Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind
Abstract
:Simple Summary
Abstract
1. Introduction
2. Prevalence of Non-AIDS Defining Cancers Increases Despite Successful Antiretroviral Therapy
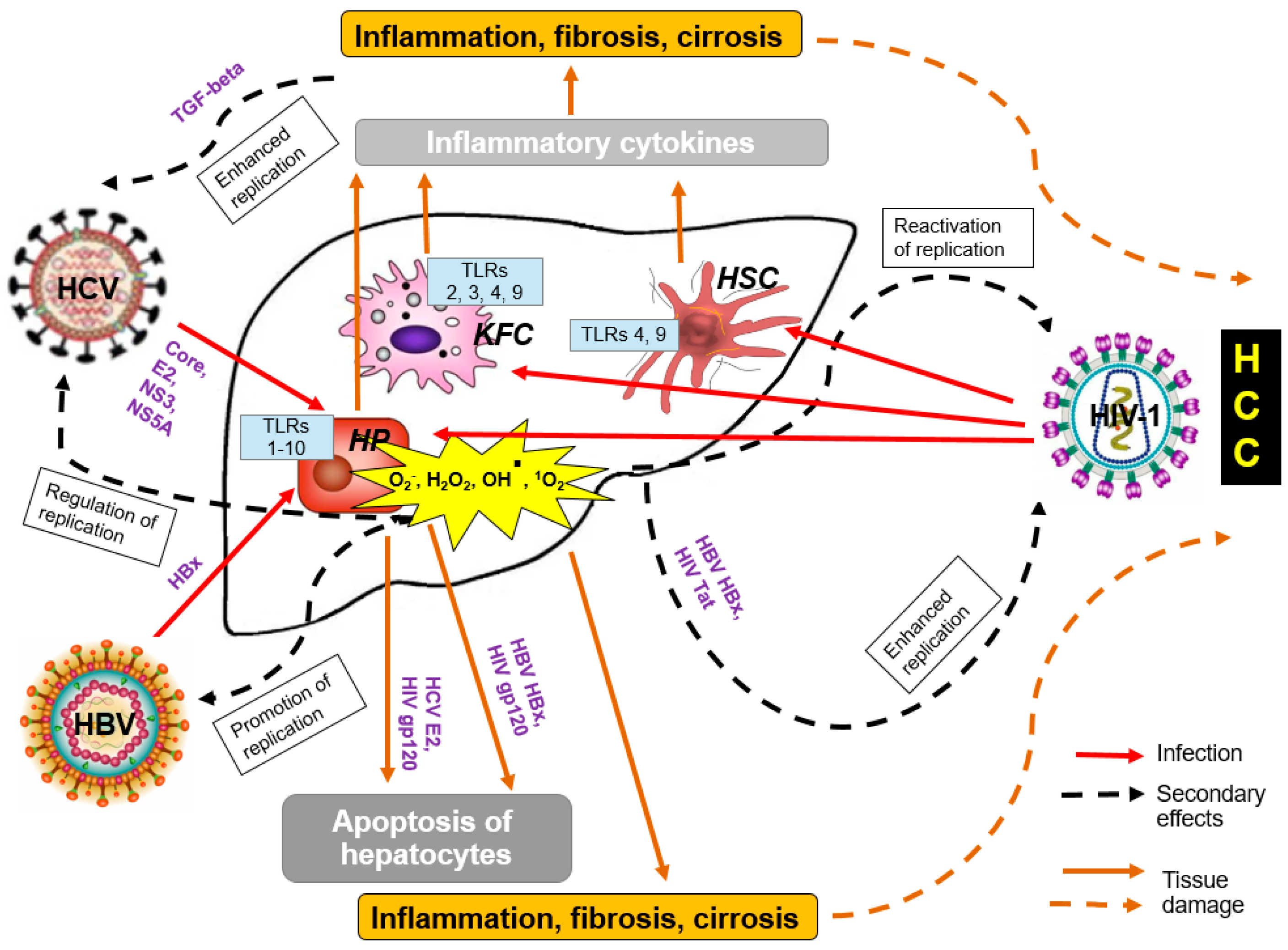
2.1. Liver Cancer
2.2. Brain Cancer
2.3. Squamous Cell Carcinomas
3. Mechanisms Underlying HIV-1 Pathogenicity in Epithelial Cells
4. Potentiation of Carcinogenesis by Interactions of HIV-1 with Other Oncogenic Viruses
5. HIV-1 Antigens Involved in Cell Transformation and Tumor Propagation
5.1. Transactivator of Transcription (Tat)
5.2. Envelope Glycoprotein gp120
5.3. Accessory Protein Negative Factor (Nef)
5.4. Reverse Transcriptase (RT)
5.5. Matrix Protein p17
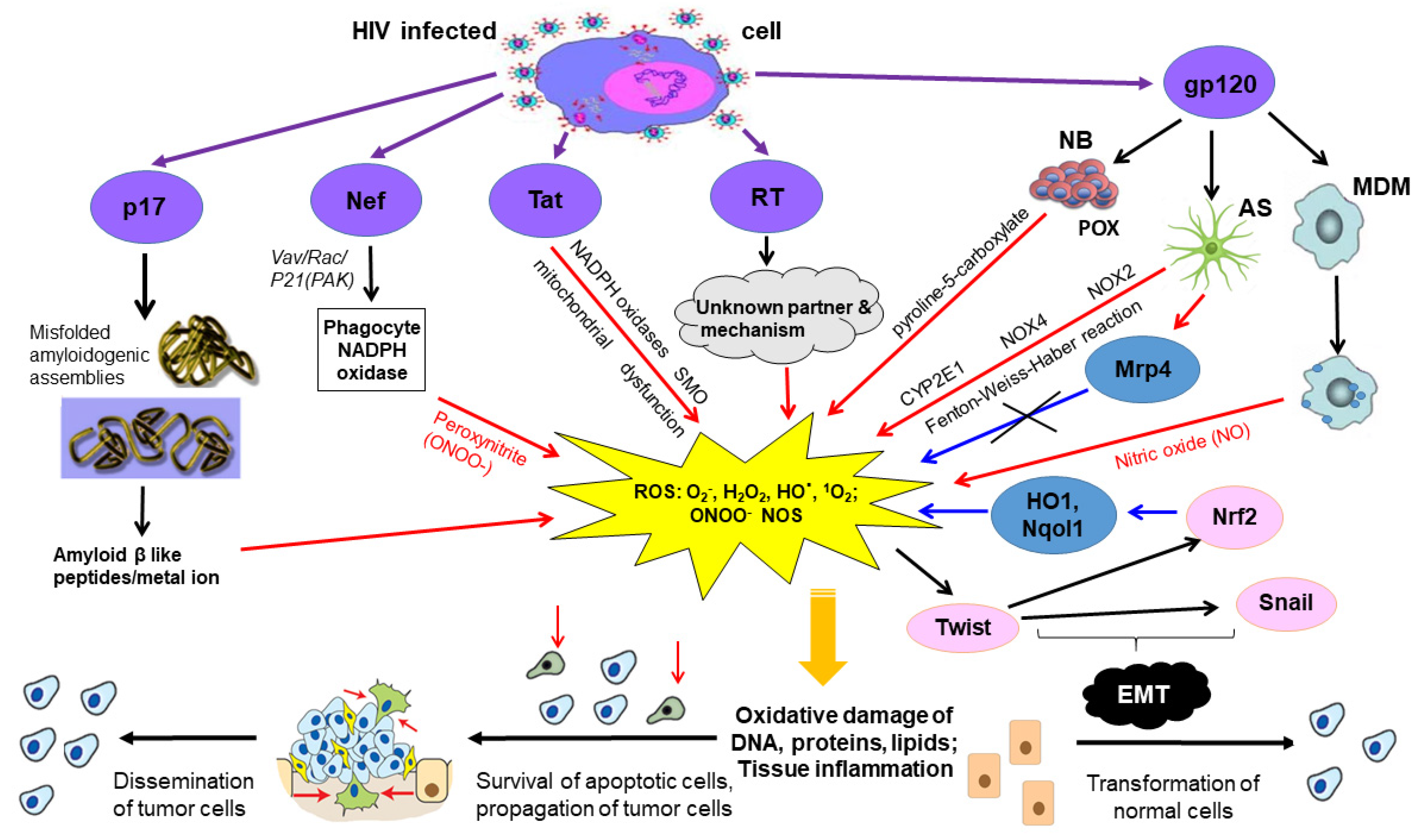
6. Oncogenic HIV-1 Proteins Induce Oxidative Stress
6.1. Transactivator of Transcription
6.2. Envelope Protein Gp120
6.3. Accessory Protein Negative Factor
6.4. Reverse Transcriptase
6.5. Matrix Protein p17
7. Oncogenic HIV-1 Proteins Inducing Oxidative Stress Are Found in the Extracellular Space
7.1. Transactivator of Transcription
7.2. Envelope Protein gp120
7.3. Accessory Protein Negative Factor
7.4. Reverse Transcriptase (RT)
7.5. Matrix Protein p17
8. Conclusions
- First, HIV proteins Tat, Nef, gp120, matrix protein p17, reverse transcriptase/RT induce oxidative stress with serious consequences in the form of DNA, protein and lipid damage, as well as changes in the intracellular signaling.
