Prevalence and Predictive Factors for Upfront Dose Reduction of the First Cycle of First-Line Chemotherapy in Older Adults with Metastatic Solid Cancer: Korean Cancer Study Group (KCSG) Multicenter Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Association between UDR and the Variables of the GA
2.3. Changes in Adverse Events According to UDR
2.4. Effect of Cisplatin Containing Chemotherapy on Hematologic Adverse Events
2.5. Tolerance and Compliance According to UDR
2.6. Overall Survival (OS) and Progression-Free Survival (PFS)
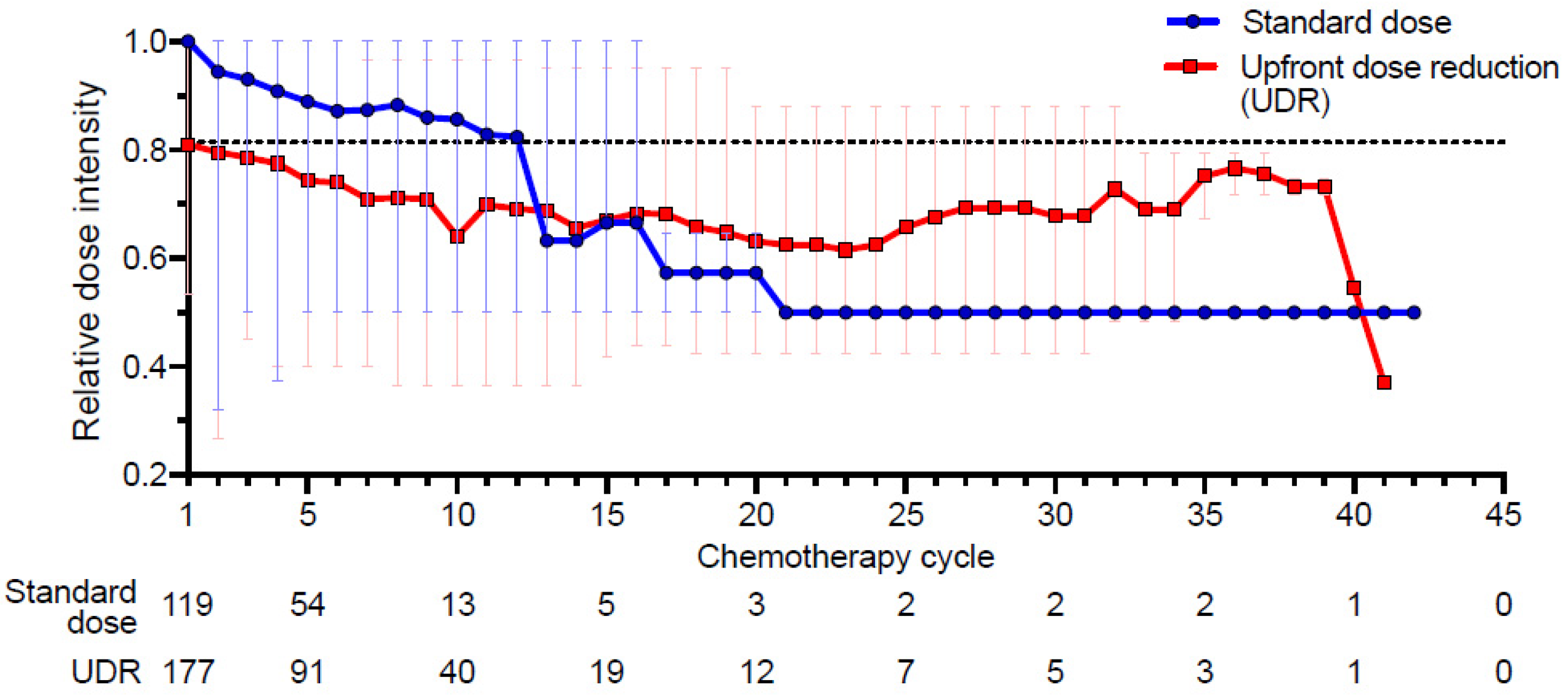
2.7. Changes in the Relative Dose Intensity (RDI)
3. Discussion
4. Materials and Methods
4.1. Patients and Chemotherapy
4.2. Measurements
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurria, A.; Dale, W.; Mooney, M.; Rowland, J.H.; Ballman, K.V.; Cohen, H.J.; Muss, H.B.; Schilsky, R.L.; Ferrell, B.; Extermann, M.; et al. Designing Therapeutic Clinical Trials for Older and Frail Adults With Cancer: U13 Conference Recommendations. J. Clin. Oncol. 2014, 32, 2587–2594. [Google Scholar] [CrossRef] [PubMed]
- Hurria, A.; Levit, L.A.; Dale, W.; Mohile, S.G.; Muss, H.B.; Fehrenbacher, L.; Magnuson, A.; Lichtman, S.M.; Bruinooge, S.S.; Soto-Perez-De-Celis, E.; et al. Improving the Evidence Base for Treating Older Adults with Cancer: American Society of Clinical Oncology Statement. J. Clin. Oncol. 2015, 33, 3826–3833. [Google Scholar] [CrossRef] [PubMed]
- Klepin, H.D.; Rodin, M.; Hurria, A. Treating Older Adults with Cancer: Geriatric Perspectives. Am. Soc. Clin. Oncol. Educ. Book 2015, e544–e552. [Google Scholar] [CrossRef] [PubMed]
- Gajra, A.; Klepin, H.D.; Feng, T.; Tew, W.P.; Mohile, S.G.; Owusu, C.; Gross, C.P.; Lichtman, S.M.; Wildes, T.M.; Chapman, A.E.; et al. Predictors of chemotherapy dose reduction at first cycle in patients age 65years and older with solid tumors. J. Geriatr. Oncol. 2015, 6, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Feliu, J.; Heredia-Soto, V.; Sarrió, R.G.; Jiménez-Munarriz, B.; Saldaña, J.; Guillén-Ponce, C.; Molina-Garrido, M. Can we avoid the toxicity of chemotherapy in elderly cancer patients? Crit. Rev. Oncol. 2018, 131, 16–23. [Google Scholar] [CrossRef]
- Hurria, A.; Togawa, K.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Bhatia, S.; Katheria, V.; et al. Predicting Chemotherapy Toxicity in Older Adults with Cancer: A Prospective Multicenter Study. J. Clin. Oncol. 2011, 29, 3457–3465. [Google Scholar] [CrossRef] [Green Version]
