In Vivo PET Imaging of Monocytes Labeled with [89Zr]Zr-PLGA-NH2 Nanoparticles in Tumor and Staphylococcus aureus Infection Models
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Nanoparticles
2.2. Characterization of Nanoparticles
2.3. Characterization of Nanoparticles Stability in Human Serum and PBS over Time
2.4. [89Zr]ZrCl4 Preparation from 89Zr-Oxalate
2.5. Intrinsic 89Zr-Labeling of PLGA and PLGA-NH2 NPs
2.6. 89Zr-Retention of PLGA-NH2 NPs in PBS and Human Serum
2.7. EDTA Challenge
2.8. Cell Culture
2.9. [89Zr]Zr-PLGA-NH2 NP Labeling of THP-1 Cell Line and Retention of Radiolabel over Time
2.10. Cell Counting
2.11. CellTiter-Glo Assay
2.12. Animal Experiments
2.13. [89Zr]Zr-PLGA-NH2 NPs Biodistribution and Blood Clearance in C57BL/6JRj Mice
2.14. PET and MRI Imaging of [89Zr]Zr-PLGA-NH2 NPs Labeled THP-1 Cells in Matrigel
2.15. In Vivo Tracking of [89Zr]Zr-THP-1 Cells in S. aureus and MDA-MB-231 Tumor Models
2.16. Statistical Analysis
3. Results
3.1. Characterization of Particles
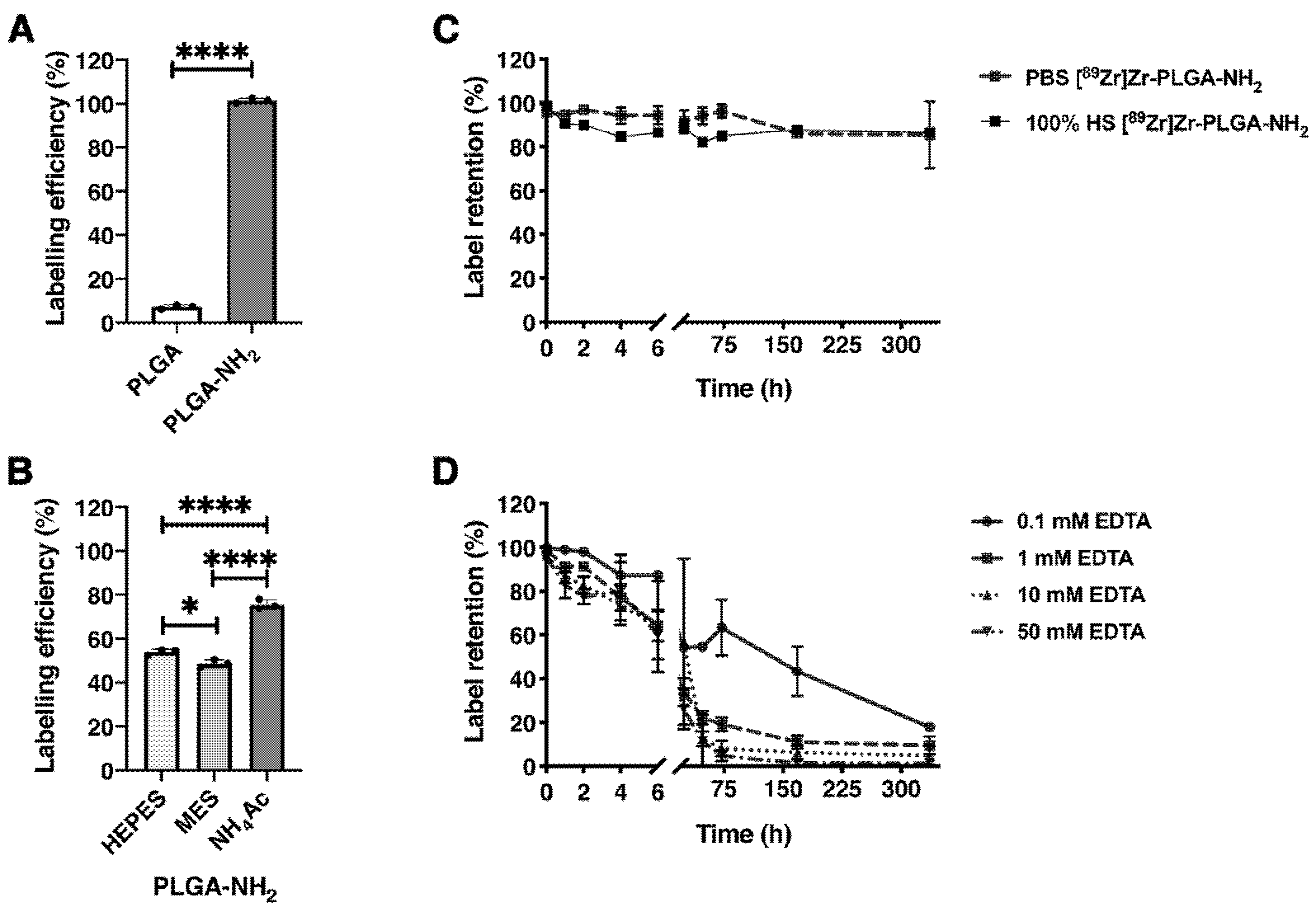
3.2. Stability of the PLGA-NH2 and Zr-PLGA-NH2 NPs in PBS and Human Serum
3.3. [89Zr]ZrCl4 Labeling of PLGA and PLGA-NH2 NPs
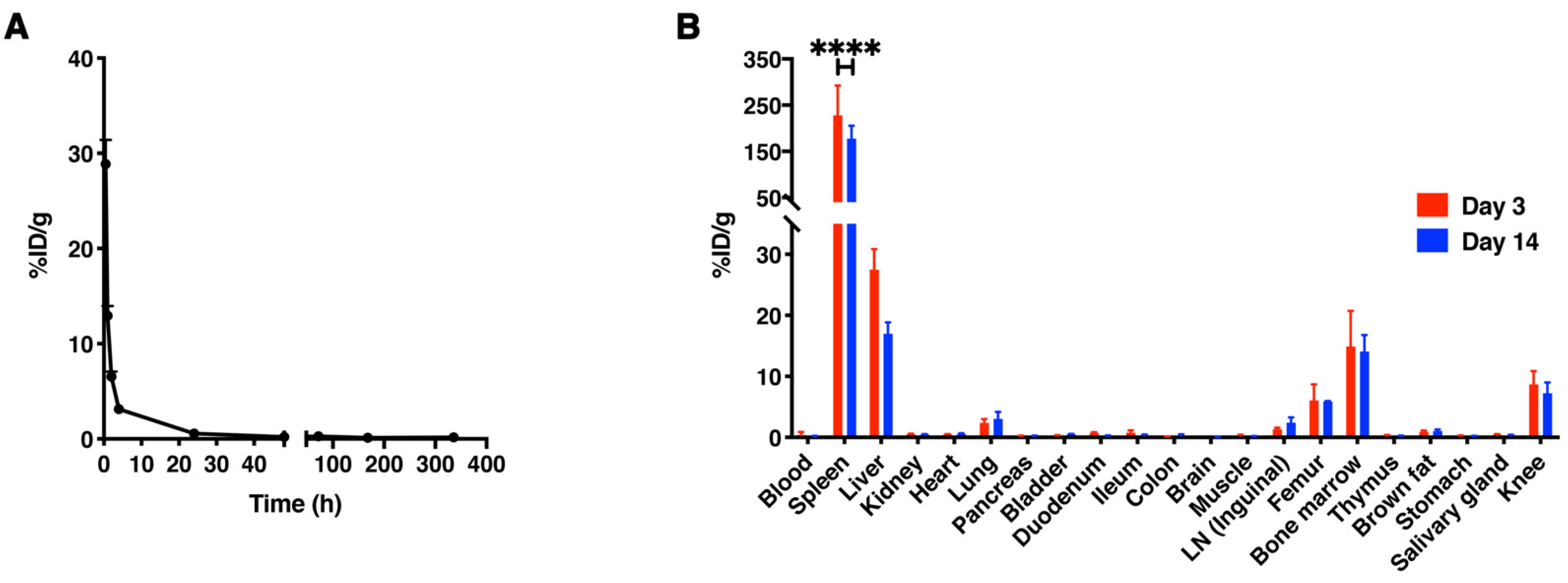
3.4. In Vivo Biodistribution of [89Zr]Zr-PLGA-NH2 NPs in C57BL/6 Mice
3.5. [89Zr]Zr-PLGA-NH2 NPs Labeling of THP-1 Cells and Retention over Time
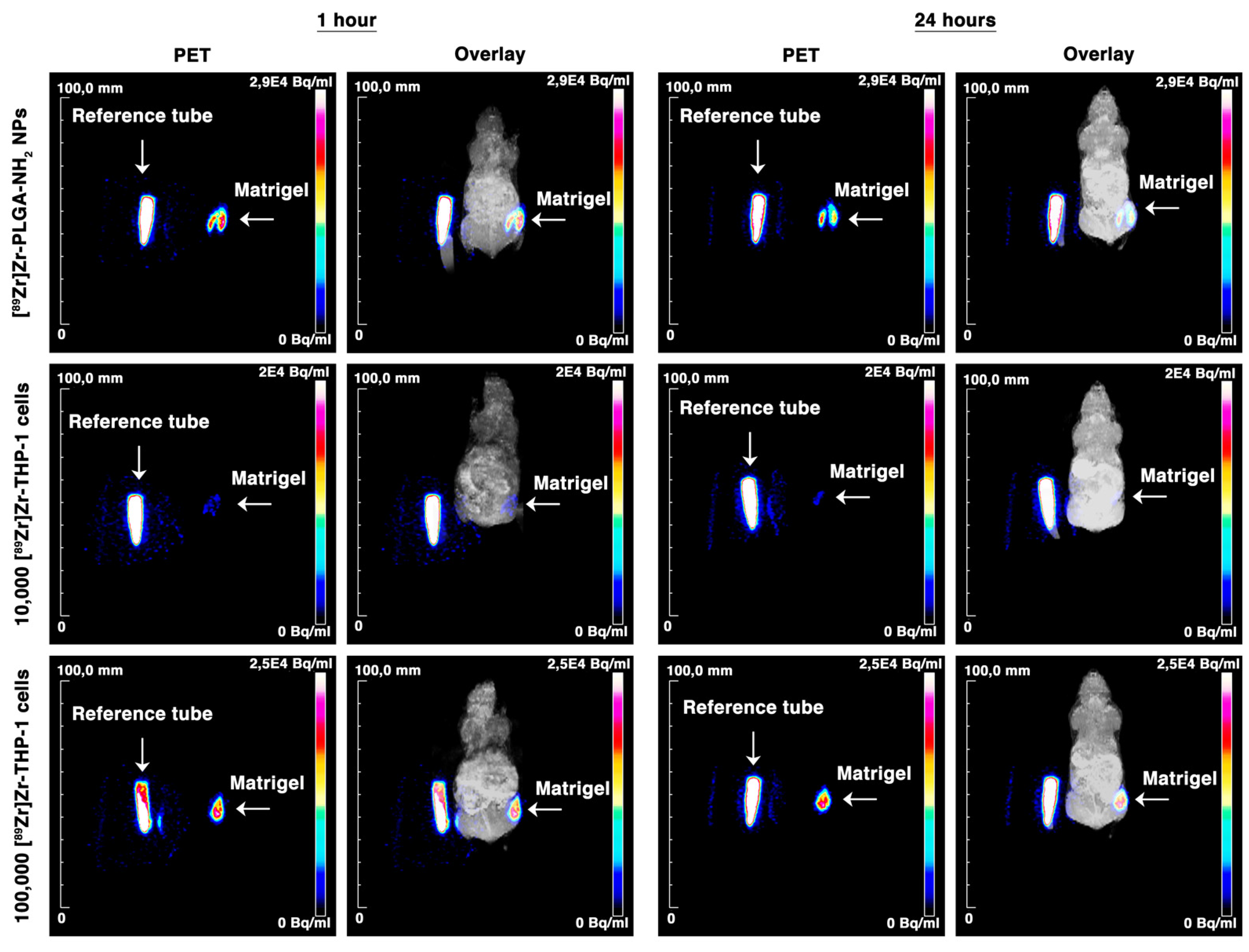
3.6. [89Zr]Zr-THP-1 Cells for In Vivo PET/MRI Imaging
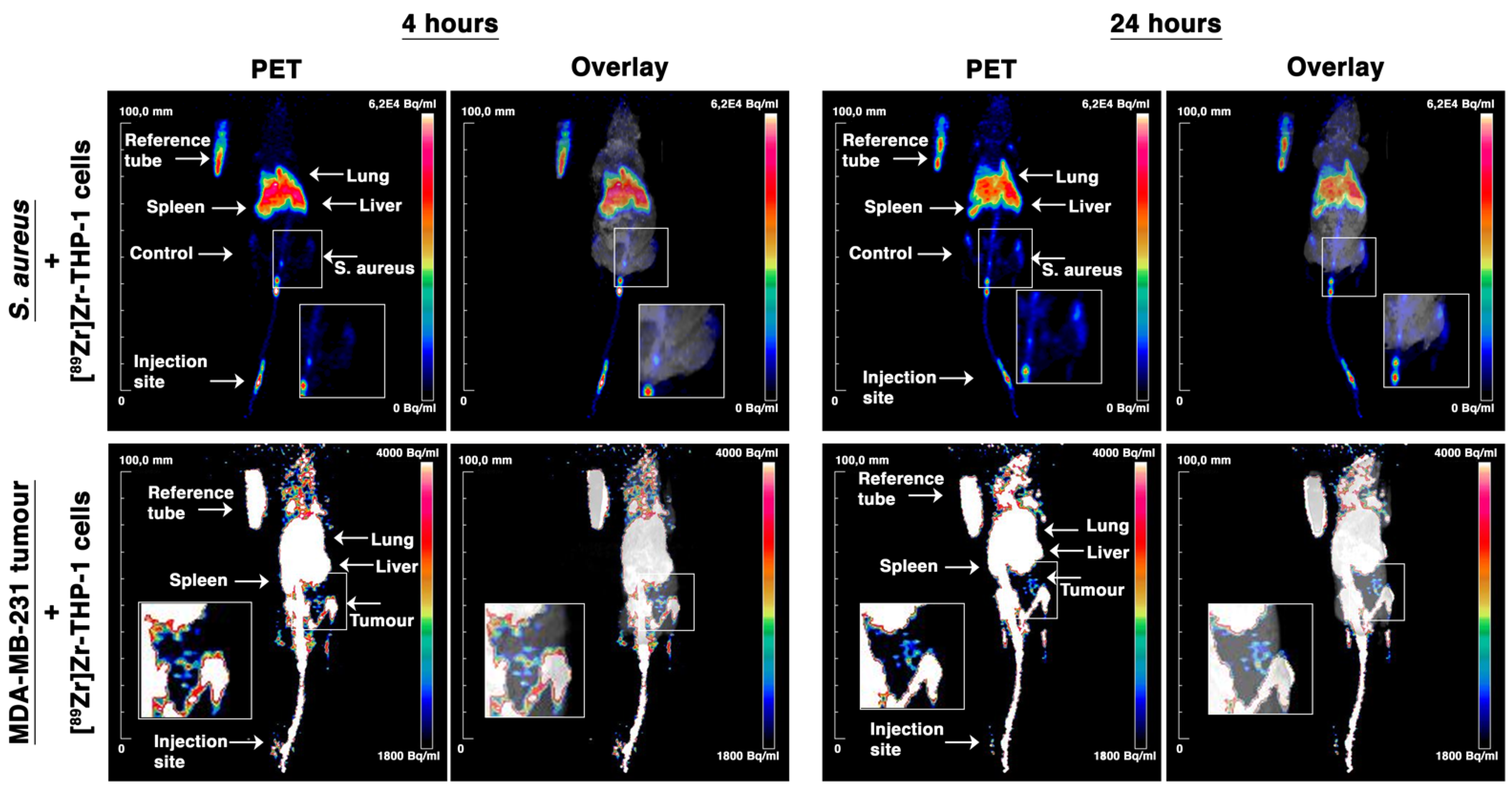
3.7. In Vivo PET/MRI Tracking of [89Zr]Zr-THP-1 Cells in Local S. aureus Infection and MDA-MB-231 Tumor Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harari, A.; Graciotti, M.; Bassani-Sternberg, M.; Kandalaft, L.E. Antitumour Dendritic Cell Vaccination in a Priming and Boosting Approach. Nat. Rev. Drug Discov. 