The Clinical and Theranostic Values of Activated Leukocyte Cell Adhesion Molecule (ALCAM)/CD166 in Human Solid Cancers
Abstract
:Simple Summary
Abstract
1. Introduction
2. ALCAM, Its Discovery and Cellular Connections
3. ALCAM Expression Pattern in Cells and Tissues and Influence on Other Genetic and Biochemical Events
4. ALCAM Protein as a Receptor for Tumour Cell Homing
5. ALCAM Expression and Its Functional Influence in Cancer Cells
6. ALCAM Expression in Human Solid Malignant Tumours and the Clinical/Prognostic Significance
6.1. Pancreatic Cancer
6.2. Colorectal Cancer
6.3. Gastric Cancer
6.4. Breast Cancer
6.5. Sarcomas
6.6. Myeloma
6.7. Prostate Cancer
6.8. Thyroid Cancers
6.9. Ovarian Cancer
6.10. Endometrial Cancers
6.11. Hepatocellular Carcinoma
6.12. Neurological Cancers
6.13. Lung Cancer
6.14. Mesothelioma
6.15. Oesophageal Cancer
6.16. Head and Neck SCC (HNSCC) and Laryngeal Squamous Cell Carcinoma (SCC)
6.17. Cutaneous Melanoma
6.18. Oral Squamous Cell Carcinoma
6.19. Bladder Cancer
7. ALCAM and Cancer Metastasis
7.1. Liver Metastases
7.2. Brain Metastasis
7.3. Lung Metastases
7.4. Skin Metastasis
8. Circulating and Soluble ALCAM
9. ALCAM and Diagnostic and Therapeutic Considerations
9.1. ALCAM as a Therapeutic Target
9.2. Truncated ALCAM
9.3. Targeting ALCAM Partner Proteins
9.4. ALCAM as Therapeutic Response Indicator
9.5. ALCAM and Chemoresistance
10. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowen, M.A.; Patel, D.D.; Li, X.; Modrell, B.; Malacko, A.R.; Wang, W.C.; Marquardt, H.; Neubauer, M.; Pesando, J.M.; Francke, U.; et al. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J. Exp. Med. 1995, 181, 2213–2220. [Google Scholar] [CrossRef]
- Patel, D.D.; Wee, S.F.; Whichard, L.P.; Bowen, M.A.; Pesando, J.M.; Aruffo, A.; Haynes, B.F. Identification and characterization of a 100-kD ligand for CD6 on human thymic epithelial cells. J. Exp. Med. 1995, 181, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Fagerberg, L.; Hallstrom, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, A.; Kampf, C.; Sjostedt, E.; Asplund, A.; et al. Proteomics. Tissue-based map of the human proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef] [PubMed]
- von Lersner, A.; Droesen, L.; Zijlstra, A. Modulation of cell adhesion and migration through regulation of the immunoglobulin superfamily member ALCAM/CD166. Clin. Exp. Metastasis 2019, 36, 87–95. [Google Scholar] [CrossRef]
- Paget, S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989, 8, 98–101. [Google Scholar] [PubMed]
- Fidler, I.J. The pathogenesis of cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal. Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Ruma, I.M.; Putranto, E.W.; Kondo, E.; Murata, H.; Watanabe, M.; Huang, P.; Kinoshita, R.; Futami, J.; Inoue, Y.; Yamauchi, A.; et al. MCAM, as a novel receptor for S100A8/A9, mediates progression of malignant melanoma through prominent activation of NF-kappaB and ROS formation upon ligand binding. Clin. Exp. Metastasis 2016, 33, 609–627. [Google Scholar] [CrossRef]
- Renard, H.F.; Tyckaert, F.; Lo Giudice, C.; Hirsch, T.; Valades-Cruz, C.A.; Lemaigre, C.; Shafaq-Zadah, M.; Wunder, C.; Wattiez, R.; Johannes, L.; et al. Endophilin-A3 and Galectin-8 control the clathrin-independent endocytosis of CD166. Nat. Commun 2020, 11, 1457. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, J.; Wang, Y.; Zhou, C.; Ma, X.; Fu, J.; Yao, B.; Zhao, P. MicroRNA expression profiling and the role of ALCAM modulating tumor growth and metastasis in benzo[a]pyrene-transformed 16HBE cells. Toxicology 2020, 442, 152539. [Google Scholar] [CrossRef]
- Munsterberg, J.; Loreth, D.; Brylka, L.; Werner, S.; Karbanova, J.; Gandrass, M.; Schneegans, S.; Besler, K.; Hamester, F.; Robador, J.R.; et al. ALCAM contributes to brain metastasis formation in non-small-cell lung cancer through interaction with the vascular endothelium. Neuro Oncol. 2020, 22, 955–966. [Google Scholar] [CrossRef]