- Extermann, M.; Boler, I.; Reich, R.R.; Lyman, G.H.; Brown, R.H.; DeFelice, J.; Levine, R.M.; Lubiner, E.T.; Reyes, P.; Schreiber, F.J.; et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012, 118, 3377–3386. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, Y.-G.; Hwang, I.G.; Song, H.S.; Koh, S.J.; Ko, Y.H.; Shin, S.H.; Woo, I.S.; Hong, S.; Kim, T.Y.; et al. Predicting cumulative incidence of adverse events in older patients with cancer undergoing first-line palliative chemotherapy: Korean Cancer Study Group (KCSG) multicentre prospective study. Br. J. Cancer 2018, 118, 1169–1175. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, S.H.; Lee, Y.-G.; Hwang, I.G.; Kim, J.Y.; Koh, S.-J.; Ko, Y.H.; Shin, S.H.; Woo, I.S.; Hong, S.; et al. Prospective Validation of The Korean Cancer Study Group Geriatric Score (KG)-7, a Novel Geriatric Screening Tool, in Older Patients with Advanced Cancer Undergoing First-line Palliative Chemotherapy. Cancer Res. Treat. 2019, 51, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
- Brown, G.C. Living too long. EMBO Rep. 2015, 16, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, H.; Hurria, A.; Klepin, H.D. Progress Through Collaboration: An ASCO and U.S. Food and Drug Administration Workshop to Improve the Evidence Base for Treating Older Adults with Cancer. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Levit, L.A.; Singh, H.; Klepin, H.D.; Hurria, A. Expanding the Evidence Base in Geriatric Oncology: Action Items From an FDA-ASCO Workshop. J. Natl. Cancer Inst. 2018, 110, 1163–1170. [Google Scholar] [CrossRef]
- De Gramont, A.; Figer, A.; Seymour, M.; Homerin, M.; Hmissi, A.; Cassidy, J.; Boni, C.; Cortes-Funes, H.; Cervantes, A.; Freyer, G.; et al. Leucovorin and Fluorouracil with or Without Oxaliplatin as First-Line Treatment in Advanced Colorectal Cancer. J. Clin. Oncol. 2000, 18, 2938–2947. [Google Scholar] [CrossRef] [PubMed]
- Saltz, L.; Clarke, S.; Díaz-Rubio, E.; Scheithauer, W.; Figer, A.; Wong, R.; Koski, S.; Lichinitser, M.; Yang, T.-S.; Rivera, F.; et al. Bevacizumab in Combination with Oxaliplatin-Based Chemotherapy As First-Line Therapy in Metastatic Colorectal Cancer: A Randomized Phase III Study. J. Clin. Oncol. 2008, 26, 2013–2019. [Google Scholar] [CrossRef] [Green Version]
- Heinemann, V.; Von Weikersthal, L.F.; Decker, T.; Kiani, A.; Vehling-Kaiser, U.; Al-Batran, S.-E.; Heintges, T.; Lerchenmüller, C.; Kahl, C.; Seipelt, G.; et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): A randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1065–1075. [Google Scholar] [CrossRef]
- Sandler, A.; Gray, R.; Perry, M.C.; Brahmer, J.; Schiller, J.H.; Dowlati, A.; Lilenbaum, R.; Johnson, D.H. Paclitaxel–Carboplatin Alone or with Bevacizumab for Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2006, 355, 2542–2550. [Google Scholar] [CrossRef] [Green Version]
