Presence of the Human Cytomegalovirus in Glioblastomas—A Systematic Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Analyses of Sample Preparation and Identification of Methods Used for HCMV Analyses
2.2. Identifying Optimal Methods for More Accurate HCMV Testing
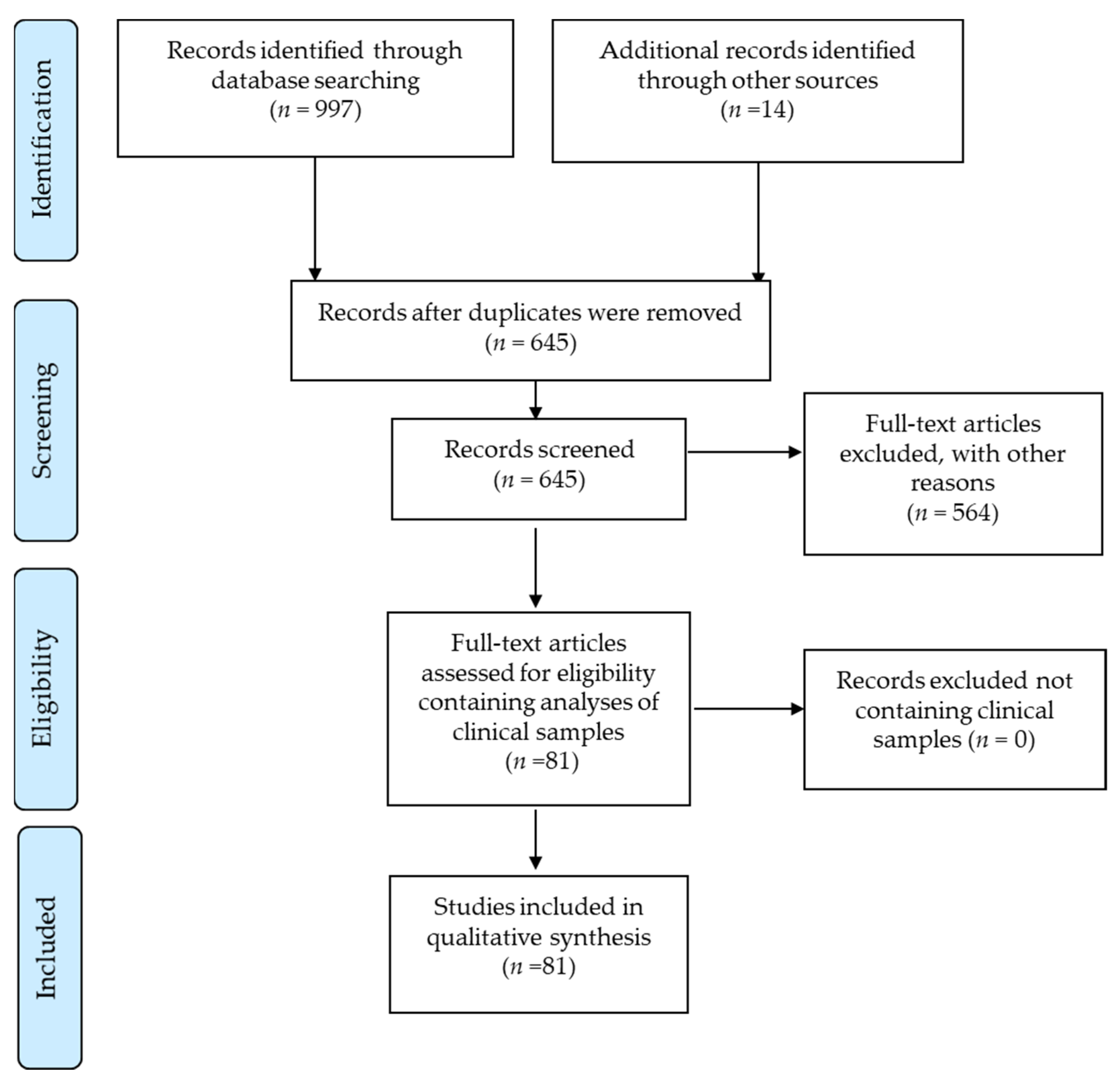
3. Results
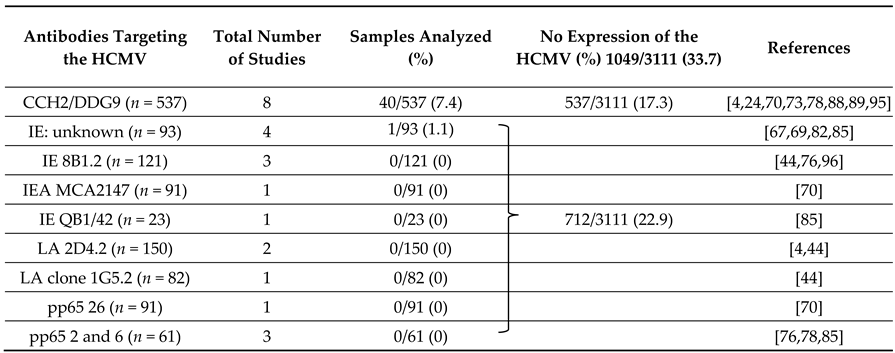
3.1. HCMV Protein Detection in Tumor Specimens
3.2. HCMV IE Protein Detection
3.3. HCMV Late Protein Detection
3.4. Optimized Techniques More Often Reveal Viral Proteins in Glioblastomas
3.5. Detection of HCMV Nucleic Acids in Tumor Samples
3.5.1. Detection of HCMV Nucleic Acids by PCR Methods
3.5.2. Detection of the HCMV Genome by Next-Generation Sequencing
3.5.3. Detection of the HCMV Genome by ISH
3.6. Analyses of Blood Samples to Detect HCMV DNA, HCMV-Specific Antibodies, or HCMV-Reactive T-Cells to HCMV
3.6.1. PCR Analyses
3.6.2. HCMV Serology Analyses
3.6.3. Detection of HCMV-Reactive T cells
3.7. Detection of HCMV Proteins Using Western Blot Analyses
4. Additional Finding
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K. Who Classification of Tumours of the Central Nervous System; WHO Regional Office Europe: Copenhagen, Denamrk, 2016. [Google Scholar]
- Cobbs, C.S.; Harkins, L.; Samanta, M.; Gillespie, G.Y.; Bharara, S.; King, P.H.; Nabors, L.B.; Cobbs, C.G.; Britt, W.J. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002, 62, 3347–3350. [Google Scholar] [PubMed]
- Dziurzynski, K.; Chang, S.M.; Heimberger, A.B.; Kalejta, R.F.; McGregor Dallas, S.R.; Smit, M.; Soroceanu, L.; Cobbs, C.S.; HCMV and Gliomas Symposium. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro-Oncology 2012, 14, 246–255. [Google Scholar] [CrossRef]
- Libard, S.; Popova, S.N.; Amini, R.-M.; Karja, V.; Pietilainen, T.; Hamalainen, K.M.; Sundstrom, C.; Hesselager, G.; Bergqvist, M.; Ekman, S.; et al. Human cytomegalovirus tegument protein pp65 is detected in all intra- and extra-axial brain tumours independent of the tumour type or grade. PLoS ONE 2014, 9, e108861. [Google Scholar] [CrossRef]
- Farias, K.; Moreli, M.L.; Floriano, V.G.; da Costa, V.G. Evidence based on a meta-analysis of human cytomegalovirus infection in glioma. Arch. Virol. 2019, 164, 1249–1257. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Schmid, D.S.; Hyde, T.B. Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev. Med. Virol. 2010, 20, 202–213. [Google Scholar] [CrossRef]
- La Rosa, C.; Diamond, D.J. The immune response to human cmv. Future Virol. 2012, 7, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Dolan, A.; Cunningham, C.; Hector, R.D.; Hassan-Walker, A.F.; Lee, L.; Addison, C.; Dargan, D.J.; McGeoch, D.J.; Gatherer, D.; Emery, V.C.; et al. Genetic content of wild-type human cytomegalovirus. J. Gen. Virol. 2004, 85, 1301–1312. [Google Scholar] [CrossRef] [PubMed]
- Stern-Ginossar, N.; Weisburd, B.; Michalski, A.; Le, V.T.; Hein, M.Y.; Huang, S.X.; Ma, M.; Shen, B.; Qian, S.B.; Hengel, H.; et al. Decoding human cytomegalovirus. Science 2012, 338, 1088–1093. [Google Scholar] [CrossRef] [PubMed]
- Barami, K. Oncomodulatory mechanisms of human cytomegalovirus in gliomas. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2010, 17, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J.; Scholz, M.; Kotchetkov, R.; Vogel, J.U.; Doerr, H.W. Molecular mechanisms of the modulatory effects of hcmv infection in tumor cell biology. Trends Mol. Med. 2004, 10, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G. The human cytomegalovirus, from oncomodulation to oncogenesis. Viruses 2018, 10, 408. [Google Scholar] [CrossRef] [PubMed]
- Mesri, E.A.; Feitelson, M.A.; Munger, K. Human viral oncogenesis: A cancer hallmarks analysis. Cell Host Microbe 2014, 15, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, P. The oncogenicity of human cytomegalovirus. In Manifestations of Cytomegalovirus Infection; IntechOpen Book Series: London, UK, 2013. [Google Scholar]
- Geder, K.M.; Lausch, R.; O’Neill, F.; Rapp, F. Oncogenic transformation of human embryo lung cells by human cytomegalovirus. Science 1976, 192, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Tripathy, M.K.; Pasquereau, S.; Al Moussawi, F.; Abbas, W.; Coquard, L.; Khan, K.A.; Russo, L.; Algros, M.P.; Valmary-Degano, S.; et al. The human cytomegalovirus strain db activates oncogenic pathways in mammary epithelial cells. EBioMedicine 2018, 30, 167–183. [Google Scholar] [CrossRef]
