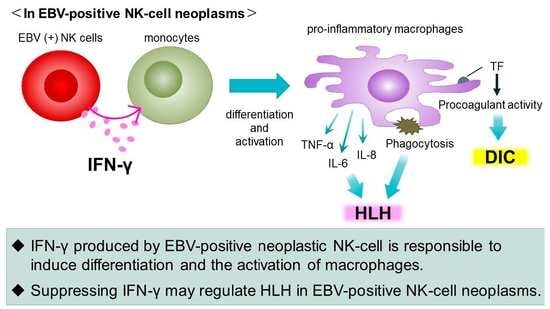
Interferon-γ Produced by EBV-Positive Neoplastic NK-Cells Induces Differentiation into Macrophages and Procoagulant Activity of Monocytes, Which Leads to HLH
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials & Methods
2.1. Cells and Reagents
2.2. Stimulation of Target Cells with the Supernatants of EBV-Positive Cell Lines
2.3. Morphological Analysis
2.4. Detection of Cell Surface Antigens by Flow Cytometry
2.5. Analysis of the Phagocytosis Ability
2.6. qRT-PCR
2.7. Procoagulant Activity (PCA) Assay
2.8. Cell Surface PCA Blocking Assay
2.9. Cytokine Multiplex Analysis
2.10. Statistical Analysis
3. Results
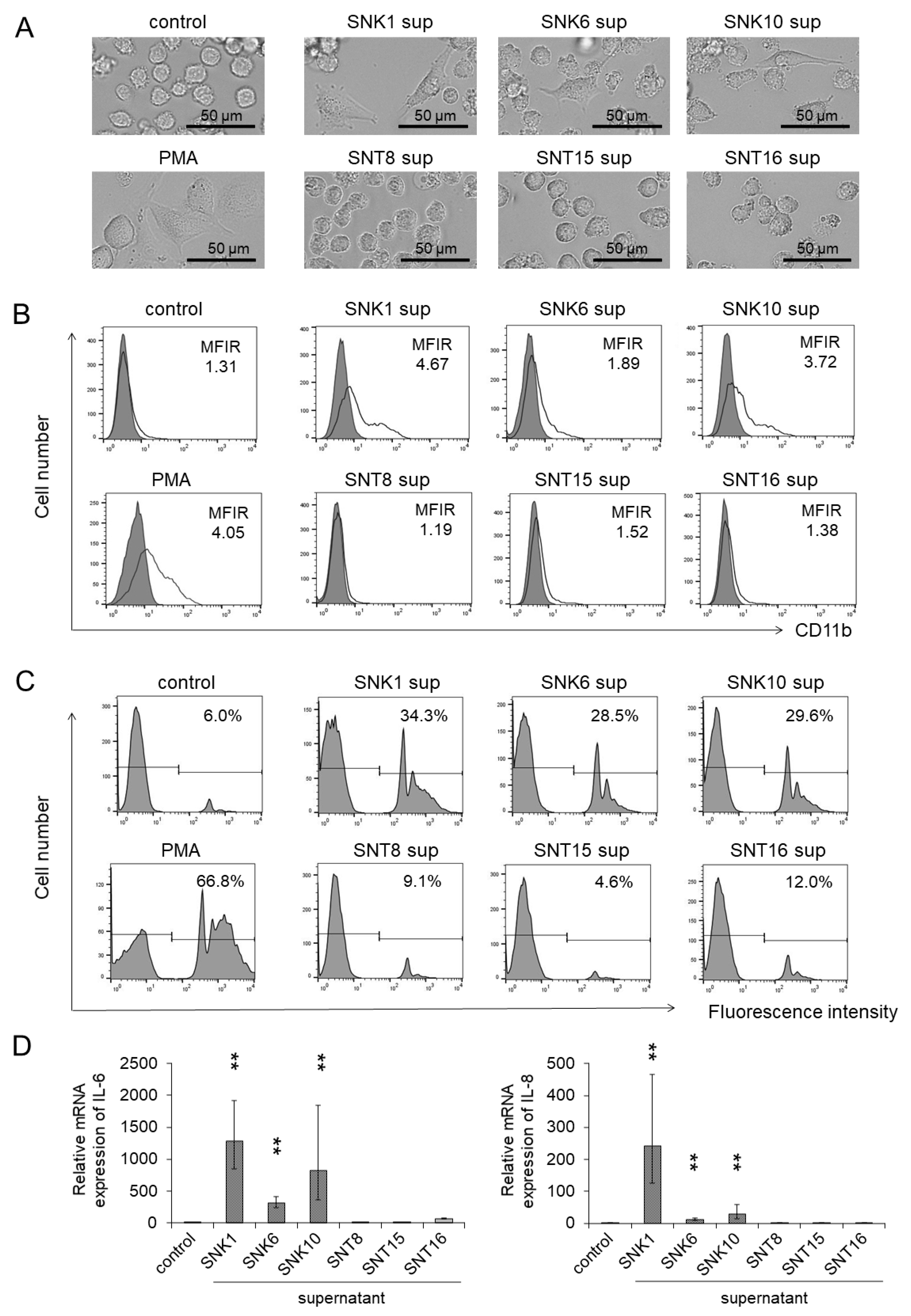
3.1. Monocytes Showed Differentiation into Macrophages by Co-Culture with the Supernatants of EBV-Positive NK-Cells
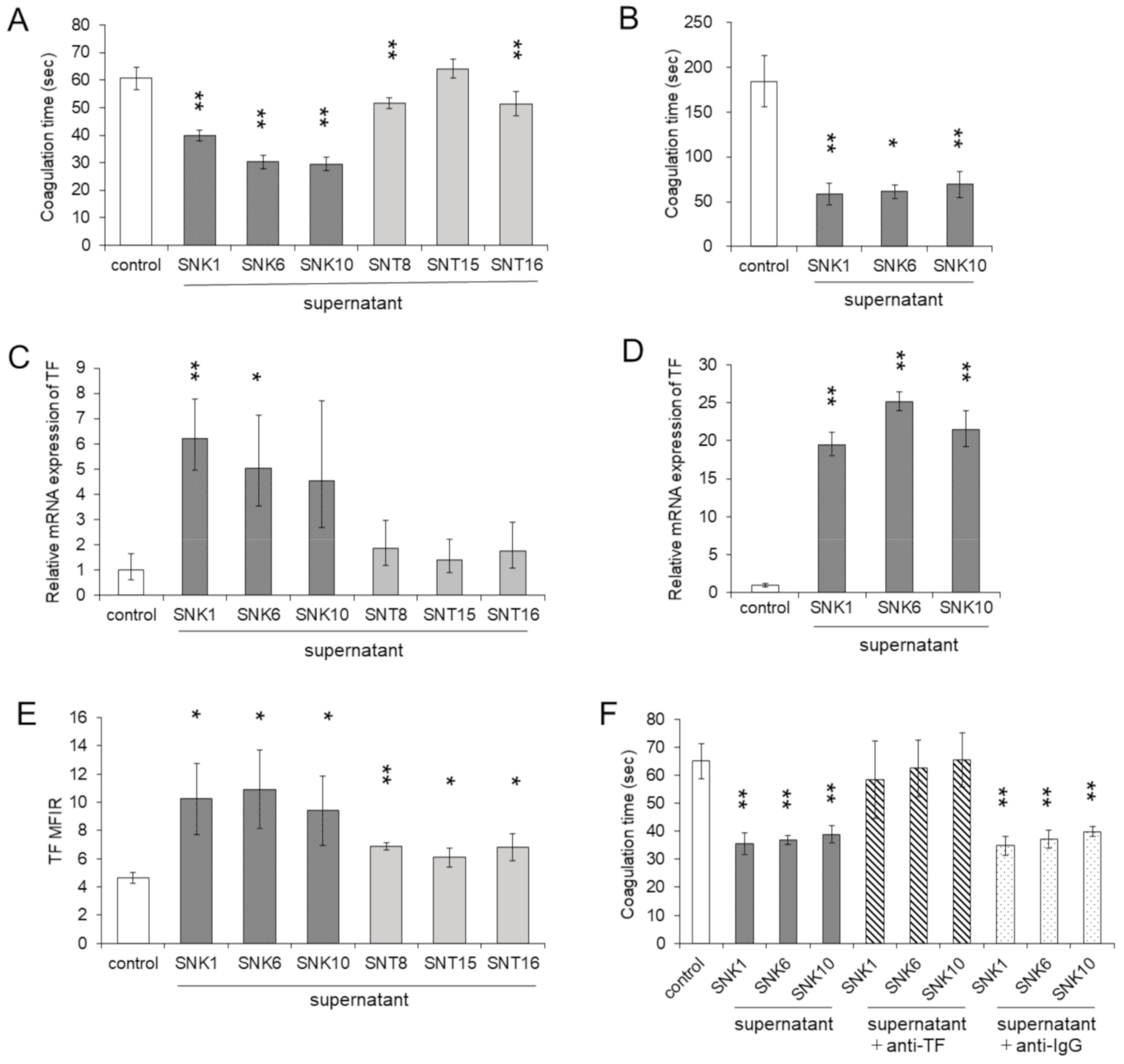
3.2. Co-Culture with the Supernatants of EBV-Positive NK-Cells Promoted Procoagulant Activity of Monocytes through the Upregulation of TF Expression
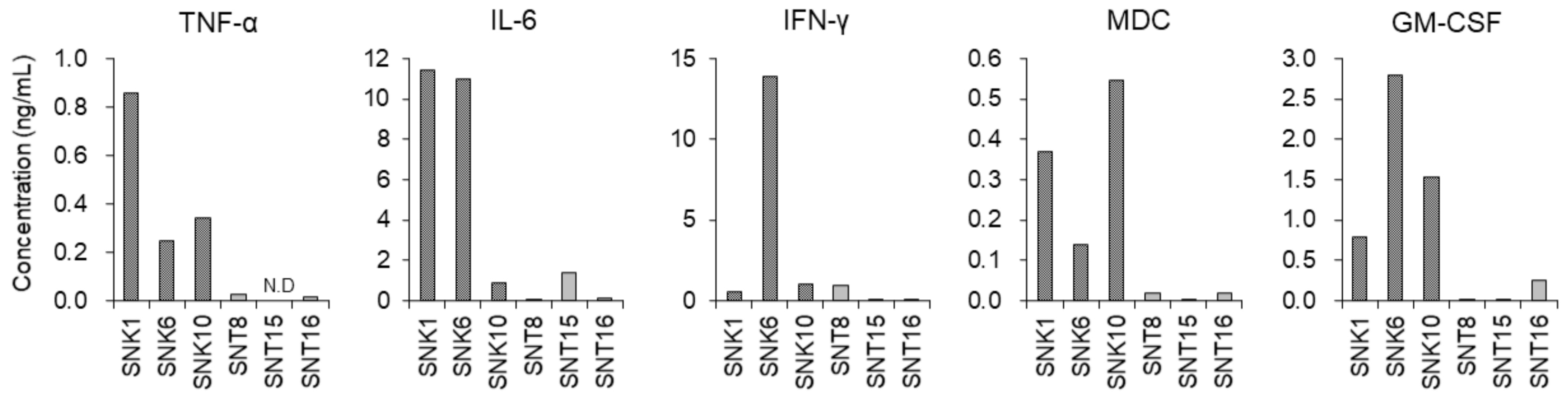
3.3. Inflammatory Cytokines in the Supernatants of Cultured EBV-Positive NK-Cell Neoplasms
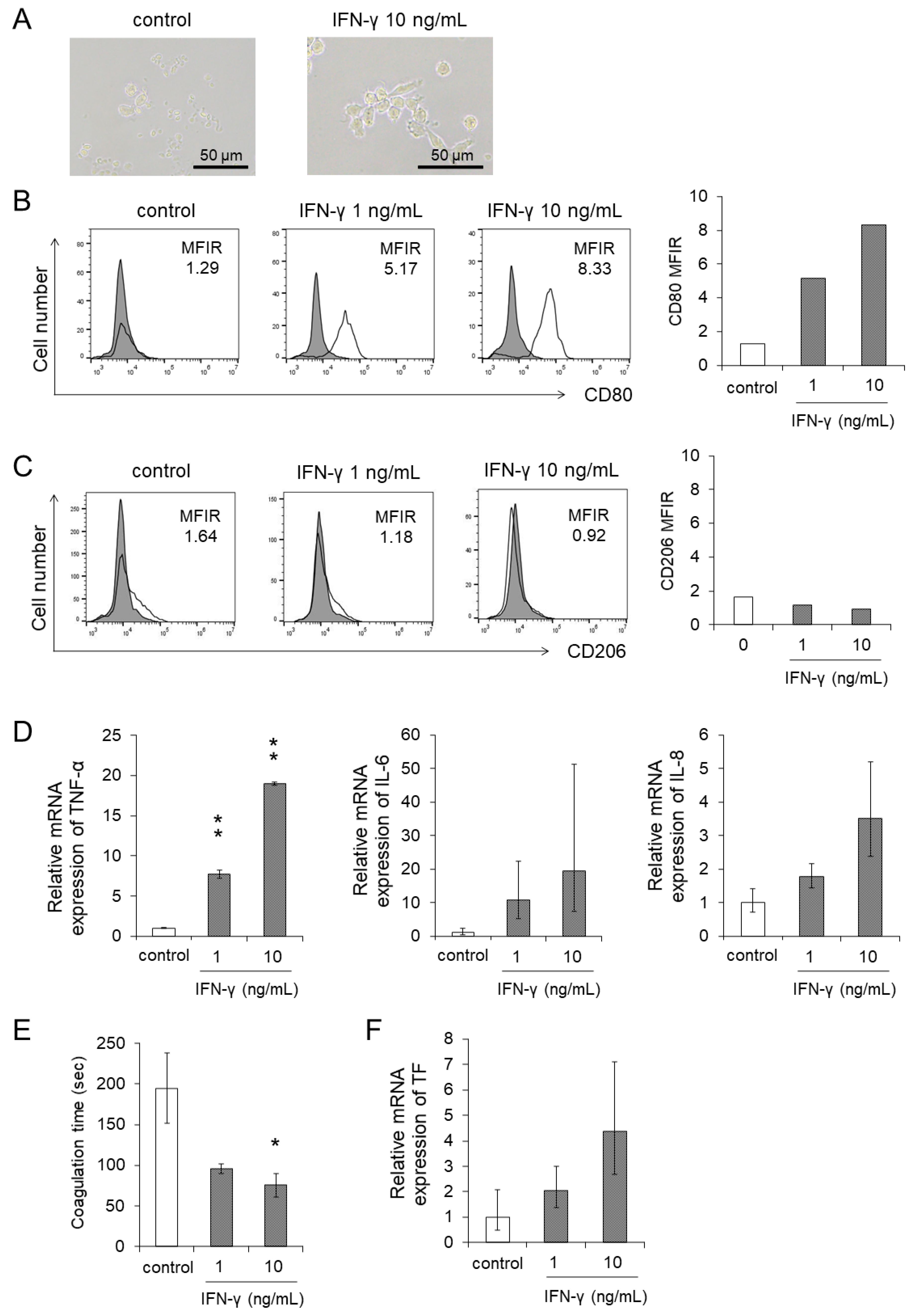
3.4. IFN-γ Directly Enhanced the Differentiation and the Procoagulant Activity of Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Esteban, Y.M.; de Jong, J.L.O.; Tesher, M.S. An Overview of Hemophagocytic Lymphohistiocytosis. Pediatr. Ann. 2017, 46, e309–e313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morimoto, A.; Nakazawa, Y.; Ishii, E. Hemophagocytic lymphohistiocytosis: Pathogenesis, diagnosis, and management. Pediatr. Int. 2016, 58, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Daver, N.; McClain, K.; Allen, C.E.; Parikh, S.A.; Otrock, Z.; Rojas-Hernandez, C.; Blechacz, B.; Wang, S.; Minkov, M.; Jordan, M.B.; et al. A consensus review on malignancy-associated hemophagocytic lymphohistiocytosis in adults. Cancer 2017, 123, 3229–3240. [Google Scholar] [CrossRef] [Green Version]
- Ishii, E.; Ohga, S.; Imashuku, S.; Yasukawa, M.; Tsuda, H.; Miura, I.; Yamamoto, K.; Horiuchi, H.; Takada, K.; Ohshima, K.; et al. Nationwide survey of hemophagocytic lymphohistiocytosis in Japan. Int. J. Hematol. 2007, 86, 58–65. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, M. The reciprocal relationship between inflammation and coagulation. Top. Companion Anim. Med. 2012, 27, 46–52. [Google Scholar] [CrossRef]
- van den Bosch, T.P.; Kannegieter, N.M.; Hesselink, D.A.; Baan, C.C.; Rowshani, A.T. Targeting the Monocyte-Macrophage Lineage in Solid Organ Transplantation. Front. Immunol. 2017, 8, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.H.; He, M.; Wang, Y.; Liao, A.H. Modulators of the Balance between M1 and M2 Macrophages during Pregnancy. Front. Immunol. 2017, 8, 120. [Google Scholar] [CrossRef] [Green Version]
- Conkling, P.R.; Greenberg, C.S.; Weinberg, J.B. Tumor necrosis factor induces tissue factor-like activity in human leukemia cell line U937 and peripheral blood monocytes. Blood 1988, 72, 128–133. [Google Scholar] [CrossRef] [Green Version]
- Neumann, F.J.; Ott, I.; Marx, N.; Luther, T.; Kenngott, S.; Gawaz, M.; Kotzsch, M.; Schomig, A. Effect of human recombinant interleukin-6 and interleukin-8 on monocyte procoagulant activity. Arter. Thromb. Vasc. Biol. 1997, 17, 3399–3405. [Google Scholar] [CrossRef]