- Schiller, J.H.; Harrington, D.; Belani, C.P.; Langer, C.; Sandler, A.; Krook, J.; Zhu, J.; Johnson, D.H. Comparison of Four Chemotherapy Regimens for Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2002, 346, 92–98. [Google Scholar] [CrossRef]
- Socinski, M.A.; Bondarenko, I.; Karaseva, N.A.; Makhson, A.M.; Vynnychenko, I.; Okamoto, I.; Hon, J.K.; Hirsh, V.; Bhar, P.; Zhang, H.; et al. Weekly nab-Paclitaxel in Combination With Carboplatin Versus Solvent-Based Paclitaxel Plus Carboplatin as First-Line Therapy in Patients With Advanced Non–Small-Cell Lung Cancer: Final Results of a Phase III Trial. J. Clin. Oncol. 2012, 30, 2055–2062. [Google Scholar] [CrossRef] [Green Version]
- Scagliotti, G.V.; Kortsik, C.; Dark, G.G.; Price, A.; Manegold, C.; Rosell, R.; O’Brien, M.; Peterson, P.M.; Castellano, D.; Selvaggi, G.; et al. Pemetrexed combined with oxaliplatin or carboplatin as first-line treatment in advanced non-small cell lung cancer: A multicenter, randomized, phase II trial. Clin. Cancer Res. 2005, 11, 690–696. [Google Scholar]
- Lichtman, S.M.; Wildiers, H.; Launay-Vacher, V.; Steer, C.; Chatelut, E.; Aapro, M. International Society of Geriatric Oncology (SIOG) recommendations for the adjustment of dosing in elderly cancer patients with renal insufficiency. Eur. J. Cancer 2007, 43, 14–34. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.L.; Hurria, A.; Feng, T.; Mohile, S.G.; Owusu, C.; Klepin, H.D.; Gross, C.P.; Lichtman, S.M.; Gajra, A.; Glezerman, I.; et al. Association between renal function and chemotherapy-related toxicity in older adults with cancer. J. Geriatr. Oncol. 2017, 8, 96–101. [Google Scholar] [CrossRef] [Green Version]
- Cockcroft, D.W.; Gault, H. Prediction of Creatinine Clearance from Serum Creatinine. Nephron 1976, 16, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Jelliffe, R.; Jelliffe, S. Estimation of creatinine clearance from changing serum-creatinine levels. Lancet 1971, 298, 710. [Google Scholar] [CrossRef]
- Wright, J.G.; Boddy, A.V.; Highley, M.; Fenwick, J.; McGill, A.; Calvert, A.H. Estimation of glomerular filtration rate in cancer patients. Br. J. Cancer 2001, 84, 452–459. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, M. [Rationale, pros and cons of GFR estimation: The Cockcroft-Gault and MDRD equations]. G. Ital. Nefrol. Organo Uff. Soc. Ital. Nefrol. 2009, 26, 310–317. [Google Scholar]