- Rapp, F.; Li, J.L. Demonstration of the oncogenic potential of herpes simplex viruses and human cytomegalovirus. Cold Spring Harb. Symp. Quant. Biol. 1975, 39 Pt 2, 747–763. [Google Scholar] [CrossRef]
- Stragliotto, G.; Pantalone, M.R.; Rahbar, A.; Bartek, J.; Soderberg-Naucler, C. Valganciclovir as add-on to standard therapy in glioblastoma patients. Clin. Cancer Res. 2020, 26, 4031–4039. [Google Scholar] [CrossRef]
- Stragliotto, G.; Rahbar, A.; Solberg, N.W.; Lilja, A.; Taher, C.; Orrego, A.; Bjurman, B.; Tammik, C.; Skarman, P.; Peredo, I.; et al. Effects of valganciclovir as an add-on therapy in patients with cytomegalovirus-positive glioblastoma: A randomized, double-blind, hypothesis-generating study. Int. J. Cancer 2013, 133, 1204–1213. [Google Scholar] [CrossRef]
- Ahani, N.; Karimi Arzenani, M.; Shirkoohi, R.; Rokouei, M.; Alipour Eskandani, M.; Nikravesh, A. Expression of insulin-like growth factor binding protein-2 (igfbp-2) gene in negative and positive human cytomegalovirus glioblastoma multiforme tissues. Med. Oncol. 2014, 31, 812. [Google Scholar] [CrossRef]
- Ahani, N.; Nikravesh, A.; Shirkoohi, R.; Karimi Arzenani, M.; Rokouei, M.; Alipour Eskandani, M. Detection of human cytomegalovirus in glioma tumor tissues. Comp. Clin. Pathol. 2013, 23, 1321–1330. [Google Scholar] [CrossRef]
- Ahn, S.; Jeun, S.S. Detection of human cytomegalovirus antigens in malignant gliomas as an immunotherapeutic target. Neuro-Oncology 2018, 20, vi134–vi135. [Google Scholar] [CrossRef]
- Al-Sherify, A.M.; Nimamezher, M.; Darwesh, M.F. Detection and comparative study of human herpesviruses 6,7 and cytomegalovirusin patients with brain tumors. Int. J. Pharm. Res. 2019, 11, 60–66. [Google Scholar]
- Bahador, M.; Navarro, A.G.; Rahman, M.A.; Dominguez-Valentin, M.; Sarowar, S.; Ulvestad, E.; Njolstad, G.; Lie, S.A.; Kristoffersen, E.K.; Bratland, E.; et al. Increased infiltration and tolerised antigen-specific cd8(+) t-em cells in tumor but not peripheral blood have no impact on survival of hcmv+ glioblastoma patients. Oncoimmunology 2017, 6, e1336272. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, B.; Renzette, N.; Kowalik, T.F. Genetic analysis of cytomegalovirus in malignant gliomas. J. Virol. 2012, 86, 6815–6824. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, E.; Roncarati, P.; Hougrand, O.; Guerin-El Khourouj, V.; Boreux, R.; Kroonen, J.; Martin, D.; Robe, P.; Rogister, B.; Delvenne, P.; et al. Human cytomegalovirus and primary intracranial tumours: Frequency of tumour infection and lack of correlation with systemic immune anti-viral responses. Neuropathol. Appl. Neurobiol. 2015, 41, e29–e40. [Google Scholar] [CrossRef]
- Chen, T.Q.; Li, Z.X.; Jiang, Y.; Xu, X.D.; Gan, L.; Wang, B.L. Detection of human cytomegalovirus in tumor tissue and peripheral blood samples of patients with gliomas. Int. J. Clin. Exp. Pathol. 2017, 10, 1499–1508. [Google Scholar]
- Deshpande, R.P.; Panigrahi, M.; Chandrasekhar, Y.B.V.K.; Babu, P.P. Profiling of micrornas modulating cytomegalovirus infection in astrocytoma patients. Neurol. Sci. 2018, 39, 1895–1902. [Google Scholar] [CrossRef]
- Ding, D.; Han, S.; Wang, Z.; Guo, Z.; Wu, A. Does the existence of hcmv components predict poor prognosis in glioma? J. Neuro-Oncol. 2014, 116, 515–522. [Google Scholar] [CrossRef]
- Dominguez-Valentin, M.; Gras Navarro, A.; Rahman, A.M.; Kumar, S.; Retiere, C.; Ulvestad, E.; Kristensen, V.; Lund-Johansen, M.; Lie, B.A.; Enger, P.O.; et al. Identification of a natural killer cell receptor allele that prolongs survival of cytomegalovirus-positive glioblastoma patients. Cancer Res. 2016, 76, 5326–5336. [Google Scholar] [CrossRef]
- dos Santos, C.J.; Stangherlin, L.M.; Figueiredo, E.G.; Correa, C.; Teixeira, M.J.; da Silva, M.C.C. High prevalence of hcmv and viral load in tumor tissues and peripheral blood of glioblastoma multiforme patients. J. Med. Virol. 2014, 86, 1953–1961. [Google Scholar] [CrossRef]
- Dziurzynski, K.; Wei, J.; Qiao, W.; Hatiboglu, M.A.; Kong, L.-Y.; Wu, A.; Wang, Y.; Cahill, D.; Levine, N.; Prabhu, S.; et al. Glioma-associated cytomegalovirus mediates subversion of the monocyte lineage to a tumor propagating phenotype. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 4642–4649. [Google Scholar] [CrossRef]
- Fonseca, R.F.; Kawamura, M.T.; Oliveira, J.A.; Teixeira, A.; Alves, G.; Carvalho, M.d.G.d.C. The prevalence of human cytomegalovirus DNA in gliomas of brazilian patients. Mem. Inst. Oswaldo Cruz 2012, 107, 953–954. [Google Scholar] [CrossRef]