- Second, Tat, Nef, gp120, matrix protein p17, RT have a direct carcinogenic potential as demonstrated in the series of in vitro experiments and experiments in the laboratory animals.
- Third, Tat, Nef, gp120, matrix protein p17, reverse transcriptase/RT were shown to exit HIV expressing cells by different mechanisms, and, once present in the extracellular space, can be up-taken by innocent neighbor cells.
Funding
Conflicts of Interest
References
- Shiels, M.S.; Engels, E.A. Evolving epidemiology of HIV-associated malignancies. Curr. Opin. HIV AIDS 2017, 12, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Borrow, P. Innate immunity in acute HIV-1 infection. Curr. Opin. HIV AIDS 2011, 6, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Jenabian, M.A.; El-Far, M.; Vyboh, K.; Kema, I.; Costiniuk, C.T.; Thomas, R.; Baril, J.G.; LeBlanc, R.; Kanagaratham, C.; Radzioch, D.; et al. Immunosuppressive Tryptophan Catabolism and Gut Mucosal Dysfunction Following Early HIV Infection. J. Infect. Dis. 2015, 212, 355–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boasso, A.; Shearer, G.M.; Chougnet, C. Immune dysregulation in human immunodeficiency virus infection: Know it, fix it, prevent it? J. Intern. Med. 2009, 265, 78–96. [Google Scholar] [CrossRef]
- Titanji, K.; Chiodi, F.; Bellocco, R.; Schepis, D.; Osorio, L.; Tassandin, C.; Tambussi, G.; Grutzmeier, S.; Lopalco, L.; De Milito, A. Primary HIV-1 infection sets the stage for important B lymphocyte dysfunctions. AIDS 2005, 19, 1947–1955. [Google Scholar] [CrossRef]
- De Milito, A.; Nilsson, A.; Titanji, K.; Thorstensson, R.; Reizenstein, E.; Narita, M.; Grutzmeier, S.; Sonnerborg, A.; Chiodi, F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood 2004, 103, 2180–2186. [Google Scholar] [CrossRef]
- Amu, S.; Lantto Graham, R.; Bekele, Y.; Nasi, A.; Bengtsson, C.; Rethi, B.; Sorial, S.; Meini, G.; Zazzi, M.; Hejdeman, B.; et al. Dysfunctional phenotypes of CD4 + and CD8 + T cells are comparable in patients initiating ART during early or chronic HIV-1 infection. Medicine 2016, 95, e3738. [Google Scholar] [CrossRef] [Green Version]
- Lemma, M.; Petkov, S.; Bekele, Y.; Petros, B.; Howe, R.; Chiodi, F. Profiling of Inflammatory Proteins in Plasma of HIV-1-Infected Children Receiving Antiretroviral Therapy. Proteomes 2020, 8, 24. [Google Scholar] [CrossRef]
- Yang, X.; Su, B.; Zhang, X.; Liu, Y.; Wu, H.; Zhang, T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non-responders. J. Leukoc. Biol. 2020, 107, 597–612. [Google Scholar] [CrossRef]
- Zicari, S.; Sessa, L.; Cotugno, N.; Ruggiero, A.; Morrocchi, E.; Concato, C.; Rocca, S.; Zangari, P.; Manno, E.C.; Palma, P. Immune Activation, Inflammation, and Non-AIDS Co-Morbidities in HIV-Infected Patients under Long-Term ART. Viruses 2019, 11, 200. [Google Scholar] [CrossRef] [Green Version]
- Nabatanzi, R.; Cose, S.; Joloba, M.; Jones, S.R.; Nakanjako, D. Effects of HIV infection and ART on phenotype and function of circulating monocytes, natural killer, and innate lymphoid cells. AIDS Res. Ther. 2018, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Amu, S.; Ruffin, N.; Rethi, B.; Chiodi, F. Impairment of B-cell functions during HIV-1 infection. AIDS 2013, 27, 2323–2334. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, M.J.; Lau, B.; Achenbach, C.J.; Jing, Y.; Althoff, K.N.; D’Souza, G.; Engels, E.A.; Hessol, N.A.; Brooks, J.T.; Burchell, A.N. Cumulative incidence of cancer among persons with HIV in North America: A cohort study. Ann. Intern. Med. 2015, 163, 507–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Symons, J.; Chopra, A.; Malatinkova, E.; De Spiegelaere, W.; Leary, S.; Cooper, D.; Abana, C.O.; Rhodes, A.; Rezaei, S.D.; Vandekerckhove, L.; et al. HIV integration sites in latently infected cell lines: Evidence of ongoing replication. Retrovirology 2017, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schawkat, K.; Reiner, C.S. Diffuse liver disease: Cirrhosis, focal lesions in cirrhosis, and vascular liver disease. IDKD Springer Ser. 2018, 229–236. [Google Scholar]
- Pinato, D.J.; Allara, E.; Chen, T.Y.; Trevisani, F.; Minguez, B.; Zoli, M.; Harris, M.; Dalla Pria, A.; Merchante, N.; Platt, H.; et al. Influence of HIV Infection on the Natural History of Hepatocellular Carcinoma: Results From a Global Multicohort Study. J. Clin. Oncol. 2019, 37, 296–304. [Google Scholar] [CrossRef] [Green Version]
- Clifford, G.M.; Rickenbach, M.; Polesel, J.; Dal Maso, L.; Steffen, I.; Ledergerber, B.; Rauch, A.; Probst-Hensch, N.M.; Bouchardy, C.; Levi, F. Influence of HIV-related immunodeficiency on the risk of hepatocellular carcinoma. AIDS 2008, 22, 2135–2141. [Google Scholar] [CrossRef] [Green Version]
- Pinato, D.J.; Dalla Pria, A.; Sharma, R.; Bower, M. Hepatocellular carcinoma: An evolving challenge in viral hepatitis and HIV coinfection. AIDS 2017, 31, 603–611. [Google Scholar] [CrossRef]
- Gjærde, L.I.; Shepherd, L.; Jablonowska, E.; Lazzarin, A.; Rougemont, M.; Darling, K.; Battegay, M.; Braun, D.; Martel-Laferriere, V.; Lundgren, J.D. Trends in Incidences and Risk Factors for Hepatocellular Carcinoma and Other Liver Events in HIV and Hepatitis C Virus–coinfected Individuals From 2001 to 2014: A Multicohort Study. Clin. Infect. Dis. 2016, 63, 821–829. [Google Scholar] [CrossRef]
- Joshi, D.; O’Grady, J.; Dieterich, D.; Gazzard, B.; Agarwal, K. Increasing burden of liver disease in patients with HIV infection. Lancet 2011, 377, 1198–1209. [Google Scholar] [CrossRef]
- Brenchley, J.M.; Price, D.A.; Schacker, T.W.; Asher, T.E.; Silvestri, G.; Rao, S.; Kazzaz, Z.; Bornstein, E.; Lambotte, O.; Altmann, D.; et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006, 12, 1365–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetti, G.; Tincati, C.; Silvestri, G. Microbial translocation in the pathogenesis of HIV infection and AIDS. Clin. Microbiol. Rev. 2013, 26, 2–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soares, J.B.; Pimentel-Nunes, P.; Roncon-Albuquerque, R.; Leite-Moreira, A. The role of lipopolysaccharide/toll-like receptor 4 signaling in chronic liver diseases. Hepatol. Int. 2010, 4, 659–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramendra, R.; Isnard, S.; Mehraj, V.; Chen, J.; Zhang, Y.; Finkelman, M.; Routy, J.P. Circulating LPS and (1-->3)-beta-d-Glucan: A Folie a Deux Contributing to HIV-Associated Immune Activation. Front. Immunol. 2019, 10, 465. [Google Scholar] [CrossRef] [PubMed]
- Kovari, H.; Ledergerber, B.; Battegay, M.; Rauch, A.; Hirschel, B.; Foguena, A.K.; Vernazza, P.; Bernasconi, E.; Mueller, N.J.; Weber, R. Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis b or c virus co-infection. Clin. Infect. Dis. 2010, 50, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Crane, M.; Visvanathan, K.; Lewin, S.R. HIV Infection and TLR Signalling in the Liver. Gastroenterol. Res. Pr. 2012, 2012, 473925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iser, D.M.; Warner, N.; Revill, P.A.; Solomon, A.; Wightman, F.; Saleh, S.; Crane, M.; Cameron, P.U.; Bowden, S.; Nguyen, T.; et al. Coinfection of hepatic cell lines with human immunodeficiency virus and hepatitis B virus leads to an increase in intracellular hepatitis B surface antigen. J. Virol. 2010, 84, 5860–5867. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, M.; Poluektova, L.Y.; Kharbanda, K.K.; Osna, N.A. Liver as a target of human immunodeficiency virus infection. World J. Gastroenterol. 2018, 24, 4728–4737. [Google Scholar] [CrossRef]