- Sargent, D.J.; Goldberg, R.M.; Jacobson, S.D.; Macdonald, J.S.; Labianca, R.; Haller, D.G.; Shepherd, L.E.; Seitz, J.F.; Francini, G. A Pooled Analysis of Adjuvant Chemotherapy for Resected Colon Cancer in Elderly Patients. N. Engl. J. Med. 2001, 345, 1091–1097. [Google Scholar] [CrossRef]
- Muss, H.; Woolf, S.; Berry, D.; Cirrincione, C.; Weiss, R.B.; Budman, D.; Wood, W.C.; Henderson, I.C.; Hudis, C.A.; Winer, E.; et al. Adjuvant Chemotherapy in Older and Younger Women with Lymph Node–Positive Breast Cancer. JAMA 2005, 293, 1073–1081. [Google Scholar] [CrossRef]
- Seymour, M.T.; Thompson, L.C.; Wasan, H.S.; Middleton, G.; E Brewster, A.; Shepherd, S.F.; O’Mahony, M.S.; Maughan, T.S.; Parmar, M.; E Langley, R. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): An open-label, randomised factorial trial. Lancet 2011, 377, 1749–1759. [Google Scholar] [CrossRef] [Green Version]
- Van Bekkum, M.L.; Van Munster, B.C.; Thunnissen, P.L.; Smorenburg, C.H.; Hamaker, M.E. Current palliative chemotherapy trials in the elderly neglect patient-centred outcome measures. J. Geriatr. Oncol. 2015, 6, 15–22. [Google Scholar] [CrossRef]
- Hoppe, S.; Rainfray, M.; Fonck, M.; Hoppenreys, L.; Blanc, J.-F.; Ceccaldi, J.; Mertens, C.; Blanc-Bisson, C.; Imbert, Y.; Cany, L.; et al. Functional Decline in Older Patients with Cancer Receiving First-Line Chemotherapy. J. Clin. Oncol. 2013, 31, 3877–3882. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, J.-D.; Gagnon, P.; Harel, F.; Tremblay, A.; Roy, M.-A. Fast, Systematic, and Continuous Delirium Assessment in Hospitalized Patients: The Nursing Delirium Screening Scale. J. Pain Symptom Manag. 2005, 29, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Kim, C.-H.; Kim, K.-I.; Yoo, H.-J.; Park, S.-Y.; Park, Y.-H. Development and validation of the Korean Nursing Delirium Scale. J. Korean Acad. Nurs. 2012, 42, 414–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnuson, A.; Sattar, S.; Nightingale, G.; Saracino, R.; Skonecki, E.; Trevino, K.M. A Practical Guide to Geriatric Syndromes in Older Adults with Cancer: A Focus on Falls, Cognition, Polypharmacy, and Depression. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e96–e109. [Google Scholar] [CrossRef] [PubMed]
- Snædal, J. Does my older cancer patient have cognitive impairment? J. Geriatr. Oncol. 2018, 9, 183–185. [Google Scholar] [CrossRef]
- Lawlor, P.G.; Gagnon, B.; Mancini, I.L.; Pereira, J.L.; Hanson, J.; Suarez-Almazor, M.E.; Bruera, E.D. Occurrence, Causes, and Outcome of Delirium in Patients With Advanced Cancer. Arch. Intern. Med. 2000, 160, 786–794. [Google Scholar] [CrossRef] [Green Version]
- Bush, S.H.; Bruera, E. The Assessment and Management of Delirium in Cancer Patients. Oncologist 2009, 14, 1039–1049. [Google Scholar] [CrossRef]
