerousi, M., Psomopoulos, F., Kotta, K., Tsagiopoulou, M., Stavroyianni, N., Anagnostopoulos, A., Anastasiadis, A., Gkanidou, M., Kotsianidis, I., Ntoufa, S., & Stamatopoulos, K. (2021). The Calcitriol/Vitamin D Receptor System Regulates Key Immune Signaling Pathways in Chronic Lymphocytic Leukemia. Cancers, 13(2), 285. https://doi.org/10.3390/cancers13020285