- Kandathil, A.; Durand, C.; Quinn, J.; Cameron, A.; Thomas, D.; Balagopal, A. Liver macrophages and HIV-1 persistence. In Proceedings of the CROI, Seattle, WA, USA, 23–26 February 2015. [Google Scholar]
- Chew, K.W.; Bhattacharya, D. Virologic and immunologic aspects of HIV-hepatitis C virus coinfection. AIDS 2016, 30, 2395–2404. [Google Scholar] [CrossRef]
- Lin, W.; Weinberg, E.M.; Chung, R.T. Pathogenesis of accelerated fibrosis in HIV/HCV co-infection. J. Infect. Dis. 2013, 207, S13–S18. [Google Scholar] [CrossRef]
- Hong, F.; Saiman, Y.; Si, C.; Mosoian, A.; Bansal, M.B. X4 Human immunodeficiency virus type 1 gp120 promotes human hepatic stellate cell activation and collagen I expression through interactions with CXCR4. PLoS ONE 2012, 7, e33659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosoian, A.; Zhang, L.; Hong, F.; Cunyat, F.; Rahman, A.; Bhalla, R.; Panchal, A.; Saiman, Y.; Fiel, M.I.; Florman, S.; et al. Frontline Science: HIV infection of Kupffer cells results in an amplified proinflammatory response to LPS. J. Leukoc. Biol. 2017, 101, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Tuyama, A.C.; Hong, F.; Saiman, Y.; Wang, C.; Ozkok, D.; Mosoian, A.; Chen, P.; Chen, B.K.; Klotman, M.E.; Bansal, M.B. Human immunodeficiency virus (HIV)-1 infects human hepatic stellate cells and promotes collagen I and monocyte chemoattractant protein-1 expression: Implications for the pathogenesis of HIV/hepatitis C virus–induced liver fibrosis. Hepatology 2010, 52, 612–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.; Wu, G.; Li, S.; Weinberg, E.M.; Kumthip, K.; Peng, L.F.; Mendez-Navarro, J.; Chen, W.C.; Jilg, N.; Zhao, H.; et al. HIV and HCV cooperatively promote hepatic fibrogenesis via induction of reactive oxygen species and NFkappaB. J. Biol. Chem. 2011, 286, 2665–2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, S.; Martinez-Sanz, J.; Serrano-Villar, S. HIV, Cancer, and the Microbiota: Common Pathways Influencing Different Diseases. Front. Immunol. 2019, 10, 1466. [Google Scholar] [CrossRef] [Green Version]
- Koziel, M.J.; Peters, M.G. Viral hepatitis in HIV infection. N. Engl. J. Med. 2007, 356, 1445–1454. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.Y.; Sheng, W.H.; Tsai, M.S.; Lee, K.Y.; Chang, S.Y.; Hung, C.C. Hepatitis B virus coinfection in human immunodeficiency virus-infected patients: A review. World J. Gastroenterol. 2014, 20, 14598–14614. [Google Scholar] [CrossRef]
- Garcia-Samaniego, J.; Rodriguez, M.; Berenguer, J.; Rodriguez-Rosado, R.; Carbo, J.; Asensi, V.; Soriano, V. Hepatocellular carcinoma in HIV-infected patients with chronic hepatitis C. Am. J. Gastroenterol. 2001, 96, 179–183. [Google Scholar] [CrossRef]
- Nabih, H.K. The Significance of HCV Viral Load in the Incidence of HCC: A Correlation Between Mir-122 and CCL2. J. Gastrointest. Cancer 2020, 51, 412–417. [Google Scholar] [CrossRef]
- Lemon, S.M.; McGivern, D.R. Is hepatitis C virus carcinogenic? Gastroenterology 2012, 142, 1274–1278. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Tyurina, D.A.; Ivanova, O.N.; Kochetkov, S.N.; Bartosch, B.; Isaguliants, M.G. Oxidative stress, a trigger of hepatitis C and B virus-induced liver carcinogenesis. Oncotarget 2017, 8, 3895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, J.; Short, S. Management of glioblastoma multiforme in HIV patients: A case series and review of published studies. Clin. Oncol. 2009, 21, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Del Valle, L.; Piña-Oviedo, S. HIV disorders of the brain: Pathology and pathogenesis. Front. Biosci. 2006, 11, 718–732. [Google Scholar] [CrossRef] [PubMed]
- Cedeno-Laurent, F.; Trujillo, J.R. Gliomas and brain lymphomas in HIV-1/AIDS patients: Reflections from a 20-year follow up in Mexico and Brazil. Microbiol. Res. 2011, 2, 11. [Google Scholar] [CrossRef]
- Patel, A.P.; Fisher, J.L.; Nichols, E.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; Abraha, H.N.; Agius, D.; Alahdab, F.; Alam, T. Global, regional, and national burden of brain and other CNS cancer, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 376–393. [Google Scholar] [CrossRef] [Green Version]
- Choy, W.; Lagman, C.; Lee, S.J.; Bui, T.T.; Safaee, M.; Yang, I. Impact of human immunodeficiency virus in the pathogenesis and outcome of patients with glioblastoma multiforme. Brain Tumor Res. Treat. 2016, 4, 77–86. [Google Scholar] [CrossRef]
- Acevedo, N.; Pillai, C.; Welch, M. HCP-01diagnosis and management of high-grade glioma in patients with HIV. Neuro Oncol. 2015, 17, v101. [Google Scholar] [CrossRef] [Green Version]
- Tacconi, L.; Stapleton, S.; Signorelli, F.; Thomas, D. Acquired immune deficiency syndrome (AIDS) and cerebral astrocytoma. Clin. Neurol. Neurosurg. 1996, 98, 149–151. [Google Scholar] [CrossRef]
- Chamberlain, M.C. Gliomas in patients with acquired immune deficiency syndrome. Cancer 1994, 74, 1912–1914. [Google Scholar] [CrossRef]
- Wolff, R.; Zimmermann, M.; Marquardt, G.; Lanfermann, H.; Nafe, R.; Seifert, V. Glioblastoma multiforme of the brain stem in a patient with aquired immunodeficiency syndrome. Acta Neurochir. 2002, 144, 941–945. [Google Scholar] [CrossRef]
- Chiodi, F.; Fuerstenberg, S.; Gidlund, M.; Asjo, B.; Fenyo, E.M. Infection of brain-derived cells with the human immunodeficiency virus. J. Virol. 1987, 61, 1244–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messam, C.A.; Major, E.O. Stages of restricted HIV-1 infection in astrocyte cultures derived from human fetal brain tissue. J. Neurovirol. 2000, 6, S90–S94. [Google Scholar] [PubMed]
- Robbins, H.A.; Shiels, M.S.; Pfeiffer, R.M.; Engels, E.A. Epidemiologic contributions to recent cancer trends among HIV-infected people in the United States. AIDS 2014, 28, 881–890. [Google Scholar] [CrossRef] [Green Version]
- Palefsky, J.M. HPV-associated anal and cervical cancers in HIV-infected individuals: Incidence and prevention in the antiretroviral therapy era. Curr. Opin. HIV AIDS 2017, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Osazuwa-Peters, N.; Massa, S.T.; Simpson, M.C.; Adjei Boakye, E.; Varvares, M.A. Survival of human papillomavirus-associated cancers: Filling in the gaps. Cancer 2018, 124, 18–20. [Google Scholar] [CrossRef] [Green Version]
- Strickler, H.D.; Burk, R.D.; Fazzari, M.; Anastos, K.; Minkoff, H.; Massad, L.S.; Hall, C.; Bacon, M.; Levine, A.M.; Watts, D.H. Natural history and possible reactivation of human papillomavirus in human immunodeficiency virus–positive women. J. Natl. Cancer Inst. 2005, 97, 577–586. [Google Scholar] [CrossRef] [Green Version]