- Tudor, C.; Te Riet, J.; Eich, C.; Harkes, R.; Smisdom, N.; Bouhuijzen Wenger, J.; Ameloot, M.; Holt, M.; Kanger, J.S.; Figdor, C.G.; et al. Syntenin-1 and ezrin proteins link activated leukocyte cell adhesion molecule to the actin cytoskeleton. J. Biol. Chem. 2014, 289, 13445–13460. [Google Scholar] [CrossRef] [Green Version]
- Hamester, F.; Legler, K.; Wichert, B.; Kelle, N.; Eylmann, K.; Rossberg, M.; Ding, Y.; Kurti, S.; Schmalfeldt, B.; Milde-Langosch, K.; et al. Prognostic relevance of the Golgi mannosidase MAN1A1 in ovarian cancer: Impact of N-glycosylation on tumour cell aggregation. Br. J. Cancer 2019, 121, 944–953. [Google Scholar] [CrossRef]
- Kahlert, C.; Weber, H.; Mogler, C.; Bergmann, F.; Schirmacher, P.; Kenngott, H.G.; Matterne, U.; Mollberg, N.; Rahbari, N.N.; Hinz, U.; et al. Increased expression of ALCAM/CD166 in pancreatic cancer is an independent prognostic marker for poor survival and early tumour relapse. Br. J. Cancer 2009, 101, 457–464. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Kadoya, T.; Amioka, A.; Hanaki, H.; Sasada, S.; Masumoto, N.; Yamamoto, H.; Arihiro, K.; Kikuchi, A.; Okada, M. Wnt5a-induced cell migration is associated with the aggressiveness of estrogen receptor-positive breast cancer. Oncotarget 2018, 9, 20979–20992. [Google Scholar] [CrossRef] [Green Version]
- Chen, G.; Yan, M.; Li, R.R.; Chen, W.T. Sonic Hedgehog Signalling Activation Contributes to ALCAM Over-Expression and Poor Clinical Outcome in Patients with Oral Squamous Cell Carcinoma. Chin. J. Dent. Res. 2018, 21, 31–40. [Google Scholar] [CrossRef]
- Fernandez, M.M.; Ferragut, F.; Cardenas Delgado, V.M.; Bracalente, C.; Bravo, A.I.; Cagnoni, A.J.; Nunez, M.; Morosi, L.G.; Quinta, H.R.; Espelt, M.V.; et al. Glycosylation-dependent binding of galectin-8 to activated leukocyte cell adhesion molecule (ALCAM/CD166) promotes its surface segregation on breast cancer cells. Biochim. Biophys. Acta 2016, 1860, 2255–2268. [Google Scholar] [CrossRef]
- Ferragut, F.; Cagnoni, A.J.; Colombo, L.L.; Sanchez Terrero, C.; Wolfenstein-Todel, C.; Troncoso, M.F.; Vanzulli, S.I.; Rabinovich, G.A.; Marino, K.V.; Elola, M.T. Dual knockdown of Galectin-8 and its glycosylated ligand, the activated leukocyte cell adhesion molecule (ALCAM/CD166), synergistically delays in vivo breast cancer growth. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1338–1352. [Google Scholar] [CrossRef]
- Jin, Z.; Selaru, F.M.; Cheng, Y.; Kan, T.; Agarwal, R.; Mori, Y.; Olaru, A.V.; Yang, J.; David, S.; Hamilton, J.P.; et al. MicroRNA-192 and -215 are upregulated in human gastric cancer in vivo and suppress ALCAM expression in vitro. Oncogene 2011, 30, 1577–1585. [Google Scholar] [CrossRef] [Green Version]
- Song, Q.; Xu, Y.; Yang, C.; Chen, Z.; Jia, C.; Chen, J.; Zhang, Y.; Lai, P.; Fan, X.; Zhou, X.; et al. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014, 74, 3031–3042. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Huang, L.; Li, M.; Yang, Y.; Chen, Z.; Shen, X. MiR-148b, MiR-152/ALCAM Axis Regulates the Proliferation and Invasion of Pituitary Adenomas Cells. Cell Physiol. Biochem. 2017, 44, 792–803. [Google Scholar] [CrossRef]
- Lu, X.Y.; Chen, D.; Gu, X.Y.; Ding, J.; Zhao, Y.J.; Zhao, Q.; Yao, M.; Chen, Z.; He, X.H.; Cong, W.M. Predicting Value of ALCAM as a Target Gene of microRNA-483-5p in Patients with Early Recurrence in Hepatocellular Carcinoma. Front. Pharmacol. 2017, 8, 973. [Google Scholar] [CrossRef]
- Kim, R.; Park, S.I.; Lee, C.Y.; Lee, J.; Kim, P.; Oh, S.; Lee, H.; Lee, M.Y.; Kim, J.; Chung, Y.A.; et al. Alternative new mesenchymal stem cell source exerts tumor tropism through ALCAM and N-cadherin via regulation of microRNA-192 and -218. Mol. Cell Biochem. 2017, 427, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Oh, B.Y.; Kim, S.Y.; Lee, Y.S.; Hong, H.K.; Kim, T.W.; Kim, S.H.; Lee, W.Y.; Cho, Y.B. Twist1-induced epithelial-mesenchymal transition according to microsatellite instability status in colon cancer cells. Oncotarget 2016, 7, 57066–57076. [Google Scholar] [CrossRef] [Green Version]
- Jannie, K.M.; Stipp, C.S.; Weiner, J.A. ALCAM regulates motility, invasiveness, and adherens junction formation in uveal melanoma cells. PLoS ONE 2012, 7, e39330. [Google Scholar] [CrossRef] [Green Version]