- Bastarache, J.A.; Sebag, S.C.; Grove, B.S.; Ware, L.B. Interferon-γ and tumor necrosis factor-α act synergistically to up-regulate tissue factor in alveolar epithelial cells. Exp. Lung. Res. 2011, 37, 509–517. [Google Scholar] [CrossRef]
- Ohga, S.; Nomura, A.; Takada, H.; Ihara, K.; Kawakami, K.; Yanai, F.; Takahata, Y.; Tanaka, T.; Kasuga, N.; Hara, T. Epstein-Barr virus (EBV) load and cytokine gene expression in activated T cells of chronic active EBV infection. J. Infect. Dis. 2001, 183, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, A.; Nogami, A.; Imadome, K.I.; Kurata, M.; Murakami, N.; Fujiwara, S.; Miura, O. Sequential monitoring of serum IL-6, TNF-α, and IFN-γ levels in a CAEBV patient treated by plasma exchange and immunochemotherapy. Int. J. Hematol. 2012, 96, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, H.; Imadome, K.I.; Onozawa, E.; Tsuzura, A.; Miura, O.; Koyama, T.; Arai, A. Virus-specific cytotoxic T cells in chronic active Epstein-Barr virus infection. Rinsho. Ketsueki. 2017, 58, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nagata, H.; Ikeuchi, T.; Mukai, H.; Oyoshi, M.K.; Demachi, A.; Morio, T.; Wakiguchi, H.; Kimura, N.; Shimizu, N.; et al. Common cytological and cytogenetic features of Epstein-Barr virus (EBV)-positive natural killer (NK) cells and cell lines derived from patients with nasal T/NK-cell lymphomas, chronic active EBV infection and hydroa vacciniforme-like eruptions. Br. J. Haematol. 2003, 121, 805–814. [Google Scholar] [CrossRef]
- Tsunaka, M.; Shinki, H.; Koyama, T. Cell-based evaluation of changes in coagulation activity induced by antineoplastic drugs for the treatment of acute myeloid leukemia. PLoS ONE 2017, 12, e0175765. [Google Scholar] [CrossRef]