2020, 19, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Boudewijns, S.; Bloemendal, M.; de Haas, N.; Westdorp, H.; Bol, K.F.; Schreibelt, G.; Aarntzen, E.H.J.G.; Lesterhuis, W.J.; Gorris, M.A.J.; Croockewit, A.; et al. Autologous Monocyte-Derived DC Vaccination Combined with Cisplatin in Stage III and IV Melanoma Patients: A Prospective, Randomized Phase 2 Trial. Cancer Immunol. Immunother. 2020, 69, 477–488. [Google Scholar] [CrossRef] [Green Version]
- D’Agostino, M.; Raje, N. Anti-BCMA CAR T-Cell Therapy in Multiple Myeloma: Can We Do Better? Leukemia 2020, 34, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; McGuirk, J.P. CAR T Cells: Continuation in a Revolution of Immunotherapy. Lancet Oncol. 2020, 21, e168–e178. [Google Scholar] [CrossRef]
- Lee, S.; Ko, Y.; Kim, T.J. Homeostasis and Regulation of Autoreactive B Cells. Cell. Mol. Immunol. 2020, 17, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Volkov, M.; Schie, K.A.; Woude, D. Autoantibodies and B Cells: The ABC of Rheumatoid Arthritis Pathophysiology. Immunol. Rev. 2020, 294, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, D.M.; Burma, A.M.; Kitakule, M.M.; Luo, Y.; Mehta, N.N. T Cells in Autoimmunity-Associated Cardiovascular Diseases. Front. Immunol. 2020, 11, 588776. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-Infiltrating Lymphocytes in the Immunotherapy Era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Du, L.; Li, H.; Zhu, X.; Cui, L.; Li, X. Tumor-Infiltrating Lymphocytes: Warriors Fight against Tumors Powerfully. Biomed. Pharmacother. 2020, 132, 110873. [Google Scholar] [CrossRef]
- Kircher, M.F.; Allport, J.R.; Graves, E.E.; Love, V.; Josephson, L.; Lichtman, A.H.; Weissleder, R. In Vivo High Resolution Three-Dimensional Imaging of Antigen-Specific Cytotoxic T-Lymphocyte Trafficking to Tumors. Cancer Res. 2003, 63, 6838–6846. [Google Scholar]