- Maltoni, M.; Caraceni, A.; Brunelli, C.; Broeckaert, B.; Christakis, N.; Eychmueller, S.; Glare, P.; Nabal, M.; Viganò, A.; Larkin, P.; et al. Prognostic Factors in Advanced Cancer Patients: Evidence-Based Clinical Recommendations—A Study by the Steering Committee of the European Association for Palliative Care. J. Clin. Oncol. 2005, 23, 6240–6248. [Google Scholar] [CrossRef]
- Shayne, M.; Culakova, E.; Poniewierski, M.S.; Wolff, D.; Dale, D.C.; Crawford, J.; Lyman, G.H. Dose intensity and hematologic toxicity in older cancer patients receiving systemic chemotherapy. Cancer 2007, 110, 1611–1620. [Google Scholar] [CrossRef]
- Hurria, A.; Mohile, S.; Gajra, A.; Klepin, H.; Muss, H.; Chapman, A.; Feng, T.; Smith, D.; Sun, C.-L.; De Glas, N.; et al. Validation of a Prediction Tool for Chemotherapy Toxicity in Older Adults with Cancer. J. Clin. Oncol. 2016, 34, 2366–2371. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Standard Dose (N = 119) | Upfront Dose Reduction (N = 177) | Total (N = 296) | p-Value |
|---|---|---|---|---|
| Age | 0.15 ※ | |||
| Mean (range) | 74.8 (70–87) | 75.5 (70–93) | 75.0 (70–93) | |
| SD | 3.7 | 4.1 | 4.00 | |
| Sex | 0.10 * | |||
| Male | 89 (74.8%) | 116 (65.5%) | 205 (69.0%) | |
| Female | 30 (25.2%) | 61 (34.5%) | 91 (30.7%) | |
| BMI | 0.21 † | |||
| Mean | 22.8 | 22.3 | 22.5 | |
| Range | 15.7–31.2 | 13.9–29.6 | 13.9–31.2 | |
| SD | 3.2 | 3.0 | 3.1 | |
| ECOG-PS | <0.01 * | |||
| 0~1 | 106 (89.1%) | 134 (75.7%) | 240 (81.1%) | |
| 2~4 | 13 (10.9%) | 43 (24.3%) | 56 (18.9%) | |
| Hypertension | 0.23 * | |||
| Yes | 62 (52.1%) | 105 (59.3%) | 167 (56.4%) | |
| No | 57 (47.9%) | 72 (40.7%) | 129 (43.6%) | |
| Diabetes melitus | 0.25 * | |||
| Yes | 31 (26.1%) | 58 (32.8%) | 89 (30.1%) | |
| No | 88 (73.9%) | 119 (67.2%) | 207 (69.9%) | |
| Cancer type | 0.19 * | |||
| Colorectal | 22 (18.5%) | 62 (35.0%) | 84 (28.4%) | |
| Lung | 32 (26.9%) | 42 (23.7%) | 74 (25.0%) | |
| Biliary | 17 (14.3%) | 16 (9.0%) | 33 (11.1%) | |
| Stomach | 16 (13.4%) | 16 (9.0%) | 32 (10.8%) | |
| Pancreas | 12 (10.1%) | 14 (7.9%) | 26 (8.8%) | |
| GU | 5 (4.2%) | 11 (6.2%) | 16 (5.4%) | |
| Head and Neck | 4 (3.4%) | 4 (2.3%) | 8 (2.7%) | |
| Breast | 2 (1.7%) | 1 (0.6%) | 3 (1.0%) | |
| GYN | 2 (1.7%) | 1 (0.6%) | 3 (1.0%) | |
| Other | 7 (5.9%) | 10 (5.6%) | 17 (5.7%) | |
| Hemoglobin, g/dL | 1.00 * | |||
| ≥10 (female), ≥11 (male) | 90 (75.6%) | 134 (75.7%) | 224 (75.7%) | |
| <10 (female), <11 (male) | 29 (24.4%) | 43 (24.3%) | 72 (24.3%) | |
| WBC, ×103/μL Median (range) | 7.0 (2.9–27.8) | 7.44 (3.43–20.5) | 7.3 (2.9–27.7) | 0.203 ‡ |