- King, J.A.; Tan, F.; Mbeunkui, F.; Chambers, Z.; Cantrell, S.; Chen, H.; Alvarez, D.; Shevde, L.A.; Ofori-Acquah, S.F. Mechanisms of transcriptional regulation and prognostic significance of activated leukocyte cell adhesion molecule in cancer. Mol. Cancer 2010, 9, 266. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, N.; Mohd Yusoff, N.; Zakaria, Z.; Widera, D.; Yahaya, B.H. Inhibition of NF-kappaB Signaling Reduces the Stemness Characteristics of Lung Cancer Stem Cells. Front. Oncol. 2018, 8, 166. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Chang, C.C.; Li, M.; Zhang, Q.Y.; Vasilescu, E.M.; D’Agati, V.; Floratos, A.; Vlad, G.; Suciu-Foca, N. ILT3.Fc-CD166 Interaction Induces Inactivation of p70 S6 Kinase and Inhibits Tumor Cell Growth. J. Immunol. 2018, 200, 1207–1219. [Google Scholar] [CrossRef] [Green Version]
- Pinto, M.; Carmo, A.M. CD6 as a therapeutic target in autoimmune diseases: Successes and challenges. BioDrugs 2013, 27, 191–202. [Google Scholar] [CrossRef]
- Enyindah-Asonye, G.; Li, Y.; Ruth, J.H.; Spassov, D.S.; Hebron, K.E.; Zijlstra, A.; Moasser, M.M.; Wang, B.; Singer, N.G.; Cui, H.; et al. CD318 is a ligand for CD6. Proc. Natl. Acad. Sci. USA 2017, 114, E6912–E6921. [Google Scholar] [CrossRef] [Green Version]
- Dippel, V.; Milde-Langosch, K.; Wicklein, D.; Schumacher, U.; Altevogt, P.; Oliveira-Ferrer, L.; Janicke, F.; Schroder, C. Influence of L1-CAM expression of breast cancer cells on adhesion to endothelial cells. J. Cancer Res. Clin. Oncol. 2013, 139, 107–121. [Google Scholar] [CrossRef]
- Sawhney, M.; Matta, A.; Macha, M.A.; Kaur, J.; DattaGupta, S.; Shukla, N.K.; Ralhan, R. Cytoplasmic accumulation of activated leukocyte cell adhesion molecule is a predictor of disease progression and reduced survival in oral cancer patients. Int. J. Cancer 2009, 124, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Uhlen, M.; Zhang, C.; Lee, S.; Sjostedt, E.; Fagerberg, L.; Bidkhori, G.; Benfeitas, R.; Arif, M.; Liu, Z.; Edfors, F.; et al. A pathology atlas of the human cancer transcriptome. Science 2017, 357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.R.; Dent, C.; Watkins, G.; King, J.A.; Mokbel, K.; Jiang, W.G. Expression of the cell to cell adhesion molecule, ALCAM, in breast cancer patients and the potential link with skeletal metastasis. Oncol. Rep. 2008, 19, 555–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, S.; Jiang, W.G. ALCAM, activated leukocyte cell adhesion molecule, influences the aggressive nature of breast cancer cells, a potential connection to bone metastasis. Anticancer Res. 2010, 30, 1163–1168. [Google Scholar] [PubMed]
- Weichert, W.; Knosel, T.; Bellach, J.; Dietel, M.; Kristiansen, G. ALCAM/CD166 is overexpressed in colorectal carcinoma and correlates with shortened patient survival. J. Clin. Pathol. 2004, 57, 1160–1164. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, R.A.; Pintado-Berninches, L.; Jaen, M.; de Los Rios, V.; Imbaud, J.I.; Casal, J.I. SOSTDC1 promotes invasion and liver metastasis in colorectal cancer via interaction with ALCAM/CD166. Oncogene 2020, 39, 6085–6098. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, K.B.; da Silva Zanetti, J.; Ribeiro-Silva, A.; Rapatoni, L.; de Oliveira, H.F.; da Cunha Tirapelli, D.P.; Garcia, S.B.; Feres, O.; da Rocha, J.J.; Peria, F.M. KRAS mutation associated with CD44/CD166 immunoexpression as predictors of worse outcome in metastatic colon cancer. Cancer Biomark 2016, 16, 513–521. [Google Scholar] [CrossRef]
- Sim, S.H.; Kang, M.H.; Kim, Y.J.; Lee, K.W.; Kim, D.W.; Kang, S.B.; Eom, K.Y.; Kim, J.S.; Lee, H.S.; Kim, J.H. P21 and CD166 as predictive markers of poor response and outcome after fluorouracil-based chemoradiotherapy for the patients with rectal cancer. BMC Cancer 2014, 14, 241. [Google Scholar] [CrossRef] [Green Version]
- Hansen, A.G.; Freeman, T.J.; Arnold, S.A.; Starchenko, A.; Jones-Paris, C.R.; Gilger, M.A.; Washington, M.K.; Fan, K.H.; Shyr, Y.; Beauchamp, R.D.; et al. Elevated ALCAM shedding in colorectal cancer correlates with poor patient outcome. Cancer Res. 2013, 73, 2955–2964. [Google Scholar] [CrossRef] [Green Version]