- Foster, H.; Piper, K.; DePledge, L.; Li, H.F.; Scanlan, J.; Jae-Guen, Y.; Boeckh, M.; Cobbs, C. Human cytomegalovirus seropositivity is associated with decreased survival in glioblastoma patients. Neuro-Oncol. Adv. 2019, 1, vdz020. [Google Scholar] [CrossRef]
- Frey, B.; Goerig, N.; Ina, B.; Anna-Jasmina, D.; Klaus, K.; Klaus, Ü.; Manuel, S.; Arnd, D.; Tobias, E.; Ilker, E.; et al. Reactivation of hcmv during rt of the brain results in critical illness and early mortality. Radiother. Oncol. 2020, 152, S193–S194. [Google Scholar] [CrossRef]
- Ghazi, A.; Ashoori, A.; Hanley, P.J.; Brawley, V.S.; Shaffer, D.R.; Kew, Y.; Powell, S.Z.; Grossman, R.; Grada, Z.; Scheurer, M.E.; et al. Generation of polyclonal cmv-specific t cells for the adoptive immunotherapy of glioblastoma. J. Immunother. 2012, 35, 159–168. [Google Scholar] [CrossRef]
- Goerig, N.L.; Frey, B.; Korn, K.; Fleckenstein, B.; Uberla, K.; Schmidt, M.A.; Dorfler, A.; Engelhorn, T.; Eyupoglu, I.; Ruhle, P.F.; et al. Early mortality of brain cancer patients and its connection to cytomegalovirus reactivation during radiochemotherapy. Clin. Cancer Res. 2020, 26, 3259–3270. [Google Scholar] [CrossRef]
- Han, S.; Deng, J.; Wang, Z.; Liu, H.; Cheng, W.; Wu, A. Decreased human leukocyte antigen a*02:01 frequency is associated with risk of glioma and existence of human cytomegalovirus: A case-control study in northern china. Cancer Immunol. Immunother. 2017, 66, 1265–1273. [Google Scholar] [CrossRef]
- Han, S.; Wang, P.F.; Xing, Y.X.; Song, H.W.; Yao, K.; Lin, Z.X. Human cytomegalovirus (hcmv) infection was not correlated with overall survival in glioblastomas. Neoplasma 2018, 65, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Wang, B.; Qian, D.; Wang, M.; Huang, R.; Wei, L.; Li, L.; Zhang, L.; Liu, D.X. Human cytomegalovirus immediate-early protein promotes survival of glioma cells through interacting and acetylating atf5. Oncotarget 2017, 8, 32157–32170. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Qian, D.; Hu, M.; Zhang, X.; Song, J.; Li, L.; Chen, H.; Wang, B. Association between human cytomegalovirus infection and histone acetylation level in various histological types of glioma. Oncol. Lett. 2015, 10, 2812–2820. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Idriss, F.A.; Gassoum, A. Sawsan al daeaf, nahla e abdelraheem, imad fadl elmula. Detection of human cytomegalovirus DNA among sudanese glioma patients in khartoum state. Afr. J. Med. Sci. 2020, 5. Ajmsc.info. [Google Scholar]
- Liang, Q.; Wang, K.; Wang, B.; Cai, Q. Hcmv-encoded mir-ul112-3p promotes glioblastoma progression via tumour suppressor candidate 3. Sci. Rep. 2017, 7, 44705. [Google Scholar] [CrossRef] [PubMed]
- Limam, S.; Missaoui, N.; Hmissa, S.; Yacoubi, M.T.; Krifa, H.; Mokni, M.; Selmi, B. Investigation of human cytomegalovirus and human papillomavirus in glioma. Cancer Investig. 2020, 38, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Poiret, T.; Meng, Q.; Rao, M.; von Landenberg, A.; Schoutrop, E.; Valentini, D.; Dodoo, E.; Peredo-Harvey, I.; Maeurer, M. Epstein-barr virus- and cytomegalovirus-specific immune response in patients with brain cancer. J. Transl. Med. 2018, 16. [Google Scholar] [CrossRef]
- Lucas, K.G.; Bao, L.; Bruggeman, R.; Dunham, K.; Specht, C. The detection of cmv pp65 and ie1 in glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 231–238. [Google Scholar] [CrossRef]
- Maleki, F.; Sadigh, Z.A.; Sadeghi, F.; Muhammadnejad, A.; Farahmand, M.; Parvin, M.; Shirkoohi, R. Human cytomegalovirus infection in iranian glioma patients correlates with aging and tumor aggressiveness. J. Med. Virol. 2020, 92, 1266–1276. [Google Scholar] [CrossRef]
- Matlaf, L.A.; Harkins, L.E.; Bezrookove, V.; Cobbs, C.S.; Soroceanu, L. Cytomegalovirus pp71 protein is expressed in human glioblastoma and promotes pro-angiogenic signaling by activation of stem cell factor. PLoS ONE 2013, 8, e68176. [Google Scholar] [CrossRef]
- Meng, Q.; Valentini, D.; Rao, M.; Dodoo, E.; Maeurer, M. Cmv and ebv targets recognized by tumor-infiltrating b lymphocytes in pancreatic cancer and brain tumors. Sci. Rep. 2018, 8, 17079. [Google Scholar] [CrossRef]
- Mitchell, D.A.; Xie, W.; Schmittling, R.; Learn, C.; Friedman, A.; McLendon, R.E.; Sampson, J.H. Sensitive detection of human cytomegalovirus in tumors and peripheral blood of patients diagnosed with glioblastoma. Neuro-Oncology 2008, 10, 10–18. [Google Scholar] [CrossRef]