| Platelet, ×103/μL Median (range) | 244 (79–493) | 257 (103–636) | 252 (79–636) | 0.195 ‡ |
| AST, IU/mL Median (range) | 23 (11–96) | 22 (7–239) | 22 (7–239) | 0.840 ‡ |
| Albumin, g/dL Median (range) | 3.7 (2.3–4.5) | 3.7 (2.2–4.6) | 3.7 (2.3–4.6) | 0.369 ‡ |
| Estimated GFR | 0.114 * | |||
| ≥60 mL/min/1.73 m2 | 99 (83.2%) | 133 (75.1%) | 232 (78.4%) | |
| <60 mL/min/1.73 m2 | 20 (16.8%) | 44 (24.9%) | 64 (21.6%) | |
| C-reactive protein, mg/L Median (range) | 1.29 (0.01–111.84) | 1.05 (0.01–191.58) | 1.14 (0.01–191.58) | 0.376 ‡ |
| Variables | Standard Dose (N = 119) | Upfront Dose Reduction (N = 177) | Total (N = 296) | p-Value |
|---|---|---|---|---|
| Comorbidity (Charlson risk index) | 0.30 † | |||
| Low (0 points) | 61 (51.3%) | 93 (52.5%) | 154 (51.7%) | |
| Medium (1 to 2 points) | 42 (35.3%) | 70 (39.5%) | 112 (38.1%) | |
| High (3 to 4 points) | 14 (11.8%) | 14 (7.9%) | 28 (9.5%) | |
| Very high (≥5 points) | 2 (1.7%) | 0 (0.0%) | 2 (0.7%) | |
| Activities of daily living | <0.01 * | |||
| Independent | 96 (80.7%) | 115 (65.0%) | 211 (71.3%) | |
| Dependent | 23 (19.3%) | 62 (35.0%) | 85 (28.7%) | |
| Instrumental activities of daily living (KIADL) | 0.55 * | |||
| Independent | 71 (59.7%) | 99 (55.9%) | 170 (57.4%) | |
| Dependent | 48 (40.3%) | 78 (44.1%) | 126 (42.9%) | |
| Cognitive function (MMSE-KC) | 0.77 † | |||
| Intact (25–30) | 48 (40.3%) | 83 (46.9%) | 131 (44.3%) | |
| Mild impairment (17–24) | 62 (52.1%) | 73 (41.2%) | 135 (45.6%) | |
| Severe impairment (≤16) | 9 (7.6%) | 21 (11.9%) | 30 (10.1%) | |
| Depression (SGDS) | 0.39 † | |||
| Intact (<5) | 69 (58.0%) | 95 (53.7%) | 164 (55.4%) | |
| Mild depression (5–9) | 38 (31.9%) | 52 (29.4%) | 90 (30.4%) | |
| Severe depression (≥10) | 11 (9.2%) | 29 (16.4%) | 40 (13.5%) | |
| Not available | 1 (0.8) | 1 (0.6%) | 2 (0.7%) | |
| Delirium risk screen (Korean Nu-DESC) | <0.01 * | |||
| No risk | 117 (98.3%) | 160 (90.4%) | 277 (93.6%) | |
| With Risk | 2 (1.7%) | 17 (9.6%) | 19 (6.4%) | |
| Nutritional status (MNA) | 0.83 † | |||
| Normal (≥24) | 28 (23.5%) | 41 (23.2%) | 69 (23.3%) | |
| Risk of malnutrition (17–23) | 72 (60.5%) | 96 (54.2%) | 168 (56.8%) | |
| Malnutrition (<17) | 18 (15.1%) | 40 (22.6%) | 58 (19.6%) | |
| Not available | 1 (0.8%) | 0 (0.0%) | 1 (0.3%) | |
| Living alone | 0.13 | |||
| Yes | 12 (10.1%) | 30 (16.9%) | 42 (14.2%) | |
| No | 107 (89.9%) | 147 (83.1%) | 254 (85.8%) | |
| Living with a spouse | <0.01 * | |||
| Yes | 96 (80.7%) | 118 (66.7%) | 214 (72.3%) | |
| No | 23 (19.3%) | 59 (33.3%) | 82 (27.7%) | |
| Male | 0.02 * | |||