- Tachezy, M.; Zander, H.; Gebauer, F.; Marx, A.; Kaifi, J.T.; Izbicki, J.R.; Bockhorn, M. Activated leukocyte cell adhesion molecule (CD166)--its prognostic power for colorectal cancer patients. J. Surg. Res. 2012, 177, e15–e20. [Google Scholar] [CrossRef]
- Badic, B.; Hatt, M.; Durand, S.; Jossic-Corcos, C.L.; Simon, B.; Visvikis, D.; Corcos, L. Radiogenomics-based cancer prognosis in colorectal cancer. Sci. Rep. 2019, 9, 9743. [Google Scholar] [CrossRef]
- Gerger, A.; Zhang, W.; Yang, D.; Bohanes, P.; Ning, Y.; Winder, T.; LaBonte, M.J.; Wilson, P.M.; Benhaim, L.; Paez, D.; et al. Common cancer stem cell gene variants predict colon cancer recurrence. Clin. Cancer Res. 2011, 17, 6934–6943. [Google Scholar] [CrossRef] [Green Version]
- Tachezy, M.; Effenberger, K.; Zander, H.; Minner, S.; Gebauer, F.; Vashist, Y.K.; Sauter, G.; Pantel, K.; Izbicki, J.R.; Bockhorn, M. ALCAM (CD166) expression and serum levels are markers for poor survival of esophageal cancer patients. Int. J. Cancer 2012, 131, 396–405. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, A.; Zhao, X.; Li, Z.; Cui, G. Tumoral Expression of CD166 in Human Esophageal Squamous Cell Carcinoma: Implications for Cancer Progression and Prognosis. Cancer Biother. Radiopharm. 2020, 35, 214–222. [Google Scholar] [CrossRef]
- Verma, A.; Shukla, N.K.; Deo, S.V.; Gupta, S.D.; Ralhan, R. MEMD/ALCAM: A potential marker for tumor invasion and nodal metastasis in esophageal squamous cell carcinoma. Oncology 2005, 68, 462–470. [Google Scholar] [CrossRef]
- Nicolau-Neto, P.; de Souza-Santos, P.T.; Severo Ramundo, M.; Valverde, P.; Martins, I.; Santos, I.C.; Dias, F.; de Almeida Simao, T.; Ribeiro Pinto, L.F. Transcriptome Analysis Identifies ALCAM Overexpression as a Prognosis Biomarker in Laryngeal Squamous Cell Carcinoma. Cancers 2020, 12, 470. [Google Scholar] [CrossRef] [Green Version]
- Clauditz, T.S.; von Rheinbaben, K.; Lebok, P.; Minner, S.; Tachezy, M.; Borgmann, K.; Knecht, R.; Sauter, G.; Wilczak, W.; Blessmann, M.; et al. Activated leukocyte cell adhesion molecule (ALCAM/CD166) expression in head and neck squamous cell carcinoma (HNSSC). Pathol. Res. Pract. 2014, 210, 649–655. [Google Scholar] [CrossRef]
- Yan, M.; Yang, X.; Wang, L.; Clark, D.; Zuo, H.; Ye, D.; Chen, W.; Zhang, P. Plasma membrane proteomics of tumor spheres identify CD166 as a novel marker for cancer stem-like cells in head and neck squamous cell carcinoma. Mol. Cell Proteomics 2013, 12, 3271–3284. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Sawhney, M.; DattaGupta, S.; Shukla, N.K.; Srivastava, A.; Walfish, P.G.; Ralhan, R. Clinical significance of altered expression of beta-catenin and E-cadherin in oral dysplasia and cancer: Potential link with ALCAM expression. PLoS ONE 2013, 8, e67361. [Google Scholar] [CrossRef]
- Bologna, S.B.; Nico, M.M.; Hsieh, R.; Coutinho-Camillo, C.M.; Buim, M.E.; Fernandes, J.D.; Sangueza, M.; Soares, F.A.; Lourenco, S.V. Adhesion molecules in primary oral mucosal melanoma: Study of claudins, integrins and immunoglobulins in a series of 35 cases. Am. J. Dermatopathol. 2013, 35, 541–554. [Google Scholar] [CrossRef] [PubMed]
- King, J.A.; Ofori-Acquah, S.F.; Stevens, T.; Al-Mehdi, A.B.; Fodstad, O.; Jiang, W.G. Activated leukocyte cell adhesion molecule in breast cancer: Prognostic indicator. Breast Cancer Res. 2004, 6, R478–R487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkhardt, M.; Mayordomo, E.; Winzer, K.J.; Fritzsche, F.; Gansukh, T.; Pahl, S.; Weichert, W.; Denkert, C.; Guski, H.; Dietel, M.; et al. Cytoplasmic overexpression of ALCAM is prognostic of disease progression in breast cancer. J. Clin. Pathol. 2006, 59, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihnen, M.; Muller, V.; Wirtz, R.M.; Schroder, C.; Krenkel, S.; Witzel, I.; Lisboa, B.W.; Janicke, F.; Milde-Langosch, K. Predictive impact of activated leukocyte cell adhesion molecule (ALCAM/CD166) in breast cancer. Breast Cancer Res. Treat. 2008, 112, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ihnen, M.; Wirtz, R.M.; Kalogeras, K.T.; Milde-Langosch, K.; Schmidt, M.; Witzel, I.; Eleftheraki, A.G.; Papadimitriou, C.; Janicke, F.; Briassoulis, E.; et al. Combination of osteopontin and activated leukocyte cell adhesion molecule as potent prognostic discriminators in HER2- and ER-negative breast cancer. Br. J. Cancer 2010, 103, 1048–1056. [Google Scholar] [CrossRef]