- Clarke, B.; Chetty, R. Postmodern cancer: The role of human immunodeficiency virus in uterine cervical cancer. Mol. Pathol. 2002, 55, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Palefsky, J. Biology of HPV in HIV infection. Adv. Dent. Res. 2006, 19, 99–105. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Holly, E.A. Chapter 6: Immunosuppression and co-infection with HIV. J. Natl. Cancer Inst. Monogr. 2003, 2003, 41–46. [Google Scholar] [CrossRef]
- Chambuso, R.; Gray, C.M.; Kaambo, E.; Rebello, G.; Ramesar, R. Impact of Host Molecular Genetic Variations and HIV/HPV Co-infection on Cervical Cancer Progression: A Systematic review. Oncomedicine 2018, 3, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Joag, S.V.; Adany, I.; Li, Z.; Foresman, L.; Pinson, D.M.; Wang, C.; Stephens, E.B.; Raghavan, R.; Narayan, O. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: Intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J. Virol. 1997, 71, 4016–4023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, M.L.; Schmidt, A.; Agy, M.B.; Kimball, L.E.; Morton, W.R. Infection of Macaca nemestrina neonates with HIV-1 via different routes of inoculation. AIDS 1997, 11, 1555–1563. [Google Scholar] [CrossRef] [PubMed]
- Carias, A.M.; McCoombe, S.; McRaven, M.; Anderson, M.; Galloway, N.; Vandergrift, N.; Fought, A.J.; Lurain, J.; Duplantis, M.; Veazey, R.S. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. J. Virol. 2013, 87, 11388–11400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, M.; Mahoney, J.; Wei, Q.; Van Der Ryst, E.; Muchmore, E.; Barré-Sinoussi, F.; Fultz, P.N. Genital infection of female chimpanzees with human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 1998, 14, 1357–1367. [Google Scholar] [CrossRef]
- Dinh, M.H.; Anderson, M.R.; McRaven, M.D.; Cianci, G.C.; McCoombe, S.G.; Kelley, Z.L.; Gioia, C.J.; Fought, A.J.; Rademaker, A.W.; Veazey, R.S.; et al. Visualization of HIV-1 interactions with penile and foreskin epithelia: Clues for female-to-male HIV transmission. PLoS Pathog. 2015, 11, e1004729. [Google Scholar] [CrossRef] [PubMed]
- Ganor, Y.; Zhou, Z.; Tudor, D.; Schmitt, A.; Vacher-Lavenu, M.C.; Gibault, L.; Thiounn, N.; Tomasini, J.; Wolf, J.P.; Bomsel, M. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol. 2010, 3, 506–522. [Google Scholar] [CrossRef] [Green Version]
- Hladik, F.; Sakchalathorn, P.; Ballweber, L.; Lentz, G.; Fialkow, M.; Eschenbach, D.; McElrath, M.J. Initial events in establishing vaginal entry and infection by human immunodeficiency virus type-1. Immunity 2007, 26, 257–270. [Google Scholar] [CrossRef] [Green Version]
- Maher, D.; Wu, X.; Schacker, T.; Horbul, J.; Southern, P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc. Natl. Acad. Sci. USA 2005, 102, 11504–11509. [Google Scholar] [CrossRef] [Green Version]
- Stoddard, E.; Ni, H.; Cannon, G.; Zhou, C.; Kallenbach, N.; Malamud, D.; Weissman, D. gp340 promotes transcytosis of human immunodeficiency virus type 1 in genital tract-derived cell lines and primary endocervical tissue. J. Virol. 2009, 83, 8596–8603. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Z.; De Longchamps, N.B.; Schmitt, A.; Zerbib, M.; Vacher-Lavenu, M.-C.; Bomsel, M.; Ganor, Y. HIV-1 efficient entry in inner foreskin is mediated by elevated CCL5/RANTES that recruits T cells and fuels conjugate formation with Langerhans cells. PLoS Pathog. 2011, 7, e1002100. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Huang, L.; Li, J.; Zhou, X.; Zhang, H.; Zhang, T.; Lei, Y.; Wang, K.; Xie, N.; Zheng, Y.; et al. HIV Infection in gastric epithelial cells. J. Infect. Dis. 2013, 208, 1221–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorosko, S.M.; Connor, R.I. Primary human mammary epithelial cells endocytose HIV-1 and facilitate viral infection of CD4+ T lymphocytes. J. Virol. 2010, 84, 10533–10542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, K.; Akturk, G.; Gnjatic, S.; Chen, B.; Klotman, M.; Blasi, M. Proliferation of HIV-infected renal epithelial cells following virus acquisition from infected macrophages. AIDS 2020, 34, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Brune, K.A.; Ferreira, F.; Mandke, P.; Chau, E.; Aggarwal, N.R.; D’Alessio, F.R.; Lambert, A.A.; Kirk, G.; Blankson, J.; Drummond, M.B.; et al. HIV Impairs Lung Epithelial Integrity and Enters the Epithelium to Promote Chronic Lung Inflammation. PLoS ONE 2016, 11, e0149679. [Google Scholar] [CrossRef]
- Wagner, T.A.; McLaughlin, S.; Garg, K.; Cheung, C.Y.; Larsen, B.B.; Styrchak, S.; Huang, H.C.; Edlefsen, P.T.; Mullins, J.I.; Frenkel, L.M. HIV latency. Proliferation of cells with HIV integrated into cancer genes contributes to persistent infection. Science 2014, 345, 570–573. [Google Scholar] [CrossRef] [Green Version]
- Fan, H.; Johnson, C. Insertional oncogenesis by non-acute retroviruses: Implications for gene therapy. Viruses 2011, 3, 398–422. [Google Scholar] [CrossRef] [Green Version]
- Maldarelli, F. The role of HIV integration in viral persistence: No more whistling past the proviral graveyard. J. Clin. Investig. 2016, 126, 438–447. [Google Scholar] [CrossRef] [Green Version]
- Asin, S.N.; Wildt-Perinic, D.; Mason, S.I.; Howell, A.L.; Wira, C.R.; Fanger, M.W. Human immunodeficiency virus type 1 infection of human uterine epithelial cells: Viral shedding and cell contact-mediated infectivity. J. Infect. Dis. 2003, 187, 1522–1533. [Google Scholar] [CrossRef]
- Aiken, C. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 1997, 71, 5871–5877. [Google Scholar] [CrossRef] [Green Version]
- King, B.; Daly, J. Pseudotypes: Your flexible friends. Futur. Microbiol. 2014, 9, 135–137. [Google Scholar] [CrossRef]
- Tang, Y.; George, A.; Nouvet, F.; Sweet, S.; Emeagwali, N.; Taylor, H.E.; Simmons, G.; Hildreth, J.E. Infection of female primary lower genital tract epithelial cells after natural pseudotyping of HIV-1: Possible implications for sexual transmission of HIV-1. PLoS ONE 2014, 9, e101367. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Woodward, B.O.; Pastor, L.; George, A.M.; Petrechko, O.; Nouvet, F.J.; Haas, D.W.; Jiang, G.; Hildreth, J.E.K. Endogenous Retroviral Envelope Syncytin Induces HIV-1 Spreading and Establishes HIV Reservoirs in Placenta. Cell Rep. 2020, 30, 4528–4539. [Google Scholar] [CrossRef] [PubMed]
- Grandi, N.; Tramontano, E. Type W Human Endogenous Retrovirus (HERV-W) Integrations and Their Mobilization by L1 Machinery: Contribution to the Human Transcriptome and Impact on the Host Physiopathology. Viruses 2017, 9, 162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devadoss, D.; Singh, S.P.; Acharya, A.; Do, K.C.; Periyasamy, P.; Manevski, M.; Mishra, N.; Tellez, C.; Ramakrishnan, S.; Belinsky, S. Lung Bronchial Epithelial Cells are HIV Targets for Proviral Genomic Integration. bioRxiv 2020. [Google Scholar] [CrossRef]
- Anand, A.R.; Rachel, G.; Parthasarathy, D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front. Cardiovasc. Med. 2018, 5, 185. [Google Scholar] [CrossRef]