- van den Eijnden, M.M.; Steenhauer, S.I.; Reitsma, P.H.; Bertina, R.M. Tissue factor expression during monocyte-macrophage differentiation. Thromb. Haemost. 1997, 77, 1129–1136. [Google Scholar] [CrossRef]
- Wu, X.; Yao, Z.; Zhao, L.; Zhang, Y.; Cao, M.; Li, T.; Ding, W.; Liu, Y.; Deng, R.; Dong, Z.; et al. Phosphatidylserine on blood cells and endothelial cells contributes to the hypercoagulable state in cirrhosis. Liver. Int. 2016, 36, 1800–1810. [Google Scholar] [CrossRef]
- Onozawa, E.; Shibayama, H.; Imadome, K.I.; Tsuzura, A.; Koyama, T.; Miura, O.; Arai, A. Inflammatory cytokine production in chronic active Epstein-Barr virus infection. Rinsho. Ketsueki. 2017, 58, 189–196. [Google Scholar] [CrossRef]
- Mizuno, S.; Akashi, K.; Ohshima, K.; Iwasaki, H.; Miyamoto, T.; Uchida, N.; Shibuya, T.; Harada, M.; Kikuchi, M.; Niho, Y. Interferon-gamma prevents apoptosis in Epstein-Barr virus-infected natural killer cell leukemia in an autocrine fashion. Blood 1999, 93, 3494–3504. [Google Scholar] [CrossRef]
- Choi, Y.L.; Makishima, H.; Ohashi, J.; Yamashita, Y.; Ohki, R.; Koinuma, K.; Ota, J.; Isobe, Y.; Ishida, F.; Oshimi, K.; et al. DNA microarray analysis of natural killer cell-type lymphoproliferative disease of granular lymphocytes with purified CD3-CD56+ fractions. Leukemia 2004, 18, 556–565. [Google Scholar] [CrossRef] [Green Version]
- Ohga, S.; Nomura, A.; Takada, H.; Tanaka, T.; Furuno, K.; Takahata, Y.; Kinukawa, N.; Fukushima, N.; Imai, S.; Hara, T. Dominant expression of interleukin-10 and transforming growth factor-beta genes in activated T-cells of chronic active Epstein-Barr virus infection. J. Med. Virol. 2004, 74, 449–458. [Google Scholar] [CrossRef]