- Zhou, X.Y.; Tay, Z.W.; Chandrasekharan, P.; Yu, E.Y.; Hensley, D.W.; Orendorff, R.; Jeffris, K.E.; Mai, D.; Zheng, B.; Goodwill, P.W.; et al. Magnetic Particle Imaging for Radiation-Free, Sensitive and High-Contrast Vascular Imaging and Cell Tracking. Curr. Opin. Chem. Biol. 2018, 45, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Panagiotopoulos, N.; Vogt, F.; Barkhausen, J.; Buzug, T.M.; Duschka, R.L.; Lüdtke-Buzug, K.; Ahlborg, M.; Bringout, G.; Debbeler, C.; Gräser, M.; et al. Magnetic Particle Imaging: Current Developments and Future Directions. Int. J. Nanomed. 2015, 10, 3097. [Google Scholar] [CrossRef] [Green Version]
- Graeser, M.; Thieben, F.; Szwargulski, P.; Werner, F.; Gdaniec, N.; Boberg, M.; Griese, F.; Möddel, M.; Ludewig, P.; van de Ven, D.; et al. Human-Sized Magnetic Particle Imaging for Brain Applications. Nat. Commun. 2019, 10, 1936. [Google Scholar] [CrossRef] [PubMed]
- Mason, E.E.; Cooley, C.Z.; Cauley, S.F.; Griswold, M.A.; Conolly, S.M.; Wald, L.L. Design Analysis of an MPI Human Functional Brain Scanner. Int. J. Magn. Part. Imaging 2017, 3, 12. [Google Scholar] [CrossRef]
- Thakur, M.L.; Segal, A.W.; Louis, L.; Welch, M.J.; Hopkins, J.; Peters, T.J. Indium-111-Labeled Cellular Blood Components: Mechanism of Labeling and Intracellular Location in Human Neutrophils. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1977, 18, 1022–1026. [Google Scholar]
- Roca, M.; de Vries, E.F.J.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the Labelling of Leucocytes with 111In-Oxine. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 835–841. [Google Scholar] [CrossRef] [Green Version]
- De Vries, E.F.J.; Roca, M.; Jamar, F.; Israel, O.; Signore, A. Guidelines for the Labelling of Leucocytes with 99mTc-HMPAO. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 842–848. [Google Scholar] [CrossRef] [Green Version]