- De Paoli, P.; Carbone, A. Microenvironmental abnormalities induced by viral cooperation: Impact on lymphomagenesis. Semin. Cancer Biol. 2015, 34, 70–80. [Google Scholar] [CrossRef] [Green Version]
- Mazzuca, P.; Caruso, A.; Caccuri, F. Endothelial Cell Dysfunction in HIV-1 Infection. Endothel. Dysfunct. Old Concepts New Chall. 2018, 347. [Google Scholar] [CrossRef] [Green Version]
- Liapis, K.; Clear, A.; Owen, A.; Coutinho, R.; Greaves, P.; Lee, A.M.; Montoto, S.; Calaminici, M.; Gribben, J.G. The microenvironment of AIDS-related diffuse large B-cell lymphoma provides insight into the pathophysiology and indicates possible therapeutic strategies. Blood 2013, 122, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.G.; Liapis, K.; Gribben, J.G. The role of the tumor microenvironment in HIV-associated lymphomas. Biomark. Med. 2015, 9, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Gaisa, M.M.; Wang, X.; Swartz, T.H.; Arens, Y.; Dresser, K.A.; Sigel, C.; Sigel, K. Differences in the Immune Microenvironment of Anal Cancer Precursors by HIV Status and Association With Ablation Outcomes. J. Infect. Dis. 2018, 217, 703–709. [Google Scholar] [CrossRef]
- Paiardini, M.; Frank, I.; Pandrea, I.; Apetrei, C.; Silvestri, G. Mucosal immune dysfunction in AIDS pathogenesis. AIDS Rev. 2008, 10, 36–46. [Google Scholar] [PubMed]
- Yaghoobi, M.; Le Gouvello, S.; Aloulou, N.; Duprez-Dutreuil, C.; Walker, F.; Sobhani, I. FoxP3 overexpression and CD1a+ and CD3 + depletion in anal tissue as possible mechanisms for increased risk of human papillomavirus-related anal carcinoma in HIV infection. Color. Dis. 2011, 13, 768–773. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.G.; da Costa, A.G.; Martins-Filho, O.A.; Pimentel, J.P.; Zauli, D.A.; Peruhype-Magalhaes, V.; Teixeira-Carvalho, A.; Bela, S.R.; Xavier, M.A.; Coelho-Dos-Reis, J.G.; et al. CD11c + CD123Low dendritic cell subset and the triad TNF-alpha/IL-17A/IFN-gamma integrate mucosal and peripheral cellular responses in HIV patients with high-grade anal intraepithelial neoplasia: A systems biology approach. JAIDS J. Acquir. Immune Defic. Syndr. 2015, 68, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Micsenyi, A.M.; Zony, C.; Alvarez, R.A.; Durham, N.D.; Chen, B.K.; Klotman, M.E. Postintegration HIV-1 infection of cervical epithelial cells mediates contact-dependent productive infection of T cells. J. Infect. Dis. 2013, 208, 1756–1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasen, A.; Herrera, R.; Rosbe, K.; Lien, K.; Tugizov, S.M. HIV internalization into oral and genital epithelial cells by endocytosis and macropinocytosis leads to viral sequestration in the vesicles. Virology 2018, 515, 92–107. [Google Scholar] [CrossRef]
- Tugizov, S.M. Human immunodeficiency virus interaction with oral and genital mucosal epithelia may lead to epithelial-mesenchymal transition and sequestration of virions in the endosomal compartments. Oral Dis. 2020, 26, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Tugizov, S.M.; Herrera, R.; Chin-Hong, P.; Veluppillai, P.; Greenspan, D.; Michael Berry, J.; Pilcher, C.D.; Shiboski, C.H.; Jay, N.; Rubin, M.; et al. HIV-associated disruption of mucosal epithelium facilitates paracellular penetration by human papillomavirus. Virology 2013, 446, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parvez, M.K. HBV and HIV co-infection: Impact on liver pathobiology and therapeutic approaches. World J. Hepatol. 2015, 7, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Park, I.W.; Fan, Y.; Luo, X.; Ryou, M.G.; Liu, J.; Green, L.; He, J.J. HIV-1 Nef is transferred from expressing T cells to hepatocytic cells through conduits and enhances HCV replication. PLoS ONE 2014, 9, e99545. [Google Scholar] [CrossRef] [PubMed]
- Luedde, T.; Schwabe, R.F. NF-kappaB in the liver—Linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGivern, D.R.; Lemon, S.M. Tumor suppressors, chromosomal instability, and hepatitis C virus–associated liver cancer. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 399–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tugizov, S.M.; Herrera, R.; Veluppillai, P.; Greenspan, D.; Palefsky, J.M. 46. HIV-induced epithelial–mesenchymal transition in mucosal epithelium facilitates HPV paracellular penetration. Sex. Health 2013, 10, 592. [Google Scholar] [CrossRef]
- Tornesello, M.L.; Buonaguro, F.M.; Beth-Giraldo, E.; Giraldo, G. Human immunodeficiency virus type 1 tat gene enhances human papillomavirus early gene expression. Intervirology 1993, 36, 57–64. [Google Scholar] [CrossRef]
- Buonaguro, F.M.; Tornesello, M.L.; Buonaguro, L.; Del Gaudio, E.; Beth-Giraldo, E.; Giraldo, G. Role of HIV as Cofactor in HPV Oncogenesis: In Vitro Evidences of Virus Interactions. In Advanced Technologies in Research, Diagnosis and Treatment of AIDS and in Oncology; Karger Publishers: Basel, Switzerland, 1994; Volume 46, pp. 102–109. [Google Scholar]
- Kim, R.H.; Yochim, J.M.; Kang, M.K.; Shin, K.-H.; Christensen, R.; Park, N.-H. HIV-1 Tat enhances replicative potential of human oral keratinocytes harboring HPV-16 genome. Int. J. Oncol. 1992, 33, 777–782. [Google Scholar] [CrossRef]
- Barillari, G.; Palladino, C.; Bacigalupo, I.; Leone, P.; Falchi, M.; Ensoli, B. Entrance of the Tat protein of HIV-1 into human uterine cervical carcinoma cells causes upregulation of HPV-E6 expression and a decrease in p53 protein levels. Oncol. Lett. 2016, 12, 2389–2394. [Google Scholar] [CrossRef] [Green Version]
- Vernon, S.D.; Hart, C.E.; Reeves, W.C.; Icenogle, J.P. The HIV-1 tat protein enhances E2-dependent human papillomavirus 16 transcription. Virus Res. 1993, 27, 133–145. [Google Scholar] [CrossRef]
- Barillari, G.; Ensoli, B. Angiogenic effects of extracellular human immunodeficiency virus type 1 Tat protein and its role in the pathogenesis of AIDS-associated Kaposi’s sarcoma. Clin. Microbiol. Rev. 2002, 15, 310–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krill, L.S.; Tewari, K.S. Exploring the therapeutic rationale for angiogenesis blockade in cervical cancer. Clin. Ther. 2015, 37, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Nyagol, J.; Leucci, E.; Omnis, A.; De Falco, G.; Tigli, C.; Sanseverino, F.; Torriccelli, M.; Palummo, N.; Pacenti, L.; Santopietro, R. The effects of HIV-1 Tat protein on cell cycle during cervical carcinogenesis. Cancer Biol. Ther. 2006, 5, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Bayurova, E.; Gamaleya Research Center for Epidemiology and Microbiology, M.P. Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products of Russian Academy of Sciences; Isaguliants, M.; Gamaleya Research Center for Epidemiology and Microbiology; M.P. Chumakov Federal Scientific Center for Research and Development of Immune-and-Biological Products of Russian Academy of Sciences; Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet; Department of Research, Riga Stradins University; Tugizov, S.; Department of Medicine, University of California, San Francisco; Palefsky, J.; Department of Medicine, University of California, San Francisco. Personal communication, 2020.