- Onozawa, E.; Shibayama, H.; Takada, H.; Imadome, K.I.; Aoki, S.; Yoshimori, M.; Shimizu, N.; Fujiwara, S.; Koyama, T.; Miura, O.; et al. STAT3 is constitutively activated in chronic active Epstein-Barr virus infection and can be a therapeutic target. Oncotarget 2018, 9, 31077–31089. [Google Scholar] [CrossRef]
- Huang, W.T.; Lin, C.W. EBV-encoded miR-BART20-5p and miR-BART8 inhibit the IFN-γ-STAT1 pathway associated with disease progression in nasal NK-cell lymphoma. Am. J. Pathol. 2014, 184, 1185–1197. [Google Scholar] [CrossRef]
- Kawano, Y.; Iwata, S.; Kawada, J.; Gotoh, K.; Suzuki, M.; Torii, Y.; Kojima, S.; Kimura, H.; Ito, Y. Plasma viral microRNA profiles reveal potential biomarkers for chronic active Epstein-Barr virus infection. J. Infect. Dis. 2013, 208, 771–779. [Google Scholar] [CrossRef] [Green Version]
- Lu, T.; Guo, Q.; Lin, K.; Chen, H.; Chen, Y.; Xu, Y.; Lin, C.; Su, Y.; Chen, M.; Zheng, Y.; et al. Circulating Epstein-Barr virus microRNAs BART7-3p and BART13-3p as novel biomarkers in nasopharyngeal carcinoma. Cancer Sci. 2020, 111, 1711–1723. [Google Scholar] [CrossRef]
- Komabayashi, Y.; Kishibe, K.; Nagato, T.; Ueda, S.; Takahara, M.; Harabuchi, Y. Circulating Epstein-Barr virus-encoded micro-RNAs as potential biomarkers for nasal natural killer/T-cell lymphoma. Hematol. Oncol. 2017, 35, 655–663. [Google Scholar] [CrossRef]
- Higuchi, H.; Yamakawa, N.; Imadome, K.I.; Yahata, T.; Kotaki, R.; Ogata, J.; Kakizaki, M.; Fujita, K.; Lu, J.; Yokoyama, K.; et al. Role of exosomes as a proinflammatory mediator in the development of EBV-associated lymphoma. Blood 2018, 131, 2552–2567. [Google Scholar] [CrossRef] [Green Version]
