Value of Assessing Peripheral Vascularization with Micro-Flow Imaging, Resistive Index and Absent Hilum Sign as Predictor for Malignancy in Lymph Nodes in Head and Neck Squamous Cell Carcinoma
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ultrasound and USgFNAC
2.3. Cytology
2.4. Statistical Analysis
3. Results
3.1. Analysis in Entire Set of Nodes
3.1.1. Short Axis Diameter
3.1.2. S/L Ratio
3.1.3. Resistive Index
3.1.4. Peripheral Vascularization
3.1.5. Absent Hilum Sign
3.2. Subgroup Analysis of Clinically N0-Stage
3.2.1. Short Axis Diameter
3.2.2. Resistive Index
3.2.3. S/L Ratio
3.2.4. Peripheral Vascularization by MFI
3.2.5. Absent Hilum Sign
3.3. Subgroup Nodes with Short Axis Diameter ≤ 6 mm
3.3.1. Resistive Index
3.3.2. S/L Ratio
3.3.3. Peripheral Vascularization by MFI
3.3.4. Absent Hilum Sign
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. Available online: https://pubmed.ncbi.nlm.nih.gov/10647931/ (accessed on 19 August 2021). [CrossRef]
- Lodder, W.; Pameijer, F.A.; Coen, R.N.; Rasch, C.R.N.; van den Brekel, M.W.M.; Balm, A.J.M. Prognostic significance of radiologically determined neck node volume: A systematic review. Oral Oncol. 2012, 48, 298–302. Available online: https://pure.uva.nl/ws/files/2311657/127130_09.pdf (accessed on 11 October 2018). [CrossRef]
- Godény, M. Prognostic factors in advanced pharyngeal and oral cavity cancer; Significance of multimodality imaging in terms of 7th edition of TNM. Cancer Imaging 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Van den Brekel, M.W.M.; Castelijns, J.A. What the clinician wants to know: Surgical perspective and ultrasound for lymph node imaging of the neck. Cancer Imaging 2005, 5, S41–S49. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16361135 (accessed on 11 October 2018). [CrossRef]
- Kyzas, P.A.; Evangelou, E.; Denaxa-Kyza, D.; Ioannidis, J.P.A. 18F-Fluorodeoxyglucose Positron Emission Tomography to Evaluate Cervical Node Metastases in Patients with Head and Neck Squamous Cell Carcinoma: A Meta-analysis. JNCI J. Natl. Cancer Inst. 2008, 100, 712–720. Available online: https://academic.oup.com/jnci/article-lookup/doi/10.1093/jnci/djn125 (accessed on 11 October 2018). [CrossRef] [PubMed]
- Sun, R.; Tang, X.; Yang, Y.; Zhang, C. (18)FDG-PET/CT for the detection of regional nodal metastasis in patients with head and neck cancer: A meta-analysis. Oral Oncol. 2015, 51, 314–320. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25619735 (accessed on 11 October 2018). [CrossRef]
- Castelijns, J.A.; van den Breckel, M.W.M. Imaging of lymphadenopathy in the neck. Eur. Radiol. 2002, 12, 727–738. [Google Scholar] [CrossRef]
- Van den Brekel, M.W.; Leemans, C.R.; Snow, G.B. Assessment and management of lymph node metastases in the neck in head and neck cancer patients. Crit. Rev. Oncol. Hematol. 1996, 22, 175–182. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8793273 (accessed on 11 October 2018). [CrossRef]
- Ahuja, A.; Ying, M. Sonography of neck lymph nodes. Part II: Abnormal lymph nodes. Clin. Radiol. 2003, 58, 359–366. [Google Scholar] [CrossRef]
- Khanna, R.; Sharma, A.D.; Khanna, S.; Kumar, M.; Shukla, R.C. Usefulness of ultrasonography for the evaluation of cervical lymphadenopathy. World J. Surg. Oncol. 2011, 9, 29. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21356049 (accessed on 27 April 2019). [CrossRef]
- Hohlweg-Majert, B.; Metzger, M.C.; Voss, P.J.; Hölzle, F.; Wolff, K.-D.; Schulze, D. Preoperative cervical lymph node size evaluation in patients with malignant head/neck tumors: Comparison between ultrasound and computer tomography. J. Cancer Res. Clin. Oncol. 2009, 135, 753–759. Available online: http://link.springer.com/10.1007/s00432-008-0487-y (accessed on 26 April 2019). [CrossRef]