- Collini, P.J.; Bewley, M.A.; Mohasin, M.; Marriott, H.M.; Miller, R.F.; Geretti, A.M.; Beloukas, A.; Papadimitropoulos, A.; Read, R.C.; Noursadeghi, M.; et al. HIV gp120 in the Lungs of Antiretroviral Therapy-treated Individuals Impairs Alveolar Macrophage Responses to Pneumococci. Am. J. Respir. Crit. Care Med. 2018, 197, 1604–1615. [Google Scholar] [CrossRef]
- Latanova, A.; Petkov, S.; Kuzmenko, Y.; Kilpelainen, A.; Ivanov, A.; Smirnova, O.; Krotova, O.; Korolev, S.; Hinkula, J.; Karpov, V.; et al. Fusion to Flaviviral Leader Peptide Targets HIV-1 Reverse Transcriptase for Secretion and Reduces Its Enzymatic Activity and Ability to Induce Oxidative Stress but Has No Major Effects on Its Immunogenic Performance in DNA-Immunized Mice. J. Immunol. Res. 2017, 2017, 7407136. [Google Scholar] [CrossRef] [PubMed]
- Lien, K.; Mayer, W.; Herrera, R.; Rosbe, K.; Tugizov, S.M. HIV-1 proteins gp120 and tat induce the epithelial-mesenchymal transition in oral and genital mucosal epithelial cells. PLoS ONE 2019, 14, e0226343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansons, J.; Bayurova, E.; Skrastina, D.; Kurlanda, A.; Fridrihsone, I.; Kostyushev, D.; Kostyusheva, A.; Artyuhov, A.; Dashinimaev, E.; Avdoshina, D. Expression of the Reverse Transcriptase Domain of Telomerase Reverse Transcriptase Induces Lytic Cellular Response in DNA-Immunized Mice and Limits Tumorigenic and Metastatic Potential of Murine Adenocarcinoma 4T1 Cells. Vaccines 2020, 8, 318. [Google Scholar] [CrossRef]
- Wechsler, E.I.; Tugizov, S.; Herrera, R.; Da Costa, M.; Palefsky, J.M. E5 can be expressed in anal cancer and leads to epidermal growth factor receptor-induced invasion in a human papillomavirus 16-transformed anal epithelial cell line. J. Gen. Virol. 2018, 99, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.L.; Gonzales, M.I.; Topalian, S.L. Selection of appropriate control genes to assess expression of tumor antigens using real-time RT-PCR. BioTechniques 2004, 36, 84–86, 88, 90–91. [Google Scholar] [CrossRef]
- Huynh, D.; Vincan, E.; Mantamadiotis, T.; Purcell, D.; Chan, C.-K.; Ramsay, R. Oncogenic properties of HIV-Tat in colorectal cancer cells. Curr. HIV Res. 2007, 5, 403–409. [Google Scholar] [CrossRef]
- Liu, Y.P.; Chen, C.H.; Yen, C.H.; Tung, C.W.; Chen, C.J.; Chen, Y.A.; Huang, M.S. Human immunodeficiency virus Tat-TIP30 interaction promotes metastasis by enhancing the nuclear translocation of Snail in lung cancer cell lines. Cancer Sci. 2018, 109, 3105–3114. [Google Scholar] [CrossRef]
- Mani, K.; Sandgren, S.; Lilja, J.; Cheng, F.; Svensson, K.; Persson, L.; Belting, M. HIV-Tat protein transduction domain specifically attenuates growth of polyamine deprived tumor cells. Mol. Cancer Ther. 2007, 6, 782–788. [Google Scholar] [CrossRef] [Green Version]
- Dandachi, D.; Moron, F. Effects of HIV on the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1263, 45–54. [Google Scholar] [CrossRef]
- Nunnari, G.; Smith, J.A.; Daniel, R. HIV-1 Tat and AIDS-associated cancer: Targeting the cellular anti-cancer barrier? J. Exp. Clin. Cancer Res. 2008, 27, 3. [Google Scholar] [CrossRef] [Green Version]
- Loarca, L.; Fraietta, J.A.; Pirrone, V.; Szep, Z.; Wigdahl, B. Human immunodeficiency and Virus (HIV) Infection and Cancer. HIV/AIDS Contemp. Chall. 2017, 1. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, D.K.; Tendler, C.L.; Milani, D.; English, M.A.; Licht, J.D.; Wilson, S.H. The HIV-1 transactivator protein Tat is a potent inducer of the human DNA repair enzyme beta-polymerase. AIDS 2001, 15, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Chipitsyna, G.; Slonina, D.; Siddiqui, K.; Peruzzi, F.; Skorski, T.; Reiss, K.; Sawaya, B.E.; Khalili, K.; Amini, S. HIV-1 Tat increases cell survival in response to cisplatin by stimulating Rad51 gene expression. Oncogene 2004, 23, 2664–2671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentin-Guillama, G.; Lopez, S.; Kucheryavykh, Y.V.; Chorna, N.E.; Perez, J.; Ortiz-Rivera, J.; Inyushin, M.; Makarov, V.; Valentin-Acevedo, A.; Quinones-Hinojosa, A.; et al. HIV-1 Envelope Protein gp120 Promotes Proliferation and the Activation of Glycolysis in Glioma Cell. Cancers 2018, 10, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Yuan, Z.; Petree, J.R.; Lee, F.E.; Fan, X.; Salaita, K.; Guidot, D.M.; Sadikot, R.T. Macrophages exposed to HIV viral protein disrupt lung epithelial cell integrity and mitochondrial bioenergetics via exosomal microRNA shuttling. Cell Death Dis. 2019, 10, 580. [Google Scholar] [CrossRef]
- Greenway, A.L.; McPhee, D.A.; Allen, K.; Johnstone, R.; Holloway, G.; Mills, J.; Azad, A.; Sankovich, S.; Lambert, P. Human immunodeficiency virus type 1 Nef binds to tumor suppressor p53 and protects cells against p53-mediated apoptosis. J. Virol. 2002, 76, 2692–2702. [Google Scholar] [CrossRef] [Green Version]
- Xue, M.; Yao, S.; Hu, M.; Li, W.; Hao, T.; Zhou, F.; Zhu, X.; Lu, H.; Qin, D.; Yan, Q.; et al. HIV-1 Nef and KSHV oncogene K1 synergistically promote angiogenesis by inducing cellular miR-718 to regulate the PTEN/AKT/mTOR signaling pathway. Nucleic Acids Res. 2014, 42, 9862–9879. [Google Scholar] [CrossRef]
- Santerre, M.; Chatila, W.; Wang, Y.; Mukerjee, R.; Sawaya, B.E. HIV-1 Nef promotes cell proliferation and microRNA dysregulation in lung cells. Cell Cycle 2019, 18, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Bayurova, E.; Jansons, J.; Skrastina, D.; Smirnova, O.; Mezale, D.; Kostyusheva, A.; Kostyushev, D.; Petkov, S.; Podschwadt, P.; Valuev-Elliston, V. HIV-1 Reverse Transcriptase Promotes Tumor Growth and Metastasis Formation via ROS-Dependent Upregulation of Twist. Oxidative Med. Cell. Longev. 2019, 2019, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giagulli, C.; Magiera, A.K.; Bugatti, A.; Caccuri, F.; Marsico, S.; Rusnati, M.; Vermi, W.; Fiorentini, S.; Caruso, A. HIV-1 matrix protein p17 binds to the IL-8 receptor CXCR1 and shows IL-8-like chemokine activity on monocytes through Rho/ROCK activation. Blood 2012, 119, 2274–2283. [Google Scholar] [CrossRef]
- Caccuri, F.; Giagulli, C.; Bugatti, A.; Benetti, A.; Alessandri, G.; Ribatti, D.; Marsico, S.; Apostoli, P.; Slevin, M.A.; Rusnati, M.; et al. HIV-1 matrix protein p17 promotes angiogenesis via chemokine receptors CXCR1 and CXCR2. Proc. Natl. Acad. Sci. USA 2012, 109, 14580–14585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caccuri, F.; Rueckert, C.; Giagulli, C.; Schulze, K.; Basta, D.; Zicari, S.; Marsico, S.; Cervi, E.; Fiorentini, S.; Slevin, M.; et al. HIV-1 matrix protein p17 promotes lymphangiogenesis and activates the endothelin-1/endothelin B receptor axis. Arter. Thromb. Vasc. Biol. 2014, 34, 846–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorentini, S.; Giagulli, C.; Caccuri, F.; Magiera, A.K.; Caruso, A. HIV-1 matrix protein p17: A candidate antigen for therapeutic vaccines against AIDS. Pharmacol. Ther. 2010, 128, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Caccuri, F.; Giordano, F.; Barone, I.; Mazzuca, P.; Giagulli, C.; Ando, S.; Caruso, A.; Marsico, S. HIV-1 matrix protein p17 and its variants promote human triple negative breast cancer cell aggressiveness. Infect. Agents Cancer 2017, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Carroll, V.A.; Lafferty, M.K.; Marchionni, L.; Bryant, J.L.; Gallo, R.C.; Garzino-Demo, A. Expression of HIV-1 matrix protein p17 and association with B-cell lymphoma in HIV-1 transgenic mice. Proc. Natl. Acad. Sci. USA 2016, 113, 13168–13173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative stress during HIV infection: Mechanisms and consequences. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- El-Amine, R.; Germini, D.; Zakharova, V.V.; Tsfasman, T.; Sheval, E.V.; Louzada, R.A.N.; Dupuy, C.; Bilhou-Nabera, C.; Hamade, A.; Najjar, F.; et al. HIV-1 Tat protein induces DNA damage in human peripheral blood B-lymphocytes via mitochondrial ROS production. Redox Biol. 2018, 15, 97–108. [Google Scholar] [CrossRef]