- Bin Othman, M.F.; Mitry, N.R.; Lewington, V.J.; Blower, P.J.; Terry, S.Y.A. Re-Assessing Gallium-67 as a Therapeutic Radionuclide. Nucl. Med. Biol. 2017, 46, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Kassis, A.I.; Adelstein, S.J. Chemotoxicity of Indium-111 Oxine in Mammalian Cells. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1985, 26, 187–190. [Google Scholar]
- Cherry, S.R.; Jones, T.; Karp, J.S.; Qi, J.; Moses, W.W.; Badawi, R.D. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. J. Nucl. Med. 2018, 59, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Asiedu, K.O.; Ferdousi, M.; Ton, P.T.; Adler, S.S.; Choyke, P.L.; Sato, N. Bone Marrow Cell Homing to Sites of Acute Tibial Fracture: 89Zr-Oxine Cell Labeling with Positron Emission Tomographic Imaging in a Mouse Model. EJNMMI Res. 2018, 8, 109. [Google Scholar] [CrossRef]
- Patrick, P.S.; Kolluri, K.K.; Zaw Thin, M.; Edwards, A.; Sage, E.K.; Sanderson, T.; Weil, B.D.; Dickson, J.C.; Lythgoe, M.F.; Lowdell, M.; et al. Lung Delivery of MSCs Expressing Anti-Cancer Protein TRAIL Visualised with 89Zr-Oxine PET-CT. Stem Cell Res. Ther. 2020, 11, 256. [Google Scholar] [CrossRef]
- Charoenphun, P.; Meszaros, L.K.; Chuamsaamarkkee, K.; Sharif-Paghaleh, E.; Ballinger, J.R.; Ferris, T.J.; Went, M.J.; Mullen, G.E.D.; Blower, P.J. [89Zr]Oxinate4 for Long-Term in Vivo Cell Tracking by Positron Emission Tomography. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Davidson-Moncada, J.; Sato, N.; Hoyt, R.F.; Reger, R.N.; Thomas, M.; Clevenger, R.; Metzger, M.E.; Donahue, R.E.; Eclarinal, P.C.; Szajek, L.; et al. A Novel Method to Study the in Vivo Trafficking and Homing of Adoptively Transferred NK Cells in Rhesus Macaques and Humans. Blood 2014, 124, 659. [Google Scholar] [CrossRef]
- Keliher, E.J.; Yoo, J.; Nahrendorf, M.; Lewis, J.S.; Marinelli, B.; Newton, A.; Pittet, M.J.; Weissleder, R. 89Zr-Labeled Dextran Nanoparticles Allow in Vivo Macrophage Imaging. Bioconjug. Chem. 2011, 22, 2383–2389. [Google Scholar] [CrossRef] [Green Version]