- Ying, M.; Ahuja, A.; Brook, F. Repeatability of power Doppler sonography of cervical lymph nodes. Ultrasound Med. Biol. 2002, 28, 737–744. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12113786 (accessed on 26 April 2019). [CrossRef]
- Moghaddam, M.I.; Davachi, B.; Mostaan, L.V.; Langaroodi, A.J.; Memar, B.; Azimi, S.A.; Marouzi, P. Evaluation of the Sonographic Features of Metastatic Cervical Lymph Nodes in Patients with Head and Neck Malignancy. J. Craniofac. Surg. 2011, 22, 2179–2184. Available online: http://www.ncbi.nlm.nih.gov/pubmed/22075819 (accessed on 26 April 2019). [CrossRef]
- Gupta, A.; Rahman, K.; Shahid, M.; Kumar, A.; Qaseem, S.M.D.; Hassan, S.A.; Siddiqui, F.A. Sonographic assessment of cervical lymphadenopathy: Role of high-resolution and color Doppler imaging. Head Neck 2010, 33, 297–302. Available online: http://doi.wiley.com/10.1002/hed.21448 (accessed on 26 April 2019). [CrossRef] [PubMed]
- Ying, M.; Bhatia, K.S.S.; Lee, Y.P.; Yuen, H.Y.; Ahuja, A.T. Review of ultrasonography of malignant neck nodes: Greyscale, Doppler, contrast enhancement and elastography. Cancer Imaging 2013, 13, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Moritz, J.D.; Ludwig, A.; Oestmann, J.W. Contrast-enhanced color Doppler sonography for evaluation of enlarged cervical lymph nodes in head and neck tumors. AJR Am. J. Roentgenol. 2000, 174, 1279–1284. Available online: https://pubmed.ncbi.nlm.nih.gov/10789776/ (accessed on 30 September 2021). [CrossRef] [PubMed]
- Wang, S.; Yang, W.; Fu, J.-J.; Sun, Y.; Zhang, H.; Bai, J.; Chen, M.-H.; Yan, K. Microflow imaging of contrast-enhanced ultrasound for evaluation of neovascularization in peripheral lung cancer. Medicine 2016, 95, e4361. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27512847 (accessed on 16 December 2018). [CrossRef] [PubMed]
- Bae, J.S.; Lee, J.M.; Jeon, S.K.; Jang, S. Comparison of MicroFlow Imaging with color and power Doppler imaging for detecting and characterizing blood flow signals in hepatocellular carcinoma. Ultrasonography 2020, 39, 85–93. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, H.J.; Ahn, H.W.; Kim, Y.G. Comparison of ultrasonographic MicroFlow Imaging with contrast enhanced CT in the evaluation of renal mass. Ultrasound Med. Biol. 2019, 45, S41. Available online: http://www.umbjournal.org/article/S0301562919312542/fulltext (accessed on 28 October 2020). [CrossRef]
- Van Den Brekel, M.W.M.; Castelijns, J.A.; Snow, G.B. The size of lymph nodes in the neck on sonograms as a radiologic criterion for metastasis: How reliable is it? Am. J. Neuroradiol. 1998, 19, 695–700. [Google Scholar]
- Efron, B. Better bootstrap confidence intervals. J. Am. Stat. Assoc. 1987, 82, 171–185. [Google Scholar] [CrossRef]
- Ahuja, A.T.; Ying, M.; Ho, S.Y.; Antonio, G.; Lee, Y.P.; King, A.D.; Wong, K.T. Ultrasound of malignant cervical lymph nodes. Cancer Imaging 2008, 8, 48–56. [Google Scholar] [CrossRef]
- Souren, C.; Kloss-Brandstätter, A.; Stadler, A.; Kross, K.; Yamauchi, K.; Ketelsen, D.; Kessler, P.; Lethaus, B. Ultrasound-guided fine-needle aspiration cytology as a diagnostic tool in comparison to ultrasound and MRI for staging in oral- and oropharyngeal squamous cell tumors. J. Cranio-Maxillofac. Surg. 2016, 44, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.-J.; Lo, W.-C.; Hsu, W.-L.; Wang, C.-T.; Lai, M.-S. Detection of cervical lymph node metastasis in head and neck cancer patients with clinically N0 neck-a meta-analysis comparing different imaging modalities. 2012. Available online: http://www.biomedcentral.com/1471-2407/12/236 (accessed on 26 April 2019).