- Mohammad, A.-A.; Rahbar, A.; Lui, W.-O.; Davoudi, B.; Catrina, A.; Stragliotto, G.; Mellbin, L.; Hamsten, A.; Ryden, L.; Yaiw, K.-C.; et al. Detection of circulating hcmv-mir-ul112-3p in patients with glioblastoma, rheumatoid arthritis, diabetes mellitus and healthy controls. PLoS ONE 2014, 9, e113740. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Orrego, A.; Peredo, I.; Dzabic, M.; Wolmer-Solberg, N.; Straat, K.; Stragliotto, G.; Soderberg-Naucler, C. Human cytomegalovirus infection levels in glioblastoma multiforme are of prognostic value for survival. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2013, 57, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, A.; Peredo, I.; Solberg, N.W.; Taher, C.; Dzabic, M.; Xu, X.; Skarman, P.; Fornara, O.; Tammik, C.; Yaiw, K.; et al. Discordant humoral and cellular immune responses to cytomegalovirus (cmv) in glioblastoma patients whose tumors are positive for cmv. Oncoimmunology 2015, 4, e982391. [Google Scholar] [CrossRef]
- Rahbar, A.; Stragliotto, G.; Orrego, A.; Peredo, I.; Taher, C.; Willems, J.; Soderberg-Naucler, C. Low levels of human cytomegalovirus infection in glioblastoma multiforme associates with patient survival; -a case-control study. Herpesviridae 2012, 3, 3. [Google Scholar] [CrossRef]
- Ranganathan, P.; Clark, P.A.; Kuo, J.S.; Salamat, M.S.; Kalejta, R.F. Significant association of multiple human cytomegalovirus genomic loci with glioblastoma multiforme samples. J. Virol. 2012, 86, 854–864. [Google Scholar] [CrossRef]
- Scheurer, M.E.; Bondy, M.L.; Aldape, K.D.; Albrecht, T.; El-Zein, R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol. 2008, 116, 79–86. [Google Scholar] [CrossRef]
- Shamran, H.A.; Kadhim, H.S.; Hussain, A.R.; Kareem, A.; Taub, D.D.; Price, R.L.; Nagarkatti, M.; Nagarkatti, P.S.; Singh, U.P. Detection of human cytomegalovirus in different histopathological types of glioma in iraqi patients. BioMed Res. Int. 2015, 2015, 642652. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, S.; Hjalmars, U.; Juto, P.; Wadell, G.; Hallmans, G.; Tjonneland, A.; Halkjaer, J.; Manjer, J.; Almquist, M.; Melin, B.S. Human immunoglobulin g levels of viruses and associated glioma risk. Cancer Causes Control 2011, 22, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Slinger, E.; Maussang, D.; Schreiber, A.; Siderius, M.; Rahbar, A.; Fraile-Ramos, A.; Lira, S.A.; Soderberg-Naucler, C.; Smit, M.J. Hcmv-encoded chemokine receptor us28 mediates proliferative signaling through the il-6-stat3 axis. Sci. Signal. 2010, 3, ra58. [Google Scholar] [CrossRef]
- Soroceanu, L.; Matlaf, L.; Bezrookove, V.; Harkins, L.; Martinez, R.; Greene, M.; Soteropoulos, P.; Cobbs, C.S. Human cytomegalovirus us28 found in glioblastoma promotes an invasive and angiogenic phenotype. Cancer Res. 2011, 71, 6643–6653. [Google Scholar] [CrossRef] [PubMed]
- Stangherlin, L.M.; Castro, F.L.F.; Medeiros, R.S.S.; Guerra, J.M.; Kimura, L.M.; Shirata, N.K.; Nonogaki, S.; Dos Santos, C.J.; Carlan Silva, M.C. Human cytomegalovirus DNA quantification and gene expression in gliomas of different grades. PLoS ONE 2016, 11, e0159604. [Google Scholar] [CrossRef] [PubMed]
- Ursu, R.; Doridam, J.; Chaugne, E.; Zannou, H.; Belin, C.; Cuzzubbo, S.; Sirven-Villaros, L.; Brichler, S.; Levy-Piedbois, C.; Carpentier, A.F. Predictive factors of human cytomegalovirus reactivation in newly diagnosed glioblastoma patients treated with chemoradiotherapy. J. Neurovirol. 2021, 27, 94–100. [Google Scholar] [CrossRef]
- Wakefield, A.; Pignata, A.; Ghazi, A.; Ashoori, A.; Hegde, M.; Landi, D.; Gray, T.; Scheurer, M.E.; Chintagumpala, M.; Adesina, A.; et al. Is cmv a target in pediatric glioblastoma? Expression of cmv proteins, pp65 and ie1-72 and cmv nucleic acids in a cohort of pediatric glioblastoma patients. J. Neuro-Oncol. 2015, 125, 307–315. [Google Scholar] [CrossRef]
- Weathers, S.P.; Penas-Prado, M.; Pei, B.L.; Ling, X.; Kassab, C.; Banerjee, P.; Bdiwi, M.; Shaim, H.; Alsuliman, A.; Shanley, M.; et al. Glioblastoma-mediated immune dysfunction limits cmv-specific t cells and therapeutic responses: Results from a phase i/ii trial. Clin. Cancer Res. 2020, 26, 3565–3577. [Google Scholar] [CrossRef]
- Wrensch, M.; Weinberg, A.; Wiencke, J.; Miike, R.; Barger, G.; Kelsey, K. Prevalence of antibodies to four herpesviruses among adults with glioma and controls. Am. J. Epidemiol. 2001, 154, 161–165. [Google Scholar] [CrossRef]