- Coppo, P.; Gouilleux-Gruart, V.; Huang, Y.; Bouhlal, H.; Bouamar, H.; Bouchet, S.; Perrot, C.; Vieillard, V.; Dartigues, P.; Gaulard, P.; et al. STAT3 transcription factor is constitutively activated and is oncogenic in nasal-type NK/T-cell lymphoma. Leukemia 2009, 23, 1667–1678. [Google Scholar] [CrossRef] [Green Version]
- Küçük, C.; Jiang, B.; Hu, X.; Zhang, W.; Chan, J.K.; Xiao, W.; Lack, N.; Alkan, C.; Williams, J.C.; Avery, K.N.; et al. Activating mutations of STAT5B and STAT3 in lymphomas derived from γδ-T or NK cells. Nat. Commun. 2015, 6, 6025. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Lee, H.; Herrmann, A.; Buettner, R.; Jove, R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer 2014, 14, 736–746. [Google Scholar] [CrossRef]
- Zhao, Y.; Niu, C.; Cui, J. Gamma-delta (γδ) T cells: Friend or foe in cancer development? J. Transl. Med. 2018, 16, 3. [Google Scholar] [CrossRef] [Green Version]
- Yoshimori, M.; Shibayama, H.; Imadome, K.I.; Kawano, F.; Ohashi, A.; Nishio, M.; Shimizu, N.; Kurata, M.; Fujiwara, S.; Arai, A. Antineoplastic and anti-inflammatory effects of bortezomib on systemic chronic active EBV infection. Blood Adv. 2021, 5, 1805–1815. [Google Scholar] [CrossRef]
- Locatelli, F.; Jordan, M.B.; Allen, C.; Cesaro, S.; Rizzari, C.; Rao, A.; Degar, B.; Garrington, T.P.; Sevilla, J.; Putti, M.C.; et al. Emapalumab in Children with Primary Hemophagocytic Lymphohistiocytosis. N. Engl. J. Med. 2020, 382, 1811–1822. [Google Scholar] [CrossRef]

| Case Number | Age | Gender | Disease | TNF-α (pg/mL) | IL-6 (pg/mL) | IFN-γ (pg/mL) | MDC (pg/mL) | GM-CSF (pg/mL) | Specimen |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 21 | F | ANKL | <5.5 | 3.7 | 4255.0 | 70.1 | N.D. | plasma |