- Borgemeester, M.C.; Brekel, M.W.M.V.D.; Van Tinteren, H.; Smeele, L.E.; Pameijer, F.A.; van Velthuysen, M.-L.; Balm, A.J.M. Ultrasound-guided aspiration cytology for the assessment of the clinically N0 neck: Factors influencing its accuracy. Head Neck 2008, 30, 1505–1513. [Google Scholar] [CrossRef]
- Van den Brekel, M.W.; van der Waal, I.; Meijer, C.J.; Freeman, J.L.; Castelijns, J.A.; Snow, G.B. The incidence of micrometastases in neck dissection specimens obtained from elective neck dissections. Laryngoscope 1996, 106, 987–991. Available online: http://www.ncbi.nlm.nih.gov/pubmed/8699914 (accessed on 11 April 2019). [CrossRef] [PubMed]
- Okumuş, Ö.; Dönmez, M.; Pekiner, F.N. Ultrasonographic Appearances of Cervical Lymph Nodes in Healthy Turkish Adults Subpopulation: Preliminary Study. Open Dent. J. 2017, 11, 404–412. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28839488 (accessed on 27 April 2019). [CrossRef] [PubMed][Green Version]
- Ghafoori, M.; Azizian, A.; Pourrajabi, Z.; Vaseghi, H. Sonographic Evaluation of Cervical Lymphadenopathy; Comparison of Metastatic and Reactive Lymph Nodes in Patients with Head and Neck Squamous Cell Carcinoma Using Gray Scale and Doppler Techniques. Iran J. Radiol. 2015, 12, e11044. Available online: http://www.ncbi.nlm.nih.gov/pubmed/26528381 (accessed on 26 April 2019). [CrossRef] [PubMed]
- Revzin, M.V.; Imanzadeh, A.; Menias, C.; Pourjabbar, S.; Mustafa, A.; Nezami, N.; Spektor, M.; Pellerito, J.S. Optimizing image quality when evaluating blood flow at doppler US: A Tutorial. Radiographics 2019, 39, 1501–1523. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.S.; Peacock, T.E. The role of ultrasound in the detection of cervical lymph node metastases in clinically NO squamous cell carcinoma of the head and neck. Cancer Imaging 2007, 7, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, A.T.; Ying, M.; Ho, S.S.Y.; Metreweli, C. Distribution of Intranodal Vessels in Differentiating Benign from Metastatic Neck Nodes. Clin. Radiol. 2001, 56, 197–201. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0009926000905749 (accessed on 26 April 2019). [CrossRef]
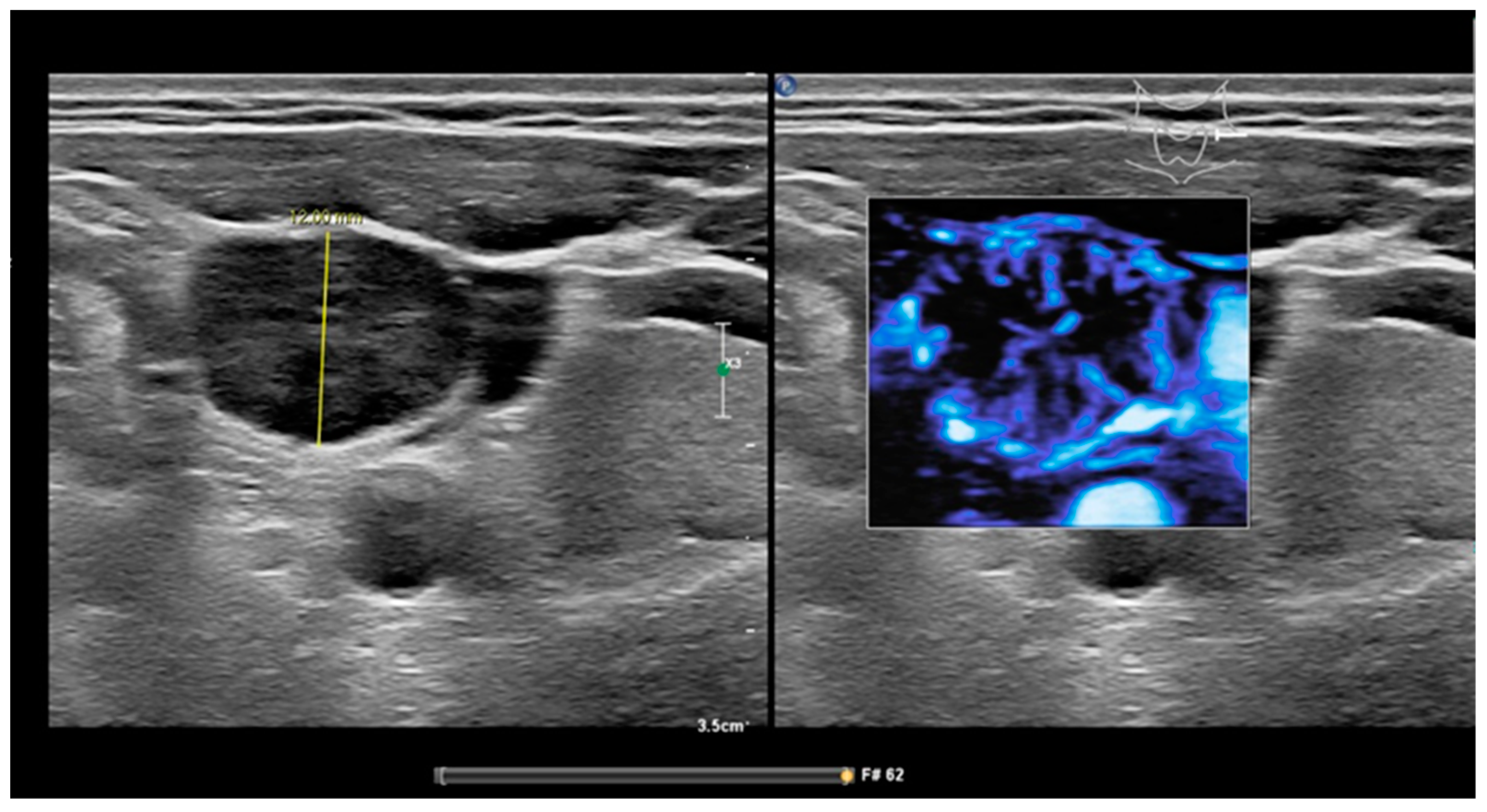

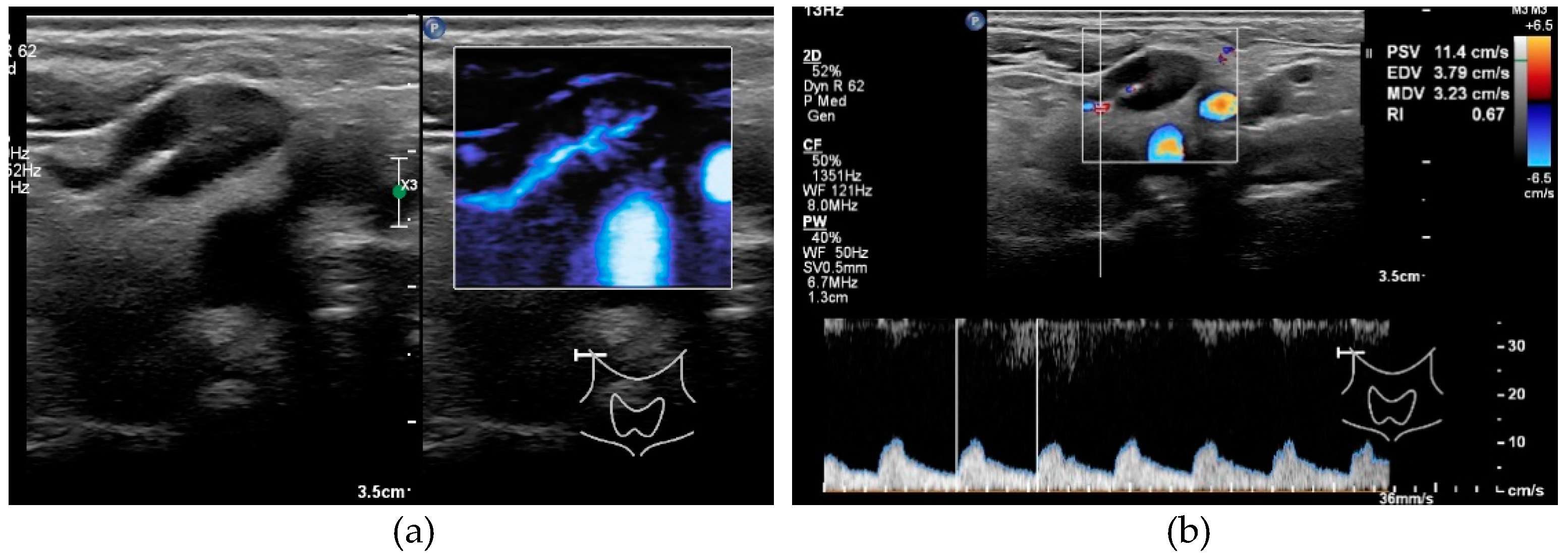
| All cN Stages | cN0-Stage | |||||
|---|---|---|---|---|---|---|
| Total | Female | Male | Total | Female | Male | |
| N patients | 102 | 27 (26%) | 73 (72%) | 56 | 16 (29%) | 38 (68%) |
| Mean age (range) | 65 (34–87) | 63 (45–87) | 65 (34–84) | 65 (34–87) | 63 (51–87) | 66 (34–84) |
| N aspirated nodes | 211 | 99 | ||||
| Mean nodes/patient (range) | 2.07 (1–5) | 1.77 (1–5) | ||||
| Data | Specificity | Sensitivity | NPV | PPV | Threshold |
|---|---|---|---|---|---|
| all nodes | |||||
| p. vascularization | 0.84 (0.77–0.90) | 0.87 (0.80–0.93) | 0.88 (0.80–0.94) | 0.83 (0.74–0.90) | |
| absent fatty hilum | 0.84 (0.77–0.90) | 0.84 (0.77–0.90) | 0.86 (0.76–0.92) | 0.82 (0.72–0.90) | |
| short axis diameter | 0.45 (0.19–0.81) | 0.88 (0.80–0.95) | 0.82 (0.59–0.89) | 0.59 (0.45–0.82) | 6.5 1 |
| short axis diameter | 0.25 (0.17–0.35) | 0.95 (0.89–0.98) | 0.84 (0.68–0.94) | 0.53 (0.43–0.62) | 6.0 2 |
| resistive index | 0.54 (0.34–0.70) | 0.88 (0.78–0.93) | 0.85 (0.72–0.92) | 0.61 (0.50–0.74) | 0.705 1 |
| S/L ratio 3 | 0.45 (0.37–0.53) | 0.88 (0.82–0.93) | 0.82 (0.69–0.90) | 0.59 (0.49–0.67) | 0.5 |
| cN0 patients | |||||
| p. vascularization | 0.79 (0.70–0.88) | 0.94 (0.56–1.00) | 0.98 (0.92–1.00) | 0.50 (0.27–0.71) | |
| asent fatty hilum | 0.82 (0.73–0.89) | 0.82 (0.60–1.00) | 0.96 (0.89–0.99) | 0.50 (0.24–0.72) | |