- Xing, Y.; Wang, Y.; Wang, S.; Wang, X.; Fan, D.; Zhou, D.; An, J. Human cytomegalovirus infection contributes to glioma disease progression via upregulating endocan expression. Transl. Res. 2016, 177, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Zavala-Vega, S.; Castro-Escarpulli, G.; Hernandez-Santos, H.; Salinas-Lara, C.; Palma, I.; Mejia-Arangure, J.M.; Gelista-Herrera, N.; Rembao-Bojorquez, D.; Ochoa, S.A.; Cruz-Cordova, A.; et al. An overview of the infection of cmv, hsv 1/2 and ebv in mexican patients with glioblastoma multiforme. Pathol. Res. Pract. 2017, 213, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Adnan Ali, S.M.; Mirza, Y.; Ahmad, Z.; Zahid, N.; Enam, S.A. Human papillomavirus and human cytomegalovirus infection and association with prognosis in patients with primary glioblastoma in pakistan. World Neurosurg. 2019, 121, e931–e939. [Google Scholar] [CrossRef]
- Bassett, K.; Blondin, N. Determining the presence of human cytomegalovirus proteins in glioblastoma. Neuro-Oncology 2017, 19, vi69. [Google Scholar] [CrossRef]
- Baumgarten, P.; Michaelis, M.; Rothweiler, F.; Starzetz, T.; Rabenau, H.F.; Berger, A.; Jennewein, L.; Braczynski, A.K.; Franz, K.; Seifert, V.; et al. Human cytomegalovirus infection in tumor cells of the nervous system is not detectable with standardized pathologico-virological diagnostics. Neuro-Oncology 2014, 16, 1469–1477. [Google Scholar] [CrossRef]
- Cimino, P.J.; Zhao, G.; Wang, D.; Sehn, J.K.; Lewis, J.S., Jr.; Duncavage, E.J. Detection of viral pathogens in high grade gliomas from unmapped next-generation sequencing data. Exp. Mol. Pathol. 2014, 96, 310–315. [Google Scholar] [CrossRef]
- Cosset, E.; Petty, T.J.; Dutoit, V.; Cordey, S.; Padioleau, I.; Otten-Hernandez, P.; Farinelli, L.; Kaiser, L.; Bruyere-Cerdan, P.; Tirefort, D.; et al. Comprehensive metagenomic analysis of glioblastoma reveals absence of known virus despite antiviral-like type i interferon gene response. Int. J. Cancer 2014, 135, 1381–1389. [Google Scholar] [CrossRef]
- Garcia-Martinez, A.; Alenda, C.; Irles, E.; Ochoa, E.; Quintanar, T.; Rodriguez-Lescure, A.; Soto, J.L.; Barbera, V.M. Lack of cytomegalovirus detection in human glioma. Virol. J. 2017, 14, 216. [Google Scholar] [CrossRef]
- Garcia-Martinez, A.; Irles, E.; Barbera, V.M.; Egoavil, C.; Alenda, C.; Castillejo, A.; Castillejo, M.I.; Ochoa, E.; Barea, M.C.; Quintanar, T.; et al. Lack of human cytomegalovirus in gliomas. A viral load analysis by quantitative real time-pcr. Lab. Investig. 2013, 93, 416A. [Google Scholar]
- Hashida, Y.; Taniguchi, A.; Yawata, T.; Hosokawa, S.; Murakami, M.; Hiroi, M.; Ueba, T.; Daibata, M. Prevalence of human cytomegalovirus, polyomaviruses, and oncogenic viruses in glioblastoma among japanese subjects. Infect. Agents Cancer 2015, 10, 3. [Google Scholar] [CrossRef]
- Holdhoff, M.; Guner, G.; Rodriguez, F.J.; Hicks, J.L.; Zheng, Q.; Forman, M.S.; Ye, X.; Grossman, S.A.; Meeker, A.K.; Heaphy, C.M.; et al. Absence of cytomegalovirus in glioblastoma and other high-grade gliomas by real-time pcr, immunohistochemistry, and in situ hybridization. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 3150–3157. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.S.; Abrams, Z.B.; Mo, X.; Zhang, Y.; Huang, K. Lack of human cytomegalovirus expression in single cells from glioblastoma tumors and cell lines. J. Neurovirol. 2017, 23, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Chen, Y.-Y.; Chen, W.-G.; Diamond, D.J.; Mamelak, A.N.; Zaia, J.A.; Weiss, L.M. Lack of association of cytomegalovirus with human brain tumors. Mod. Pathol. 2005, 18, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Lehrer, S.; Green, S.; Rosenzweig, K.E.; Rendo, A. No circulating human cytomegalovirus in 14 cases of glioblastoma. Neuro-Oncology 2015, 17, 320. [Google Scholar] [CrossRef][Green Version]
- Lehrer, S.; Labombardi, V.; Green, S.; Pessin-Minsley, M.S.; Germano, I.M.; Rosenzweig, K.E. No circulating cytomegalovirus in five patients with glioblastoma multiforme. Anticancer Res. 2011, 31, 959–960. [Google Scholar]
- Lin, C.-T.M.; Leibovitch, E.C.; Almira-Suarez, M.I.; Jacobson, S. Human herpesvirus multiplex ddpcr detection in brain tissue from low- and high-grade astrocytoma cases and controls. Infect. Agents Cancer 2016, 11, 32. [Google Scholar] [CrossRef]
- Loit, M.P.; Adle-Biassette, H.; Bouazza, S.; Mazeron, M.C.; Manivet, P.; Lehmann-Che, J.; Teissier, N.; Mandonnet, E.; Molina, J.M. Multimodal techniques failed to detect cytomegalovirus in human glioblastoma samples. J. Neurovirol. 2019, 25, 50–56. [Google Scholar] [CrossRef]
- Malekpour Afshar, R.; Mollaei, H.R.; Zandi, B.; Iranpour, M. Evaluation of jc and cytomegalo viruses in glioblastoma tissue. Asian Pac. J. Cancer Prev. 2016, 17, 4907–4911. [Google Scholar]