- Patrick, P.S.; Bogart, L.K.; Macdonald, T.J.; Southern, P.; Powell, M.J.; Zaw-Thin, M.; Voelcker, N.H.; Parkin, I.P.; Pankhurst, Q.A.; Lythgoe, M.F.; et al. Surface Radio-Mineralisation Mediates Chelate-Free Radiolabelling of Iron Oxide Nanoparticles. Chem. Sci. 2019, 10, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Medina, C.; Tang, J.; Abdel-Atti, D.; Hogstad, B.; Merad, M.; Fisher, E.A.; Fayad, Z.A.; Lewis, J.S.; Mulder, W.J.M.; Reiner, T. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J. Nucl. Med. 2015, 56, 1272–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairclough, M.; Ellis, B.; Boutin, H.; Jones, A.K.P.; McMahon, A.; Alzabin, S.; Gennari, A.; Prenant, C. Development of a Method for the Preparation of Zirconium-89 Radiolabelled Chitosan Nanoparticles as an Application for Leukocyte Trafficking with Positron Emission Tomography. Appl. Radiat. Isot. 2017, 130, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In Vivo Biodistribution and Accumulation of 89Zr in Mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perrin, J.; Capitao, M.; Mougin-Degraef, M.; Guérard, F.; Faivre-Chauvet, A.; Rbah-Vidal, L.; Gaschet, J.; Guilloux, Y.; Kraeber-Bodéré, F.; Chérel, M.; et al. Cell Tracking in Cancer Immunotherapy. Front. Med. 2020, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krekorian, M.; Sandker, G.G.W.; Cortenbach, K.R.G.; Tagit, O.; van Riessen, N.K.; Raavé, R.; Srinivas, M.; Figdor, C.G.; Heskamp, S.; Aarntzen, E.H.J.G. Characterization of Intrinsically Radiolabeled Poly(Lactic- Co -Glycolic Acid) Nanoparticles for Ex Vivo Autologous Cell Labeling and in Vivo Tracking. Bioconjug. Chem. 2021, 32, 1802–1811. [Google Scholar] [CrossRef]