| short axis diameter | 0.26 (0.15–0.55) | 0.94 (0.57–1.00) | 0.95 (0.59–1.00) | 0.22 (0.11–0.38) | 5.5 1 |
| short axis diameter | 0.05 (0.01–0.11) | 1.00 (0.80–1.00) * | 1.00 (0.40–1.00) * | 0.19 (0.09–0.32) | 4.0 2 |
| restistive index | 0.25 (0.15–0.36) * | 1.00 (0.77–1.00) * | 1.00 (0.81–1.00) * | 0.20 (0.06–0.31) | 0.615 1 |
| S/L ratio 3 | 0.46 (0.36–0.56) | 0.88 (0.71–1.00) | 0.95 (0.82–1.00) | 0.26 (0.14–0.42) | 0.5 |
| absent fatty hilum | |||||
| p. vascularization | 0.71 (0.45–0.89) | 0.92 (0.85–0.97) | 0.67 (0.38–0.85) | 0.94 (0.86–0.98) | |
| cN0 and absent fatty hilum absent | |||||
| p. vascularization | 0.64 (0.36–0.88) | 0.93 (0.50–1.00) | 0.90 (0.55–1.00) * | 0.72 (0.40–0.92) | |
| short axis diameter mm ≤ 6 | |||||
| p. vascularization | 0.90 (0.79–0.96) | 0.73 (0.33–0.93) | 0.94 (0.82–0.98) | 0.62 (0.30–0.86) | |
| absent fatty hilum | 0.80 (0.67–0.89) | 0.91 (0.00–1.00) | 0.98 (0.86–1.00) | 0.50 (0.23–0.72) | |
| resistive index | 0.26 (0.00–0.58) | 0.80 (0.38–1.00) | 0.86 (0.57–0.98) * | 0.19 (0.07–0.30) | 0.615 1 |
| S/L ratio 3 | 0.61 (0.49–0.73) | 0.82 (0.40–1.00) | 0.94 (0.79–1.00) | 0.32 (0.16–0.52) | 0.5 |
| Nodes at All cN Stages | Nodes at cN0 Stages | Nodes with Short Axis mm ≤ 6 mm | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Features | N | Mal 3 | %. | Ben 4 | %. | N | Mal 3 | %. | Ben 4 | % | N | Mal 3 | % | Ben 4 | % |
| hilus + 1 | 106 | 15 | 14% | 91 | 86% | 67 | 3 | 4% | 64 | 96% | 40 | 1 | 3% | 39 | 98% |
| hilus − 1 | 97 | 80 | 82% | 17 | 18% | 28 | 14 | 50% | 14 | 50% | 20 | 10 | 50% | 10 | 50% |
| p. vasc + 2 | 100 | 83 | 83% | 17 | 17% | 32 | 16 | 50% | 16 | 50% | 13 | 8 | 62% | 5 | 38% |
| p. vasc − 2 | 103 | 12 | 12% | 91 | 88% | 63 | 1 | 2% | 62 | 98% | 47 | 3 | 6% | 44 | 94% |