- Nikolova, E.; Ferdinandov, D.; Mitev, V.; Todorova, A. Prevalence of human cytomegalovirus infection among bulgarian patients with brain tumours. Eur. J. Hum. Genet. 2019, 27, 1900. [Google Scholar]
- Poltermann, S.; Schlehofer, B.; Steindorf, K.; Schnitzler, P.; Geletneky, K.; Schlehofer, J.R. Lack of association of herpesviruses with brain tumors. J. Neurovirol. 2006, 12, 90–99. [Google Scholar] [CrossRef]
- Priel, E.; Wohl, A.; Teperberg, M.; Nass, D.; Cohen, Z.R. Human cytomegalovirus viral load in tumor and peripheral blood samples of patients with malignant gliomas. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2015, 22, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Sabatier, J.; Uro-Coste, E.; Pommepuy, I.; Labrousse, F.; Allart, S.; Tremoulet, M.; Delisle, M.B.; Brousset, P. Detection of human cytomegalovirus genome and gene products in central nervous system tumours. Br. J. Cancer 2005, 92, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Sardi, I.; Lucchesi, M.; Becciani, S.; Facchini, L.; Guidi, M.; Buccoliero, A.M.; Moriondo, M.; Baroni, G.; Stival, A.; Farina, S.; et al. Absence of human cytomegalovirus infection in childhood brain tumors. Am. J. Cancer Res. 2015, 5, 2476–2483. [Google Scholar] [PubMed]
- Solomon, I.H.; Ramkissoon, S.H.; Milner, D.A., Jr.; Folkerth, R.D. Cytomegalovirus and glioblastoma: A review of evidence for their association and indications for testing and treatment. J. Neuropathol. Exp. Neurol. 2014, 73, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Strojnik, T.; Duh, D.; Lah, T.T. Prevalence of neurotropic viruses in malignant glioma and their onco-modulatory potential. In Vivo 2017, 31, 221–229. [Google Scholar] [CrossRef]
- Strong, M.J.; Blanchard, E.t.; Lin, Z.; Morris, C.A.; Baddoo, M.; Taylor, C.M.; Ware, M.L.; Flemington, E.K. A comprehensive next generation sequencing-based virome assessment in brain tissue suggests no major virus - tumor association. Acta Neuropathol. Commun. 2016, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.S.; Abdalhamid, B.A.; El-Badawy, S.A.; Sorour, Y.M.; Almsned, F.M.; Al-Abbadi, M.A. Expression of cytomegalovirus in glioblastoma multiforme: Myth or reality? Br. J. Neurosurg. 2016, 30, 307–312. [Google Scholar] [CrossRef]
- Tang, K.W.; Alaei-Mahabadi, B.; Samuelsson, T.; Lindh, M.; Larsson, E. The landscape of viral expression and host gene fusion and adaptation in human cancer. Nat. Commun. 2013, 4, 2513. [Google Scholar] [CrossRef]
- Tang, K.-W.; Hellstrand, K.; Larsson, E. Absence of cytomegalovirus in high-coverage DNA sequencing of human glioblastoma multiforme. Int. J. Cancer 2015, 136, 977–981. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ito, Y.; Isomura, H.; Takemura, N.; Okamoto, A.; Motomura, K.; Tsujiuchi, T.; Natsume, A.; Wakabayashi, T.; Toyokuni, S.; et al. Lack of presence of the human cytomegalovirus in human glioblastoma. Mod. Pathol. 2014, 27, 922–929. [Google Scholar] [CrossRef]
- Yang, C.-F.; Ho, H.-L.; Lin, S.-C.; Hsu, C.-Y.; Ho, D.M.-T. Detection of human cytomegalovirus in glioblastoma among taiwanese subjects. PLoS ONE 2017, 12, e0179366. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Ye, X.; Zhu, L.; Zhang, N.; An, Z.; Zheng, W.J. Virome assembly and annotation in brain tissue based on next-generation sequencing. Cancer Med. 2020, 9, 6776–6790. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.; Touma, J.; Davoudi, B.; Benard, M.; Sauer, T.; Geisler, J.; Vetvik, K.; Rahbar, A.; Soderberg-Naucler, C. Human cytomegalovirus infection is correlated with enhanced cyclooxygenase-2 and 5-lipoxygenase protein expression in breast cancer. J. Cancer Res. Clin. Oncol. 2019, 145, 2083–2095. [Google Scholar] [CrossRef] [PubMed]
- Radestad, A.F.; Estekizadeh, A.; Cui, H.L.; Kostopoulou, O.N.; Davoudi, B.; Hirschberg, A.L.; Carlson, J.; Rahbar, A.; Soderberg-Naucler, C. Impact of human cytomegalovirus infection and its immune response on survival of patients with ovarian cancer. Transl. Oncol. 2018, 11, 1292–1300. [Google Scholar] [CrossRef]
- Samanta, M.; Harkins, L.; Klemm, K.; Britt, W.J.; Cobbs, C.S. High prevalence of human cytomegalovirus in prostatic intraepithelial neoplasia and prostatic carcinoma. J. Urol. 2003, 170, 998–1002. [Google Scholar] [CrossRef]
- Cobbs, C.S.; Matlaf, L.; Harkins, L.E. Methods for the detection of cytomegalovirus in glioblastoma cells and tissues. Methods Mol. Biol. 2014, 1119, 165–196. [Google Scholar]
- Lester, S. Manual of Surgical Pathology, 3rd ed.; Specimen processing; Elsevier Health Sciences: Philadelphia, PA, USA, 2010. [Google Scholar]