| 2 | 35 | F | CAEBV (CD56 type) | 30.8 | 110.6 | 571.7 | 157.2 | N.D. | plasma |
| 3 | 36 | M | ENKL | 12.0 | 23.1 | 2863.3 | 155.0 | N.D. | plasma |
| 4 | 35 | F | CAEBV (CD56 type) | <5.5 | 12.0 | 11,608.0 | 44.0 | N.D. | serum |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshimori, M.; Nishio, M.; Ohashi, A.; Tateishi, M.; Mimura, A.; Wada, N.; Saito, M.; Shimizu, N.; Imadome, K.-I.; Arai, A. Interferon-γ Produced by EBV-Positive Neoplastic NK-Cells Induces Differentiation into Macrophages and Procoagulant Activity of Monocytes, Which Leads to HLH. Cancers 2021, 13, 5097. https://doi.org/10.3390/cancers13205097
Yoshimori M, Nishio M, Ohashi A, Tateishi M, Mimura A, Wada N, Saito M, Shimizu N, Imadome K-I, Arai A. Interferon-γ Produced by EBV-Positive Neoplastic NK-Cells Induces Differentiation into Macrophages and Procoagulant Activity of Monocytes, Which Leads to HLH. Cancers. 2021; 13(20):5097. https://doi.org/10.3390/cancers13205097
Chicago/Turabian StyleYoshimori, Mayumi, Miwako Nishio, Ayaka Ohashi, Megumi Tateishi, Ayaka Mimura, Naomi Wada, Minori Saito, Norio Shimizu, Ken-Ichi Imadome, and Ayako Arai. 2021. "Interferon-γ Produced by EBV-Positive Neoplastic NK-Cells Induces Differentiation into Macrophages and Procoagulant Activity of Monocytes, Which Leads to HLH" Cancers 13, no. 20: 5097. https://doi.org/10.3390/cancers13205097
APA StyleYoshimori, M., Nishio, M., Ohashi, A., Tateishi, M., Mimura, A., Wada, N., Saito, M., Shimizu, N., Imadome, K.-I., & Arai, A. (2021). Interferon-γ Produced by EBV-Positive Neoplastic NK-Cells Induces Differentiation into Macrophages and Procoagulant Activity of Monocytes, Which Leads to HLH. Cancers, 13(20), 5097. https://doi.org/10.3390/cancers13205097