- Low, S.K.; Chin, Y.M.; Ito, H.; Matsuo, K.; Tanikawa, C.; Matsuda, K.; Saito, H.; Sakurai-Yageta, M.; Nakaya, N.; Shimizu, A.; et al. Identification of two novel breast cancer loci through large-scale genome-wide association study in the Japanese population. Sci. Rep. 2019, 9, 17332. [Google Scholar] [CrossRef]
- Legler, K.; Rosprim, R.; Karius, T.; Eylmann, K.; Rossberg, M.; Wirtz, R.M.; Muller, V.; Witzel, I.; Schmalfeldt, B.; Milde-Langosch, K.; et al. Reduced mannosidase MAN1A1 expression leads to aberrant N-glycosylation and impaired survival in breast cancer. Br. J. Cancer 2018, 118, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.J.; Oh, H.K.; Park, S.H.; Bong, J.G. Prognostic Significance of Activated Leukocyte Cell Adhesion Molecule (ALCAM) in Association with Promoter Methylation of the ALCAM Gene in Breast Cancer. Molecules 2018, 23, 131. [Google Scholar] [CrossRef] [Green Version]
- Al-Shehri, F.S.; Abd El Azeem, E.M. Activated Leukocyte Cell Adhesion Molecule (ALCAM) in Saudi Breast Cancer Patients as Prognostic and Predictive Indicator. Breast Cancer 2015, 9, 81–86. [Google Scholar] [CrossRef]
- Burandt, E.; Bari Noubar, T.; Lebeau, A.; Minner, S.; Burdelski, C.; Janicke, F.; Muller, V.; Terracciano, L.; Simon, R.; Sauter, G.; et al. Loss of ALCAM expression is linked to adverse phenotype and poor prognosis in breast cancer: A TMA-based immunohistochemical study on 2,197 breast cancer patients. Oncol. Rep. 2014, 32, 2628–2634. [Google Scholar] [CrossRef] [Green Version]
- Amantini, C.; Morelli, M.B.; Nabissi, M.; Piva, F.; Marinelli, O.; Maggi, F.; Bianchi, F.; Bittoni, A.; Berardi, R.; Giampieri, R.; et al. Expression Profiling of Circulating Tumor Cells in Pancreatic Ductal Adenocarcinoma Patients: Biomarkers Predicting Overall Survival. Front. Oncol. 2019, 9, 874. [Google Scholar] [CrossRef]
- Tachezy, M.; Zander, H.; Marx, A.H.; Stahl, P.R.; Gebauer, F.; Izbicki, J.R.; Bockhorn, M. ALCAM (CD166) expression and serum levels in pancreatic cancer. PLoS ONE 2012, 7, e39018. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, K.; Ohuchida, K.; Sada, M.; Horioka, K.; Ulrich, C.D., 3rd; Shindo, K.; Ohtsuka, T.; Takahata, S.; Mizumoto, K.; Oda, Y.; et al. CD166/ALCAM expression is characteristic of tumorigenicity and invasive and migratory activities of pancreatic cancer cells. PLoS ONE 2014, 9, e107247. [Google Scholar] [CrossRef] [Green Version]
- Piscuoglio, S.; Lehmann, F.S.; Zlobec, I.; Tornillo, L.; Dietmaier, W.; Hartmann, A.; Wunsch, P.H.; Sessa, F.; Rummele, P.; Baumhoer, D.; et al. Effect of EpCAM, CD44, CD133 and CD166 expression on patient survival in tumours of the ampulla of Vater. J. Clin. Pathol. 2012, 65, 140–145. [Google Scholar] [CrossRef]
- Ishiguro, F.; Murakami, H.; Mizuno, T.; Fujii, M.; Kondo, Y.; Usami, N.; Taniguchi, T.; Yokoi, K.; Osada, H.; Sekido, Y. Membranous expression of activated leukocyte cell adhesion molecule contributes to poor prognosis and malignant phenotypes of non-small-cell lung cancer. J. Surg. Res. 2013, 179, 24–32. [Google Scholar] [CrossRef]
- Eom, D.W.; Hong, S.M.; Kim, G.; Bae, Y.K.; Jang, K.T.; Yu, E. Prognostic Significance of CD44v6, CD133, CD166, and ALDH1 Expression in Small Intestinal Adenocarcinoma. Appl. Immunohistochem. Mol. Morphol. 2015, 23, 682–688. [Google Scholar] [CrossRef]
- Mezzanzanica, D.; Fabbi, M.; Bagnoli, M.; Staurengo, S.; Losa, M.; Balladore, E.; Alberti, P.; Lusa, L.; Ditto, A.; Ferrini, S.; et al. Subcellular localization of activated leukocyte cell adhesion molecule is a molecular predictor of survival in ovarian carcinoma patients. Clin. Cancer Res. 2008, 14, 1726–1733. [Google Scholar] [CrossRef] [Green Version]