- Raavé, R.; Sandker, G.; Adumeau, P.; Jacobsen, C.B.; Mangin, F.; Meyer, M.; Moreau, M.; Bernhard, C.; Da Costa, L.; Dubois, A.; et al. Direct Comparison of the in Vitro and in Vivo Stability of DFO, DFO* and DFOcyclo* for 89Zr-ImmunoPET. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1966–1977. [Google Scholar] [CrossRef] [Green Version]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. 89Zr-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression In Vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Staal, A.H.J.; Becker, K.; Tagit, O.; Koen van Riessen, N.; Koshkina, O.; Veltien, A.; Bouvain, P.; Cortenbach, K.R.G.; Scheenen, T.; Flögel, U.; et al. In Vivo Clearance of 19F MRI Imaging Nanocarriers Is Strongly Influenced by Nanoparticle Ultrastructure. Biomaterials 2020, 261, 120307. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Wu, H.; Asiedu, K.O.; Szajek, L.P.; Griffiths, G.L.; Choyke, P.L. 89Zr-Oxine Complex PET Cell Imaging in Monitoring Cell-Based Therapies. Radiology 2015, 275, 490–500. [Google Scholar] [CrossRef] [Green Version]
- Man, F.; Khan, A.A.; Carrascal-Miniño, A.; Blower, P.J.; de Rosales, R.T. A Kit Formulation for the Preparation of [89Zr]Zr(Oxinate)4 for PET Cell Tracking: White Blood Cell Labelling and Comparison with [111In]In(Oxinate). Nucl. Med. Biol. 2020, 90, 31–40. [Google Scholar] [CrossRef]
- Weist, M.R.; Starr, R.; Aguilar, B.; Chea, J.; Miles, J.K.; Poku, E.; Gerdts, E.; Yang, X.; Priceman, S.J.; Forman, S.J.; et al. PET of Adoptively Transferred Chimeric Antigen Receptor T Cells with 89Zr-Oxine. J. Nucl. Med. 2018, 59, 1531–1537. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhao, X.; Ding, J.; Xing, Y.; Zhou, M.; Wang, X.; Zhu, W.; Huo, L.; Yang, J. Evidence of Accumulated Endothelial Progenitor Cells in the Lungs of Rats with Pulmonary Arterial Hypertension by 89Zr-Oxine PET Imaging. Mol. Ther. Methods Clin. Dev. 2020, 17, 1108–1117. [Google Scholar] [CrossRef]