- Estrada, V.; Monge, S.; Gomez-Garre, M.D.; Sobrino, P.; Masia, M.; Berenguer, J.; Portilla, J.; Vilades, C.; Martinez, E.; Blanco, J.R.; et al. Relationship between plasma bilirubin level and oxidative stress markers in HIV-infected patients on atazanavir- vs. efavirenz-based antiretroviral therapy. HIV Med. 2016, 17, 653–661. [Google Scholar] [CrossRef] [Green Version]
- Kolgiri, V.; Nagar, V.; Patil, V. Association of serum total bilirubin and plasma 8-OHdG in HIV/AIDS patients. Interv. Med. Appl. Sci. 2018, 10, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.M.; Sutliff, R.L. HIV-1, reactive oxygen species, and vascular complications. Free Radic. Biol. Med. 2012, 53, 143–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, T.O.; Ercal, N.; Nakaoke, R.; Banks, W.A. HIV-1 viral proteins gp120 and Tat induce oxidative stress in brain endothelial cells. Brain Res. 2005, 1045, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wu, R.F.; Xu, Y.C.; Flores, S.C.; Terada, L.S. HIV Tat activates c-Jun amino-terminal kinase through an oxidant-dependent mechanism. Virology 2001, 286, 62–71. [Google Scholar] [CrossRef] [Green Version]
- Capone, C.; Cervelli, M.; Angelucci, E.; Colasanti, M.; Macone, A.; Mariottini, P.; Persichini, T. A role for spermine oxidase as a mediator of reactive oxygen species production in HIV-Tat-induced neuronal toxicity. Free Radic. Biol. Med. 2013, 63, 99–107. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Litzburg, A.; Zhang, D.; Dewhurst, S.; Gelbard, H.A. HIV-1 transactivator of transcription protein induces mitochondrial hyperpolarization and synaptic stress leading to apoptosis. J. Immunol. 2005, 174, 4333–4344. [Google Scholar] [CrossRef] [Green Version]
- Pocernich, C.B.; Sultana, R.; Mohmmad-Abdul, H.; Nath, A.; Butterfield, D.A. HIV-dementia, Tat-induced oxidative stress, and antioxidant therapeutic considerations. Brain Res. Rev. 2005, 50, 14–26. [Google Scholar] [CrossRef]
- Wu, R.F.; Ma, Z.; Liu, Z.; Terada, L.S. Nox4-derived H2O2 mediates endoplasmic reticulum signaling through local Ras activation. Mol. Cell. Biol. 2010, 30, 3553–3568. [Google Scholar] [CrossRef] [Green Version]
- Helmcke, I.; Heumuller, S.; Tikkanen, R.; Schroder, K.; Brandes, R.P. Identification of structural elements in Nox1 and Nox4 controlling localization and activity. Antioxidants Redox Signal. 2009, 11, 1279–1287. [Google Scholar] [CrossRef]
- Pietraforte, D.; Tritarelli, E.; Testa, U.; Minetti, M. gp120 HIV envelope glycoprotein increases the production of nitric oxide in human monocyte-derived macrophages. J. Leukoc. Biol. 1994, 55, 175–182. [Google Scholar] [CrossRef]
- Shah, A.; Kumar, S.; Simon, S.D.; Singh, D.P.; Kumar, A. HIV gp120- and methamphetamine-mediated oxidative stress induces astrocyte apoptosis via cytochrome P450 2E1. Cell Death Dis. 2013, 4, e850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foga, I.O.; Nath, A.; Hasinoff, B.B.; Geiger, J.D. Antioxidants and dipyridamole inhibit HIV-1 gp120-induced free radical-based oxidative damage to human monocytoid cells. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1997, 16, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson, P.T.; Bendayan, R. HIV-1 viral envelope glycoprotein gp120 produces oxidative stress and regulates the functional expression of multidrug resistance protein-1 (Mrp1) in glial cells. J. Neurochem. 2008, 106, 1298–1313. [Google Scholar] [CrossRef]
- Reddy, P.V.; Gandhi, N.; Samikkannu, T.; Saiyed, Z.; Agudelo, M.; Yndart, A.; Khatavkar, P.; Nair, M.P. HIV-1 gp120 induces antioxidant response element-mediated expression in primary astrocytes: Role in HIV associated neurocognitive disorder. Neurochem. Int. 2012, 61, 807–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivetta, E.; Pietraforte, D.; Schiavoni, I.; Minetti, M.; Federico, M.; Sanchez, M. HIV-1 Nef regulates the release of superoxide anions from human macrophages. Biochem. J. 2005, 390, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olivetta, E.; Mallozzi, C.; Ruggieri, V.; Pietraforte, D.; Federico, M.; Sanchez, M. HIV-1 Nef induces p47(phox) phosphorylation leading to a rapid superoxide anion release from the U937 human monoblastic cell line. J. Cell. Biochem. 2009, 106, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Vilhardt, F.; Plastre, O.; Sawada, M.; Suzuki, K.; Wiznerowicz, M.; Kiyokawa, E.; Trono, D.; Krause, K.-H. The HIV-1 Nef protein and phagocyte NADPH oxidase activation. J. Biol. Chem. 2002, 277, 42136–42143. [Google Scholar] [CrossRef] [Green Version]
- Masanetz, S.; Lehmann, M.H. HIV-1 Nef increases astrocyte sensitivity towards exogenous hydrogen peroxide. Virol. J. 2011, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Chelvanambi, S.; Gupta, S.K.; Chen, X.; Ellis, B.W.; Maier, B.F.; Colbert, T.M.; Kuriakose, J.; Zorlutuna, P.; Jolicoeur, P.; Obukhov, A.G.; et al. HIV-Nef Protein Transfer to Endothelial Cells Requires Rac1 Activation and Leads to Endothelial Dysfunction Implications for Statin Treatment in HIV Patients. Circ. Res. 2019, 125, 805–820. [Google Scholar] [CrossRef]
- Isaguliants, M.; Smirnova, O.; Ivanov, A.V.; Kilpelainen, A.; Kuzmenko, Y.; Petkov, S.; Latanova, A.; Krotova, O.; Engström, G.; Karpov, V. Oxidative stress induced by HIV-1 reverse transcriptase modulates the enzyme’s performance in gene immunization. Hum. Vacc. Immunother. 2013, 9, 2111–2119. [Google Scholar] [CrossRef] [Green Version]
- Massiah, M.A.; Starich, M.R.; Paschall, C.; Summers, M.F.; Christensen, A.M.; Sundquist, W.I. Three-dimensional structure of the human immunodeficiency virus type 1 matrix protein. J. Mol. Biol. 1994, 244, 198–223. [Google Scholar] [CrossRef] [PubMed]
- Doherty, R.S.; De Oliveira, T.; Seebregts, C.; Danaviah, S.; Gordon, M.; Cassol, S. BioAfrica’s HIV-1 proteomics resource: Combining protein data with bioinformatics tools. Retrovirology 2005, 2, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeinolabediny, Y.; Caccuri, F.; Colombo, L.; Morelli, F.; Romeo, M.; Rossi, A.; Schiarea, S.; Ciaramelli, C.; Airoldi, C.; Weston, R.; et al. HIV-1 matrix protein p17 misfolding forms toxic amyloidogenic assemblies that induce neurocognitive disorders. Sci. Rep. 2017, 7, 10313. [Google Scholar] [CrossRef]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Miller, I.P.; Pavlovic, I.; Poljsak, B.; Suput, D.; Milisav, I. Beneficial Role of ROS in Cell Survival: Moderate Increases in H2O2 Production Induced by Hepatocyte Isolation Mediate Stress Adaptation and Enhanced Survival. Antioxidants 2019, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Ajasin, D.; Eugenin, E.A. HIV-1 Tat: Role in Bystander Toxicity. Front. Cell. Infect. Microbiol. 2020, 10, 61. [Google Scholar] [CrossRef]