- Munro, S.C.; Hall, B.; Whybin, L.R.; Leader, L.; Robertson, P.; Maine, G.T.; Rawlinson, W.D. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J. Clin. Microbiol. 2005, 43, 4713–4718. [Google Scholar] [CrossRef]
- Odland, M.L.; Strand, K.M.; Nordbo, S.A.; Forsmo, S.; Austgulen, R.; Iversen, A.C. Changing patterns of cytomegalovirus seroprevalence among pregnant women in norway between 1995 and 2009 examined in the norwegian mother and child cohort study and two cohorts from sor-trondelag county: A cross-sectional study. BMJ Open 2013, 3, e003066. [Google Scholar] [CrossRef][Green Version]
- Olsson, J.; Kok, E.; Adolfsson, R.; Lovheim, H.; Elgh, F. Herpes virus seroepidemiology in the adult swedish population. Immun. Ageing 2017, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Porobic-Jahic, H.; Skokic, F.; Ahmetagic, S.; Piljic, D.; Jahic, R.; Petrovic, J. Cytomegalovirus infection in pregnancy—Our experiences. Med. Arch. 2019, 73, 149–153. [Google Scholar] [PubMed]
- Goerig, N.; Semrau, S.; Frey, B.; Korn, K.; Fleckenstein, B.; Uberla, K.; Dorfler, A.; Putz, F.; Gaipl, U.S.; Fietkau, R. Clinically significant cmv (re)activation during or after radiotherapy/chemotherapy of the brain. Strahlentherapie Und Onkologie 2016, 192, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Gumrukcu, S.; Nguyen, T.X.; White, R.L.; Howell, G.T.; Musikanth, P. Allogeneic natural killer and cytomegalovirus (cmv)-pp65 pulsed dendritic cells induced complete response through 15 months in a patient with recurrent glioblastoma: A case study. Am. J. Case Rep. 2021, 22, e931030. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, E.; Dimova, P.; Minkin, K.; Todorov, T.; Mitev, V.; Todorova, A. Human cytomegalovirus DNA detection in a recurrent glioblastoma multiforme tumour, but not in whole blood: A case report and discussion about the hcmv latency and therapy perspectives. J. Neurovirol. 2020, 26, 984–987. [Google Scholar] [CrossRef]
- Stragliotto, G.; Pantalone, M.R.; Rahbar, A.; Soderberg-Naucler, C. Valganciclovir as add-on to standard therapy in secondary glioblastoma. Microorganisms 2020, 8, 1471. [Google Scholar] [CrossRef]
- Walker, D.G.; Shakya, R.; Morrison, B.; Neller, M.A.; Matthews, K.K.; Nicholls, J.; Smith, C.; Khanna, R. Impact of pre-therapy glioblastoma multiforme microenvironment on clinical response to autologous cmv-specific t-cell therapy. Clin. Transl. Immunol. 2019, 8, e01088. [Google Scholar] [CrossRef]
- Barukčić, I. Human cytomegalovirus is the cause of glioblastoma multiforme. Mod. Health Sci. 2018, 1, p19. [Google Scholar] [CrossRef]
- Chrysanthakopoulos, N.A.; Chrysanthakopoulos, P.A. Potential risk factors of glioblastoma multiforme in greek adults: A case-control study. J. Clin. Med. Res. 2020, 1, 1–17. [Google Scholar] [CrossRef]
- Cobbs, C. Response to “human cytomegalovirus infection in tumor cells of the nervous system is not detectable with standardized pathologico-virological diagnostics”. Neuro-Oncology 2014, 16, 1435–1436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Renzette, N.; Bhattacharjee, B.; Jensen, J.D.; Gibson, L.; Kowalik, T.F. Extensive genome-wide variability of human cytomegalovirus in congenitally infected infants. PLoS Pathog. 2011, 7, e1001344. [Google Scholar] [CrossRef] [PubMed]
- Renzette, N.; Pokalyuk, C.; Gibson, L.; Bhattacharjee, B.; Schleiss, M.R.; Hamprecht, K.; Yamamoto, A.Y.; Mussi-Pinhata, M.M.; Britt, W.J.; Jensen, J.D.; et al. Limits and patterns of cytomegalovirus genomic diversity in humans. Proc. Natl. Acad. Sci. USA 2015, 112, E4120–E4128. [Google Scholar] [CrossRef] [PubMed]
| Number of Patients | |||
|---|---|---|---|
| (n = 3770) | (n = 1561) | ||
| Evidence of HCMV (%) | HCMV in Tissue Specimens (%) | HCMV in Blood Samples (%) | |
| Articles (n = 81) | 51/81 (63.0) | - | |
| Analyses (n * = 247) | 192/247 (77.7) | 141/190 (74.2) | 51/57 (89.5) |
| Samples (n º = 9444) | 3623/9444 (38.4) | 2529/7024 (36.0) | 1094/2420 (45.2) |
| Methods | Tumor Tissue Specimens | Blood Samples | ||||||
|---|---|---|---|---|---|---|---|---|
| IF | IHC | ISH | PCR | NGS | FACS | PCR | ELISA | |
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| Articles | 4/4 | 25/39 | 10/15 | 29/48 | 0/7 | 6/6 | 8/15 | 14/20 |
| (100) | (64.1) | (66.7) | (60.4) | 0 | (100) | (53.3) | (70.0) | |
| Analyses | 6/6 | 51/74 | 11/18 | 73/85 | 0/7 | 8/8 | 14/20 | 28/29 |
| (100) | (68.9) | (61.1) | (85.9) | 0 | (100) | −70 | (96.6) | |
| Positive | 119/217 | 1500/3111 | 132/339 | 778/2676 | 0/681 | 216/288 | 238/883 | 640/1249 |