- Devis, L.; Moiola, C.P.; Masia, N.; Martinez-Garcia, E.; Santacana, M.; Stirbat, T.V.; Brochard-Wyart, F.; Garcia, A.; Alameda, F.; Cabrera, S.; et al. Activated leukocyte cell adhesion molecule (ALCAM) is a marker of recurrence and promotes cell migration, invasion, and metastasis in early-stage endometrioid endometrial cancer. J. Pathol. 2017, 241, 475–487. [Google Scholar] [CrossRef]
- Devis, L.; Martinez-Garcia, E.; Moiola, C.P.; Quiles, M.T.; Arbos, M.A.; Stirbat, T.V.; Brochard-Wyart, F.; Garcia, A.; Alonso-Alconada, L.; Abal, M.; et al. ALCAM shedding at the invasive front of the tumor is a marker of myometrial infiltration and promotes invasion in endometrioid endometrial cancer. Oncotarget 2018, 9, 16648–16664. [Google Scholar] [CrossRef] [Green Version]
- van den Brand, M.; Takes, R.P.; Blokpoel-deRuyter, M.; Slootweg, P.J.; van Kempen, L.C. Activated leukocyte cell adhesion molecule expression predicts lymph node metastasis in oral squamous cell carcinoma. Oral Oncol. 2010, 46, 393–398. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, Y.; Wang, X.; Hu, J.; Kuang, M.; Wang, Z.; Li, S.; Xu, W.; Xiao, J. ALCAM(+) stromal cells: Role in giant cell tumor of bone progression. Cell Death Dis. 2018, 9, 299. [Google Scholar] [CrossRef] [Green Version]
- Hebron, K.E.; Li, E.Y.; Arnold Egloff, S.A.; von Lersner, A.K.; Taylor, C.; Houkes, J.; Flaherty, D.K.; Eskaros, A.; Stricker, T.P.; Zijlstra, A. Alternative splicing of ALCAM enables tunable regulation of cell-cell adhesion through differential proteolysis. Sci. Rep. 2018, 8, 3208. [Google Scholar] [CrossRef]
- Arnold Egloff, S.A.; Du, L.; Loomans, H.A.; Starchenko, A.; Su, P.F.; Ketova, T.; Knoll, P.B.; Wang, J.; Haddad, A.Q.; Fadare, O.; et al. Shed urinary ALCAM is an independent prognostic biomarker of three-year overall survival after cystectomy in patients with bladder cancer. Oncotarget 2017, 8, 722–741. [Google Scholar] [CrossRef] [Green Version]
- Chaker, S.; Kak, I.; MacMillan, C.; Ralhan, R.; Walfish, P.G. Activated leukocyte cell adhesion molecule is a marker for thyroid carcinoma aggressiveness and disease-free survival. Thyroid 2013, 23, 201–208. [Google Scholar] [CrossRef]
- Kristiansen, G.; Pilarsky, C.; Wissmann, C.; Stephan, C.; Weissbach, L.; Loy, V.; Loening, S.; Dietel, M.; Rosenthal, A. ALCAM/CD166 is up-regulated in low-grade prostate cancer and progressively lost in high-grade lesions. Prostate 2003, 54, 34–43. [Google Scholar] [CrossRef]
- Minner, S.; Kraetzig, F.; Tachezy, M.; Kilic, E.; Graefen, M.; Wilczak, W.; Bokemeyer, C.; Huland, H.; Sauter, G.; Schlomm, T. Low activated leukocyte cell adhesion molecule expression is associated with advanced tumor stage and early prostate-specific antigen relapse in prostate cancer. Hum. Pathol. 2011, 42, 1946–1952. [Google Scholar] [CrossRef]
- Kristiansen, G.; Pilarsky, C.; Wissmann, C.; Kaiser, S.; Bruemmendorf, T.; Roepcke, S.; Dahl, E.; Hinzmann, B.; Specht, T.; Pervan, J.; et al. Expression profiling of microdissected matched prostate cancer samples reveals CD166/MEMD and CD24 as new prognostic markers for patient survival. J. Pathol. 2005, 205, 359–376. [Google Scholar] [CrossRef]
- Hansen, A.G.; Arnold, S.A.; Jiang, M.; Palmer, T.D.; Ketova, T.; Merkel, A.; Pickup, M.; Samaras, S.; Shyr, Y.; Moses, H.L.; et al. ALCAM/CD166 is a TGF-beta-responsive marker and functional regulator of prostate cancer metastasis to bone. Cancer Res. 2014, 74, 1404–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanders, A.J.; Owen, S.; Morgan, L.D.; Ruge, F.; Collins, R.J.; Ye, L.; Mason, M.D.; Jiang, W.G. Importance of activated leukocyte cell adhesion molecule (ALCAM) in prostate cancer progression and metastatic dissemination. Oncotarget 2019, 10, 6362–6377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, H.; Chen, Z.T.; Zhu, X.D.; Li, L.; Qu, S.; Wei, Z.; Su, F.; Wei, J.N.; Liang, Z.G.; Mo, Q.Y.; et al. Serum CD166: A novel biomarker for predicting nasopharyngeal carcinoma response to radiotherapy. Oncotarget 2017, 8, 62858–62867. [Google Scholar] [CrossRef] [PubMed]
- Inaguma, S.; Lasota, J.; Wang, Z.; Czapiewski, P.; Langfort, R.; Rys, J.; Szpor, J.; Waloszczyk, P.; Okon, K.; Biernat, W.; et al. Expression of ALCAM (CD166) and PD-L1 (CD274) independently predicts shorter survival in malignant pleural mesothelioma. Hum. Pathol. 2018, 71, 1–7. [Google Scholar] [CrossRef]