| Yes | 82 (92.1%) | 93 (80.2%) | 175 (85.4%) | |
| No | 7 (7.9%) | 23 (19.8%) | 30 (14.6%) | |
| Female | 0.66 * | |||
| Yes | 14 (46.7%) | 25 (41.0%) | 39 (42.9%) | |
| No | 16 (53.3%) | 36 (59.0%) | 52 (57.1%) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variables | OR | (95% CI) | p-Value | OR | (95% CI) | p-Value |
| Poor ECOG-PS (2~4 vs 0~1) | 2.62 | (1.34–5.12) | 0.01 | 2.33 | (1.17–4.64) | 0.02 |
| Dependent in activity of daily living | 2.25 | (1.30–3.90) | <0.01 | |||
| Not living with a spouse | 2.09 | (1.02–3.62) | 0.01 | 1.89 | (1.07–3.33) | 0.03 |
| High delirium risk | 6.22 | (1.41–27.43) | 0.02 | 4.44 | (0.98–20.21) | 0.05 |
| Variables | Standard Dose (N = 119) | Upfront Dose Reduction (N = 177) | Total (N = 296) | p-Value |
|---|---|---|---|---|
| Any Grade 3 | 70 (58.8%) | 80 (45.2%) | 150 (50.7%) | |
| Any Grade 4 | 34 (28.6%) | 25 (14.1%) | 57 (19.3%) | |
| Any Grade 5 | 7 (5.9%) | 8 (4.5%) | 13 (4.4%) | <0.001 † |
| Any Grade 3–5 | 76 (63.9%) | 86 (48.6%) | 162 (54.7%) | 0.01 * |
| Hematologic Adverse Events (Grade 3–5) | ||||
| Neutropenia | 44 (37.0%) | 37 (20.9%) | 83 (27.4%) | <0.001 * |
| Febrile Neutropenia | 6 (5.0%) | 7 (4.0%) | 13 (4.4%) | 0.77 * |
| Leukopenia | 10 (8.4%) | 11 (6.2%) | 21 (7.1%) | 0.50 * |
| Anemia | 18 (15.1%) | 15 (8.5%) | 33 (11.1%) | 0.04 * |
| Thrombocytopenia | 14 (11.8%) | 9 (5.1%) | 23 (7.8%) | 0.04 * |
| Nonhematologic Adverse Events (Grade 3–5) | ||||
| Fatigue | 6 (5.0%) | 15 (8.5%) | 21 (7.1%) | 0.13 * |
| Generalized muscle weakness | 7 (5.9%) | 1 (0.6%) | 7 (2.4%) | 0.01 * |
| Thromboembolism | 3 (2.5%) | 4 (2.3%) | 7 (2.4%) | 1.00 * |
| Oral Mucositis | 0 (0) | 7 (4.0%) | 7 (2.4%) | 0.04 * |
| Peripheral sensory neuropathy | 0 (0) | 2 (1.1%) | 2 (2.4%) | 0.52 * |
| Anorexia | 12 (10.1%) | 8 (4.5%) | 20 (6.8%) | 0.09 |
| Nausea | 4 (3.8%) | 10 (5.3%) | 14 (6.7%) | 0.41 * |
| Vomiting | 3 (2.5%) | 2 (1.1%) | 5 (1.7%) | 0.39 * |
| Diarrhea | 4 (3.4%) | 6 (3.4%) | 10 (3.4%) | 1.00 * |
| Infection | 11 (9.2%) | 9 (5.1%) | 10 (6.8%) | 0.23 * |
| Sepsis | 3 (2.5%) | 3 (1.7%) | 6 (2.0%) | 0.68 * |
| Arrhythmia | 2 (1.7%) | 3 (1.7%) | 5 (1.7%) | 1.00 * |
| Variables | Standard Dose (N = 119) | Upfront Dose Reduction (N = 177) | Total (N = 296) | p-Value |
|---|---|---|---|---|
| Neutropenia | 44 (37.0%) | 37 (20.9%) | 83 (27.4%) | |
| Cisplatin (−) | 28/99 (28.3%) | 30/133 (22.6%) | 58/232 (27.2%) | 0.319 |
| Cisplatin (+) | 16/20 (80.0%) | 7/44 (15.9%) | 23/64 (28.1%) | 0.016 |
| Anemia | 18 (15.1%) | 15 (8.5%) | 33 (11.1%) | |
| Cisplatin (−) | 7/99 (7.1%) | 12/133 (9.0%) | 26/232 (11.2%) | 0.917 |
| Cisplatin (+) | 11/20 (55.0%) | 3/44 (6.8%) | 7/64 (10.9%) | 0.012 |