- Bansal, A.; Pandey, M.K.; Demirhan, Y.E.; Nesbitt, J.J.; Crespo-Diaz, R.J.; Terzic, A.; Behfar, A.; DeGrado, T.R. Novel 89Zr Cell Labeling Approach for PET-Based Cell Trafficking Studies. EJNMMI Res. 2015, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Bansal, A.; Pandey, M.K.; Yamada, S.; Goyal, R.; Schmit, N.R.; Jeon, R.; Nesbitt, J.J.; Witt, T.A.; Singh, R.D.; Gunderson, T.M.; et al. [89Zr]Zr-DBN Labeled Cardiopoietic Stem Cells Proficient for Heart Failure. Nucl. Med. Biol. 2020, 90, 23–30. [Google Scholar] [CrossRef]
- Sato, N.; Stringaris, K.; Davidson-Moncada, J.K.; Reger, R.; Adler, S.S.; Dunbar, C.; Choyke, P.L.; Childs, R.W. In Vivo Tracking of Adoptively Transferred Natural Killer Cells in Rhesus Macaques Using 89Zirconium-Oxine Cell Labeling and PET Imaging. Clin. Cancer Res. 2020, 26, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Van Bracht, E.; Versteegden, L.R.M.; Stolle, S.; Verdurmen, W.P.R.; Woestenenk, R.; Raavé, R.; Hafmans, T.; Oosterwijk, E.; Brock, R.; van Kuppevelt, T.H.; et al. Enhanced Cellular Uptake of Albumin-Based Lyophilisomes When Functionalized with Cell-Penetrating Peptide TAT in HeLa Cells. PLoS ONE 2014, 9, e110813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardarelli, F.; Digiacomo, L.; Marchini, C.; Amici, A.; Salomone, F.; Fiume, G.; Rossetta, A.; Gratton, E.; Pozzi, D.; Caracciolo, G. The Intracellular Trafficking Mechanism of Lipofectamine-Based Transfection Reagents and Its Implication for Gene Delivery. Sci. Rep. 2016, 6, 25879. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Almeida, A.; Vale, N. Combination of Cell-Penetrating Peptides with Nanoparticles for Therapeutic Application: A Review. Biomolecules 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.-T.; Lee, J.-Y.; Kim, D.-D.; Yoon, I.-S.; Cho, H.-J. Recent Progress in the Development of Poly(Lactic-Co-Glycolic Acid)-Based Nanostructures for Cancer Imaging and Therapy. Pharmaceutics 2019, 11, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, A.; Cimas, F.J.; Bravo, I.; Pandiella, A.; Ocaña, A.; Alonso-Moreno, C. An Overview of Antibody Conjugated Polymeric Nanoparticles for Breast Cancer Therapy. Pharmaceutics 2020, 12, 802. [Google Scholar] [CrossRef]