- Atukeren, P.; Turk, O.; Yanar, K.; Kemerdere, R.; Sayyahmelli, S.; Eren, B.; Tanriverdi, T. Evaluation of ALCAM, PECAM-1 and selectin levels in intracranial meningiomas. Clin. Neurol. Neurosurg. 2017, 160, 21–26. [Google Scholar] [CrossRef]
- Wachowiak, R.; Mayer, S.; Kaifi, J.; Gebauer, F.; Izbicki, J.R.; Lacher, M.; Bockhorn, M.; Tachezy, M. Prognostic Impact of Activated Leucocyte Cell Adhesion Molecule (ALCAM/CD166) in Infantile Neuroblastoma. Anticancer Res. 2016, 36, 3991–3995. [Google Scholar]
- Kijima, N.; Hosen, N.; Kagawa, N.; Hashimoto, N.; Nakano, A.; Fujimoto, Y.; Kinoshita, M.; Sugiyama, H.; Yoshimine, T. CD166/activated leukocyte cell adhesion molecule is expressed on glioblastoma progenitor cells and involved in the regulation of tumor cell invasion. Neuro Oncol. 2012, 14, 1254–1264. [Google Scholar] [CrossRef] [Green Version]
- Achiha, T.; Kijima, N.; Kodama, Y.; Kagawa, N.; Kinoshita, M.; Fujimoto, Y.; Nonaka, M.; Fukai, J.; Inoue, A.; Nishida, N.; et al. Activated leukocyte cell adhesion molecule expression correlates with the WNT subgroup in medulloblastoma and is involved in regulating tumor cell proliferation and invasion. PLoS ONE 2020, 15, e0243272. [Google Scholar] [CrossRef]
- Klein, W.M.; Wu, B.P.; Zhao, S.; Wu, H.; Klein-Szanto, A.J.; Tahan, S.R. Increased expression of stem cell markers in malignant melanoma. Mod. Pathol. 2007, 20, 102–107. [Google Scholar] [CrossRef]
- Donizy, P.; Zietek, M.; Halon, A.; Leskiewicz, M.; Kozyra, C.; Matkowski, R. Prognostic significance of ALCAM (CD166/MEMD) expression in cutaneous melanoma patients. Diagn. Pathol. 2015, 10, 86. [Google Scholar] [CrossRef] [Green Version]
- Shanesmith, R.P.; Smart, C.; Cassarino, D.S. Tissue microarray analysis of ezrin, KBA.62, CD166, nestin, and p-Akt in melanoma versus banal and atypical nevi, and nonmelanocytic lesions. Am. J. Dermatopathol. 2011, 33, 663–668. [Google Scholar] [CrossRef]
- Ma, L.; Lin, J.; Qiao, Y.; Weng, W.; Liu, W.; Wang, J.; Sun, F. Serum CD166: A novel hepatocellular carcinoma tumor marker. Clin. Chim. Acta 2015, 441, 156–162. [Google Scholar] [CrossRef]
- Andisheh-Tadbir, A.; Ashraf, M.J.; Khademi, B.; Ahmadi, S. Clinical implication of CD166 expression in salivary gland tumor. Tumour Biol. 2015, 36, 2793–2799. [Google Scholar] [CrossRef]
- Ishigami, S.; Ueno, S.; Arigami, T.; Arima, H.; Uchikado, Y.; Kita, Y.; Sasaki, K.; Nishizono, Y.; Omoto, I.; Kurahara, H.; et al. Clinical implication of CD166 expression in gastric cancer. J. Surg. Oncol. 2011, 103, 57–61. [Google Scholar] [CrossRef]
- Ye, M.; Du, Y.L.; Nie, Y.Q.; Zhou, Z.W.; Cao, J.; Li, Y.F. Overexpression of activated leukocute cell adhesion molecule in gastric cancer is associated with advanced stages and poor prognosis and miR-9 deregulation. Mol. Med. Rep. 2015, 11, 2004–2012. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, Y.; Gao, H.; Peng, T.; Shi, H.; Tang, Y.; Li, H.; Chen, L.; Hu, K.; Han, A. A novel HDGF-ALCAM axis promotes the metastasis of Ewing sarcoma via regulating the GTPases signaling pathway. Oncogene 2021, 40, 731–745. [Google Scholar] [CrossRef]
- Federman, N.; Chan, J.; Nagy, J.O.; Landaw, E.M.; McCabe, K.; Wu, A.M.; Triche, T.; Kang, H.; Liu, B.; Marks, J.D.; et al. Enhanced growth inhibition of osteosarcoma by cytotoxic polymerized liposomal nanoparticles targeting the alcam cell surface receptor. Sarcoma 2012, 2012, 126906. [Google Scholar] [CrossRef]
- Ishiguro, F.; Murakami, H.; Mizuno, T.; Fujii, M.; Kondo, Y.; Usami, N.; Yokoi, K.; Osada, H.; Sekido, Y. Activated leukocyte cell-adhesion molecule (ALCAM) promotes malignant phenotypes of malignant mesothelioma. J. Thorac. Oncol. 2012, 7, 890–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ihnen, M.; Kress, K.; Kersten, J.F.; Kilic, E.; Choschzick, M.; Zander, H.; Muller, V.; Mahner, S.; Janicke, F.; Woelber, L.; et al. Relevance of activated leukocyte cell adhesion molecule (ALCAM) in tumor tissue and sera of cervical cancer patients. BMC Cancer 2012, 12, 140. [Google Scholar] [CrossRef] [Green Version]