| Thrombocytopenia | 14 (11.8%) | 9 (5.1%) | 23 (7.8%) | |
| Cisplatin (−) | 4/99 (4.0%) | 6/133 (4.5%) | 19/232 (8.2%) | 0.774 |
| Cisplatin (+) | 10/20 (50.0%) | 3/44 (6.8%) | 4/64 (6.3%) | 0.022 |
| Variables | Standard Dose (N = 119) | Upfront Dose Reduction (N = 177) | Total (N = 296) | p-Value |
|---|---|---|---|---|
| ER visit or hospitalization | ||||
| Yes | 63 (52.9%) | 67 (37.9%) | 130 (43.9%) | |
| No | 56 (47.1%) | 110 (62.1%) | 166 (56.1%) | 0.01 * |
| Number of patients with delayed chemotherapy | ||||
| Yes | 61 (51.3%) | 88 (49.7%) | 149 (50.3%) | |
| No | 58 (48.7%) | 89 (50.3%) | 147 (49.7%) | 0.80 * |
| Number of cycles with delayed chemotherapy | ||||
| Median | 3 | 1 | 1 | 0.39 † |
| Range | 1–15 | 1–6 | 0–15 | |
| Delayed dates of chemotherapy (day) | ||||
| Median | 14.5 | 22 | 1 | 0.65 † |
| Range | 1–67 | 2–117 | 1–117 |
| Variables | Standard Dose (N = 119) | Upfront Dose Reduction (N = 177) | Total (N = 296) | p-Value * |
|---|---|---|---|---|
| Study Completion | 20 (16.8%) | 52 (29.4%) | 72 (24.3%) | 0.02 |
| Not completed | 99 (83.2%) | 125 (70.6%) | 224 (75.7%) | |
| Death | 69 (58.0%) | 97 (54.8%) | 166 (56.1%) | 0.63 |
| Follow-up loss | 12 (10.1%) | 12 (6.8%) | 24 (8.1%) | 0.18 |
| Patient decision | 8 (6.7%) | 12 (6.8%) | 20 (6.8%) | 1.00 |
| Transfer | 10 (8.4%) | 4 (2.3%) | 14 (4.7%) | 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, I.G.; Kwon, M.; Kim, J.W.; Kim, S.H.; Lee, Y.-G.; Kim, J.Y.; Koh, S.-J.; Ko, Y.H.; Shin, S.H.; Hong, S.; et al. Prevalence and Predictive Factors for Upfront Dose Reduction of the First Cycle of First-Line Chemotherapy in Older Adults with Metastatic Solid Cancer: Korean Cancer Study Group (KCSG) Multicenter Study. Cancers 2021, 13, 331. https://doi.org/10.3390/cancers13020331
Hwang IG, Kwon M, Kim JW, Kim SH, Lee Y-G, Kim JY, Koh S-J, Ko YH, Shin SH, Hong S, et al. Prevalence and Predictive Factors for Upfront Dose Reduction of the First Cycle of First-Line Chemotherapy in Older Adults with Metastatic Solid Cancer: Korean Cancer Study Group (KCSG) Multicenter Study. Cancers. 2021; 13(2):331. https://doi.org/10.3390/cancers13020331
Chicago/Turabian StyleHwang, In Gyu, Minsuk Kwon, Jin Won Kim, Se Hyun Kim, Yun-Gyoo Lee, Jin Young Kim, Su-Jin Koh, Yoon Ho Ko, Seong Hoon Shin, Soojung Hong, and et al. 2021. "Prevalence and Predictive Factors for Upfront Dose Reduction of the First Cycle of First-Line Chemotherapy in Older Adults with Metastatic Solid Cancer: Korean Cancer Study Group (KCSG) Multicenter Study" Cancers 13, no. 2: 331. https://doi.org/10.3390/cancers13020331