- Debaisieux, S.; Rayne, F.; Yezid, H.; Beaumelle, B. The ins and outs of HIV-1 Tat. Traffic 2012, 13, 355–363. [Google Scholar] [CrossRef]
- Clark, E.; Nava, B.; Caputi, M. Tat is a multifunctional viral protein that modulates cellular gene expression and functions. Oncotarget 2017, 8, 27569–27581. [Google Scholar] [CrossRef] [Green Version]
- Marino, J.; Wigdahl, B.; Nonnemacher, M.R. Extracellular HIV-1 Tat Mediates Increased Glutamate in the CNS Leading to Onset of Senescence and Progression of HAND. Front. Aging Neurosci. 2020, 12, 168. [Google Scholar] [CrossRef]
- Montano, M.A.; Novitsky, V.A.; Blackard, J.T.; Cho, N.L.; Katzenstein, D.A.; Essex, M. Divergent transcriptional regulation among expanding human immunodeficiency virus type 1 subtypes. J. Virol. 1997, 71, 8657–8665. [Google Scholar] [CrossRef] [Green Version]
- Karn, J.; Stoltzfus, C.M. Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb. Perspect. Med. 2012, 2, a006916. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Ferrini, S.; Benelli, R.; Sforzini, S.; Giunciuglio, D.; Aluigi, M.G.; Proudfoot, A.E.; Alouani, S.; Wells, T.N.; Mariani, G.; et al. HIV-1 Tat protein mimicry of chemokines. Proc. Natl. Acad. Sci. USA 1998, 95, 13153–13158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eugenin, E.A.; Dyer, G.; Calderon, T.M.; Berman, J.W. HIV-1 tat protein induces a migratory phenotype in human fetal microglia by a CCL2 (MCP-1)-dependent mechanism: Possible role in NeuroAIDS. Glia 2005, 49, 501–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Hage, N.; Wu, G.; Wang, J.; Ambati, J.; Knapp, P.E.; Reed, J.L.; Bruce-Keller, A.J.; Hauser, K.F. HIV-1 Tat and opiate-induced changes in astrocytes promote chemotaxis of microglia through the expression of MCP-1 and alternative chemokines. Glia 2006, 53, 132–146. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, D.M.; Seth, P.; Durham, L.; Diaz, F.; Boursiquot, R.; Ransohoff, R.M.; Major, E.O. Astrocyte differentiation selectively upregulates CCL2/monocyte chemoattractant protein-1 in cultured human brain-derived progenitor cells. Glia 2006, 53, 81–91. [Google Scholar] [CrossRef]
- Youn, G.S.; Ju, S.M.; Choi, S.Y.; Park, J. HDAC6 mediates HIV-1 tat-induced proinflammatory responses by regulating MAPK-NF-kappaB/AP-1 pathways in astrocytes. Glia 2015, 63, 1953–1965. [Google Scholar] [CrossRef]
- Clouse, K.A.; Cosentino, L.M.; Weih, K.A.; Pyle, S.W.; Robbins, P.B.; Hochstein, H.D.; Natarajan, V.; Farrar, W.L. The HIV-1 gp120 envelope protein has the intrinsic capacity to stimulate monokine secretion. J. Immunol. 1991, 147, 2892–2901. [Google Scholar]
- Kalyanaraman, V.S.; Rodriguez, V.; Veronese, F.; Rahman, R.; Lusso, P.; DeVico, A.L.; Copeland, T.; Oroszlan, S.; Gallo, R.C.; Sarngadharan, M.G. Characterization of the secreted, native gp120 and gp160 of the human immunodeficiency virus type 1. AIDS Res. Hum. Retroviruses 1990, 6, 371–380. [Google Scholar] [CrossRef] [Green Version]
- Oh, S.-K.; Cruikshank, W.W.; Raina, J.; Blanchard, G.C.; Adler, W.H.; Walker, J.; Kornfeld, H. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J. Acquir. Immune Defic. Syndr. 1992, 5, 251–256. [Google Scholar] [CrossRef]
- Jones, M.V.; Bell, J.E.; Nath, A. Immunolocalization of HIV envelope gp120 in HIV encephalitis with dementia. AIDS 2000, 14, 2709–2713. [Google Scholar] [CrossRef]
- Berth, S.; Caicedo, H.H.; Sarma, T.; Morfini, G.; Brady, S.T. Internalization and axonal transport of the HIV glycoprotein gp120. ASN Neuro 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Rossi, F.W.; Pecoraro, A.; Pucino, V.; Criscuolo, G.; Paulis, A.; Spadaro, G.; Marone, G.; Varricchi, G. HIV gp120 Induces the Release of Proinflammatory, Angiogenic, and Lymphangiogenic Factors from Human Lung Mast Cells. Vaccines 2020, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Caby, M.-P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdin, J.; Goricar, K.; Dolzan, V.; Plemenitas, A.; Martin, J.N.; Peterlin, B.M.; Deeks, S.G.; Lenassi, M. Viral protein Nef is detected in plasma of half of HIV-infected adults with undetectable plasma HIV RNA. PLoS ONE 2018, 13, e0191613. [Google Scholar] [CrossRef] [PubMed]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotič, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitaš, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4 + T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Dillon, S.T.; Reeves, R.K.; Manickam, C.; Morgello, S.; Kirk, G.D.; Mehta, S.H.; Gabuzda, D. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci. Rep. 2018, 8, 7227. [Google Scholar] [CrossRef] [PubMed]
- James, C.O.; Huang, M.-B.; Khan, M.; Garcia-Barrio, M.; Powell, M.D.; Bond, V.C. Extracellular Nef protein targets CD4 + T cells for apoptosis by interacting with CXCR4 surface receptors. J. Virol. 2004, 78, 3099–3109. [Google Scholar] [CrossRef] [Green Version]
- Qiao, X.; He, B.; Chiu, A.; Knowles, D.M.; Chadburn, A.; Cerutti, A. Human immunodeficiency virus 1 Nef suppresses CD40-dependent immunoglobulin class switching in bystander B cells. Nat. Immunol. 2006, 7, 302–310. [Google Scholar] [CrossRef]
- Saribas, A.S.; Cicalese, S.; Ahooyi, T.M.; Khalili, K.; Amini, S.; Sariyer, I.K. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: Evidence for Nef-mediated neurotoxicity. Cell Death Dis. 2018, 8, e2542. [Google Scholar] [CrossRef] [Green Version]
- Anyanwu, S.I.; Doherty, A.; Powell, M.D.; Obialo, C.; Huang, M.B.; Quarshie, A.; Mitchell, C.; Bashir, K.; Newman, G.W. Detection of HIV-1 and Human Proteins in Urinary Extracellular Vesicles from HIV+ Patients. Adv. Virol. 2018, 2018, 1–16. [Google Scholar] [CrossRef]
- Caccuri, F.; Iaria, M.L.; Campilongo, F.; Varney, K.; Rossi, A.; Mitola, S.; Schiarea, S.; Bugatti, A.; Mazzuca, P.; Giagulli, C.; et al. Cellular aspartyl proteases promote the unconventional secretion of biologically active HIV-1 matrix protein p17. Sci. Rep. 2016, 6, 38027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolcetti, R.; Gloghini, A.; Caruso, A.; Carbone, A. A lymphomagenic role for HIV beyond immune suppression? Blood 2016, 127, 1403–1409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovic, M.; Tenner-Racz, K.; Pelser, C.; Stellbrink, H.J.; van Lunzen, J.; Lewis, G.; Kalyanaraman, V.S.; Gallo, R.C.; Racz, P. Persistence of HIV-1 structural proteins and glycoproteins in lymph nodes of patients under highly active antiretroviral therapy. Proc. Natl. Acad. Sci. USA 2005, 102, 14807–14812. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isaguliants, M.; Bayurova, E.; Avdoshina, D.; Kondrashova, A.; Chiodi, F.; Palefsky, J.M. Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind. Cancers 2021, 13, 305. https://doi.org/10.3390/cancers13020305
Isaguliants M, Bayurova E, Avdoshina D, Kondrashova A, Chiodi F, Palefsky JM. Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind. Cancers. 2021; 13(2):305. https://doi.org/10.3390/cancers13020305
Chicago/Turabian StyleIsaguliants, Maria, Ekaterina Bayurova, Darya Avdoshina, Alla Kondrashova, Francesca Chiodi, and Joel M. Palefsky. 2021. "Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind" Cancers 13, no. 2: 305. https://doi.org/10.3390/cancers13020305
APA StyleIsaguliants, M., Bayurova, E., Avdoshina, D., Kondrashova, A., Chiodi, F., & Palefsky, J. M. (2021). Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind. Cancers, 13(2), 305. https://doi.org/10.3390/cancers13020305