| Samples | (54.8) | (48.2) | (38.9) | (29.1) | 0 | (75.0) | (27.0) | (51.2) |
| Analyzed | 217/7024 | 3111/7024 | 339/7024 | 2676/7024 | 681/7024 | 288/2420 | 883/2420 | 1249/2420 |
| Samples/ | (3.1) | (44.3) | (4.8) | (38.1) | (9.7) | (11.9) | (36.5) | (51.6) |
| Total number of samples | ||||||||
| Methods | IE1 * | IE2 ** | IEA | EA | LA£ | pp65 | CCH2 + DDG9 | Other |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | (%) | (%) | (%) | |
| Expression of the HCMV | 389/773 | 73/73 | 230/327 | 122/159 | 275/533 | 329 ∞/630 ∞ | 40/537 | 42/79 |
| 50.3 | 100 | 70.3 | 76.7 | 51.6 | 52.2 | 7.4 | 53.2 | |
| Analyzed Samples/Total samples (n = 3111) | 773/3111 | 73/3111 | 327/3111 | 159/3111 | 533/3111 | 630/3111 | 537/3111 | 79/3111 |
| (24.9) | (2.4) | (10.5) | (5.1) | (17.1) | (20.3) | (17.3) | (2.5) |
| Antibodies Targeting the HCMV | Total Number of Analysis (n = 37) | HCMV-Positive Samples (%) (n = 1391/1653 (84,2) | References |
|---|---|---|---|
| IE72 (n = 368) | 10 | 256/368 (69.6) | [2,27,29,36,50,56,63,87] |
| IE1 clone 8B1.2 (n = 68) (optimized methods) | 1 | 61/68 (89.7) | [4] |
| IE1 clone 6F8.2 (n = 68) | 1 | 61/68 (89.7) | [4] |
| IE2 (n = 73) | 3 | 73/73 (100) | [40,41,43] |
| IEA (n = 236) | 6 | 230/236 (97.5) | [2,19,52,54,59,95] |
| EA clone QB1/42 EA clone BM204 (n = 136) | 2 | 122/136 (89.7) | [4] |
| LA (n = 1 97) | 3 | 188/197 (95.4) | [19,52,54] |
| LA clone1G5.2 (n = 104) | 2 | 87/104 (83.7) | [4,57] |
| pp65 (n = 93) | 4 | 79/93 (84.9) | [2,27,50,57] |
| pp65 clone 12D10 (n = 36) | 1 | 28/36 (77.8) | [57] |
| pp65 clones 2 and 6 (n = 274) | 6 | 206/274 (75.2) | [4,29,38,46,47,66] |
 |
| HCMV Genes | No Def | Nested | RT, Taq, q | RT | ddPCR | Samples | % | %/tot 2676 | Analysis |
|---|---|---|---|---|---|---|---|---|---|
| Pos/tot | Pos/tot | Pos/tot | Pos/tot | Pos/tot | Pos/tot | ||||
| No def | 13/22 | 31/146 | 106/441 | 0/45 | 150/654 | 22.9 | 24.4 | 19 | |
| gB | 5/13 | 84/258 | 94/366 | 183/637 | 28.7 | 23.8 | 19 | ||
| pp71 | 5/5 | 22/25 | 27/30 | 90 | 1.1 | 4 | |||
| IE1 | 26/73 | 21/162 | 25/25 | 72/260 | 27.7 | 9.7 | 12 | ||
| pp65 | 6/18 | 10/11 | 169/612 | 185/641 | 28.9 | 24.0 | 9 | ||
| US28 | 8/8 | 8/8 | 18/44 | 34/60 | 56.7 | 2.2 | 3 | ||
| UL144 | 4/5 | 0/116 | 4/5 | 8/126 | 6.3 | 4.7 | 3 | ||
| Others | 49/50 | 65/198 | 5/20 | 119/268 | 44.4 | 10.0 | 16 | ||
| Samples | 90/121 | 151/604 | 489/1817 | 48/89 | 0/45 | 778/2676 | 29.1 | ||
| % | 74.4 | 25 | 26.9 | 53.9 | 0 | 29.1 | |||
| %/tot 2676 | 4.5 | 22.6 | 67.9 | 3.3 | 1.7 | 100 | |||
| Analysis | 14 | 20 | 47 | 3 | 1 | 85 |
| Methods | NGS DNA | NGS RNA | Meta RNA | ISH RNA | ISH DNA |
|---|---|---|---|---|---|
| Samples | 0/55 | 0/606 | 0/20 | 10/117 | 122/222 |
| Analysis | 2 | 4 | 1 | 5 | 13 |
| HCMV Genes | No def | Nested | RT, Taq, q | RT | Samples | % | %/tot 883 | Analysis |
|---|---|---|---|---|---|---|---|---|
| Pos/tot | Pos/tot | Pos/tot | Pos/tot | Pos/tot | 4 | |||
| No def | 6/41 | 0/19 | 6/60 | 10 | 6.8 | 7 | ||
| gB | 27/40 | 15/56 | 0/27 | 42/123 | 34,1 | 13.9 | 5 | |
| IE1 | 135/251 | 0/23 | 18/149 | 153/423 | 36,2 | 47.9 | 3 | |
| pp65 | 33/239 | 33/239 | 13,8 | 27.1 | 1 | |||
| US17 | 4/38 | 4/38 | 10,5 | 4.3 | ||||
| Samples | 168/332 | 15/79 | 55/453 | 0/19 | 238/883 | 27 | ||
| % | 50.6 | 19 | 12.1 | 0 | 27.0 | |||
| %/tot 883 | 37.6 | 9 | 51.3 | 2.2 | 100 | |||
| Analysis | 6 | 4 | 8 | 2 | 20 |
| Methods | HCMV-IgG | HCMV-IgM | HCMV-Ig | HCMV-IE Peptides (T Cell Stimulation) | HCMV-pp65 Peptides (T Cell Stimulation) |
|---|---|---|---|---|---|
| Analyses | 12/12 | 5/7 | 2/2 | 2/2 | 3/3 |
| Samples (%) | 562/883 (63.6) | 33/297 (11.1) | 45/69 (65.2) | 21/23 (91.3) | 195/265 (73.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peredo-Harvey, I.; Rahbar, A.; Söderberg-Nauclér, C. Presence of the Human Cytomegalovirus in Glioblastomas—A Systematic Review. Cancers 2021, 13, 5051. https://doi.org/10.3390/cancers13205051
Peredo-Harvey I, Rahbar A, Söderberg-Nauclér C. Presence of the Human Cytomegalovirus in Glioblastomas—A Systematic Review. Cancers. 2021; 13(20):5051. https://doi.org/10.3390/cancers13205051
Chicago/Turabian StylePeredo-Harvey, Inti, Afsar Rahbar, and Cecilia Söderberg-Nauclér. 2021. "Presence of the Human Cytomegalovirus in Glioblastomas—A Systematic Review" Cancers 13, no. 20: 5051. https://doi.org/10.3390/cancers13205051
APA StylePeredo-Harvey, I., Rahbar, A., & Söderberg-Nauclér, C. (2021). Presence of the Human Cytomegalovirus in Glioblastomas—A Systematic Review. Cancers, 13(20), 5051. https://doi.org/10.3390/cancers13205051