| hilus−/ p. vasc + | 79 | 74 | 94% | 5 | 6% | 18 | 13 | 72% | 5 | 28% | 9 | 7 | 78% | 2 | 22% |
| hilus−/ p. vasc - | 18 | 6 | 33% | 12 | 67% | 10 | 1 | 10% | 9 | 90% | 11 | 3 | 27% | 8 | 73% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Koekkoek-Doll, P.K.; Roberti, S.; van den Brekel, M.W.; Maas, M.; Smit, L.; Beets-Tan, R.; Castelijns, J. Value of Assessing Peripheral Vascularization with Micro-Flow Imaging, Resistive Index and Absent Hilum Sign as Predictor for Malignancy in Lymph Nodes in Head and Neck Squamous Cell Carcinoma. Cancers 2021, 13, 5071. https://doi.org/10.3390/cancers13205071
de Koekkoek-Doll PK, Roberti S, van den Brekel MW, Maas M, Smit L, Beets-Tan R, Castelijns J. Value of Assessing Peripheral Vascularization with Micro-Flow Imaging, Resistive Index and Absent Hilum Sign as Predictor for Malignancy in Lymph Nodes in Head and Neck Squamous Cell Carcinoma. Cancers. 2021; 13(20):5071. https://doi.org/10.3390/cancers13205071
Chicago/Turabian Stylede Koekkoek-Doll, Petra K., Sander Roberti, Michiel W. van den Brekel, Monique Maas, Laura Smit, Regina Beets-Tan, and Jonas Castelijns. 2021. "Value of Assessing Peripheral Vascularization with Micro-Flow Imaging, Resistive Index and Absent Hilum Sign as Predictor for Malignancy in Lymph Nodes in Head and Neck Squamous Cell Carcinoma" Cancers 13, no. 20: 5071. https://doi.org/10.3390/cancers13205071
APA Stylede Koekkoek-Doll, P. K., Roberti, S., van den Brekel, M. W., Maas, M., Smit, L., Beets-Tan, R., & Castelijns, J. (2021). Value of Assessing Peripheral Vascularization with Micro-Flow Imaging, Resistive Index and Absent Hilum Sign as Predictor for Malignancy in Lymph Nodes in Head and Neck Squamous Cell Carcinoma. Cancers, 13(20), 5071. https://doi.org/10.3390/cancers13205071