- Jung, K.O.; Kim, T.J.; Yu, J.H.; Rhee, S.; Zhao, W.; Ha, B.; Red-Horse, K.; Gambhir, S.S.; Pratx, G. Whole-Body Tracking of Single Cells via Positron Emission Tomography. Nat. Biomed. Eng. 2020, 4, 835–844. [Google Scholar] [CrossRef]
- Weigelin, B.; den Boer, A.T.; Wagena, E.; Broen, K.; Dolstra, H.; de Boer, R.J.; Figdor, C.G.; Textor, J.; Friedl, P. Cancer Cell Elimination by Cytotoxic T Cell Cooperation and Additive Damage. Immunology 2020. [Google Scholar] [CrossRef] [Green Version]




| Sample | Diameter (nm) | Polydispersity (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| PLGA-NH2 | 189 ± 1.9 | 0.07 | −2.3 ± 0.9 |
| Zr-PLGA-NH2 | 196 ± 4.1 | 0.07 | −0.3 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krekorian, M.; Cortenbach, K.R.G.; Boswinkel, M.; Kip, A.; Franssen, G.M.; Veltien, A.; Scheenen, T.W.J.; Raavé, R.; van Riessen, N.K.; Srinivas, M.; et al. In Vivo PET Imaging of Monocytes Labeled with [89Zr]Zr-PLGA-NH2 Nanoparticles in Tumor and Staphylococcus aureus Infection Models. Cancers 2021, 13, 5069. https://doi.org/10.3390/cancers13205069
Krekorian M, Cortenbach KRG, Boswinkel M, Kip A, Franssen GM, Veltien A, Scheenen TWJ, Raavé R, van Riessen NK, Srinivas M, et al. In Vivo PET Imaging of Monocytes Labeled with [89Zr]Zr-PLGA-NH2 Nanoparticles in Tumor and Staphylococcus aureus Infection Models. Cancers. 2021; 13(20):5069. https://doi.org/10.3390/cancers13205069
Chicago/Turabian StyleKrekorian, Massis, Kimberley R. G. Cortenbach, Milou Boswinkel, Annemarie Kip, Gerben M. Franssen, Andor Veltien, Tom W. J. Scheenen, René Raavé, Nicolaas Koen van Riessen, Mangala Srinivas, and et al. 2021. "In Vivo PET Imaging of Monocytes Labeled with [89Zr]Zr-PLGA-NH2 Nanoparticles in Tumor and Staphylococcus aureus Infection Models" Cancers 13, no. 20: 5069. https://doi.org/10.3390/cancers13205069