- Hong, X.; Michalski, C.W.; Kong, B.; Zhang, W.; Raggi, M.C.; Sauliunaite, D.; De Oliveira, T.; Friess, H.; Kleeff, J. ALCAM is associated with chemoresistance and tumor cell adhesion in pancreatic cancer. J. Surg. Oncol. 2010, 101, 564–569. [Google Scholar] [CrossRef]
- Tachezy, M.; Zander, H.; Marx, A.H.; Gebauer, F.; Rawnaq, T.; Kaifi, J.T.; Sauter, G.; Izbicki, J.R.; Bockhorn, M. ALCAM (CD166) expression as novel prognostic biomarker for pancreatic neuroendocrine tumor patients. J. Surg. Res. 2011, 170, 226–232. [Google Scholar] [CrossRef]
- Zhang, W.W.; Zhan, S.H.; Geng, C.X.; Sun, X.; Erkan, M.; Kleeff, J.; Xie, X.J. Activated leukocyte cell adhesion molecule regulates the interaction between pancreatic cancer cells and stellate cells. Mol. Med. Rep. 2016, 14, 3627–3633. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Qian, C.; Jing, L.; Ren, J.; Guan, Y. Meta-analysis indicating that high ALCAM expression predicts poor prognosis in colorectal cancer. Oncotarget 2017, 8, 48272–48281. [Google Scholar] [CrossRef] [Green Version]
- Park, J.J.; Kwon, J.H.; Oh, S.H.; Choi, J.; Moon, C.M.; Ahn, J.B.; Hong, S.P.; Cheon, J.H.; Kim, T.I.; Kim, H.; et al. Differential expression of CD133 based on microsatellite instability status in human colorectal cancer. Mol. Carcinog. 2014, 53 (Suppl. 1), E1–E10. [Google Scholar] [CrossRef] [PubMed]
- Szkandera, J.; Herzog, S.; Pichler, M.; Stiegelbauer, V.; Stotz, M.; Schaberl-Moser, R.; Samonigg, H.; Asslaber, M.; Lax, S.; Leitner, G.; et al. LGR5 rs17109924 is a predictive genetic biomarker for time to recurrence in patients with colon cancer treated with 5-fluorouracil-based adjuvant chemotherapy. Pharmacogenomics J. 2015, 15, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Hein, S.; Muller, V.; Kohler, N.; Wikman, H.; Krenkel, S.; Streichert, T.; Schweizer, M.; Riethdorf, S.; Assmann, V.; Ihnen, M.; et al. Biologic role of activated leukocyte cell adhesion molecule overexpression in breast cancer cell lines and clinical tumor tissue. Breast Cancer Res. Treat. 2011, 129, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Mosunjac, M.; Adams, A.L.; Adade, B.; Taye, O.; Hu, Y.; Rizzo, M.; Ofori-Acquah, S.F. Enhanced down-regulation of ALCAM/CD166 in African-American Breast Cancer. BMC Cancer 2014, 14, 715. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Hua, T.; Hong, T. Integrated diagnostic network construction reveals a 4-gene panel and 5 cancer hallmarks driving breast cancer heterogeneity. Sci. Rep. 2017, 7, 6827. [Google Scholar] [CrossRef]
- Zhou, P.; Du, L.F.; Lv, G.Q.; Yu, X.M.; Gu, Y.L.; Li, J.P.; Zhang, C. Functional polymorphisms in CD166/ALCAM gene associated with increased risk for breast cancer in a Chinese population. Breast Cancer Res. Treat. 2011, 128, 527–534. [Google Scholar] [CrossRef]
- Varadi, V.; Bevier, M.; Grzybowska, E.; Johansson, R.; Enquist-Olsson, K.; Henriksson, R.; Butkiewicz, D.; Pamula-Pilat, J.; Tecza, K.; Hemminki, K.; et al. Genetic variation in ALCAM and other chromosomal instability genes in breast cancer survival. Breast Cancer Res. Treat. 2012, 131, 311–319. [Google Scholar] [CrossRef]
- Xu, L.; Mohammad, K.S.; Wu, H.; Crean, C.; Poteat, B.; Cheng, Y.; Cardoso, A.A.; Machal, C.; Hanenberg, H.; Abonour, R.; et al. Cell Adhesion Molecule CD166 Drives Malignant Progression and Osteolytic Disease in Multiple Myeloma. Cancer Res. 2016, 76, 6901–6910. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Wang, Q.; Li, T.; Qian, J.; Lu, Y.; Li, Y.; Bi, E.; Reu, F.; Qin, Y.; Drazba, J.; et al. Role of Myeloma-Derived MIF in Myeloma Cell Adhesion to Bone Marrow and Chemotherapy Response. J. Natl. Cancer Inst. 2016, 108, djw131. [Google Scholar] [CrossRef] [Green Version]
- Micciche, F.; Da Riva, L.; Fabbi, M.; Pilotti, S.; Mondellini, P.; Ferrini, S.; Canevari, S.; Pierotti, M.A.; Bongarzone, I. Activated leukocyte cell adhesion molecule expression and shedding in thyroid tumors. PLoS ONE 2011, 6, e17141. [Google Scholar] [CrossRef] [Green Version]
- Allmendinger, O.; Trautmann, K.; Mittelbronn, M.; Waidelich, J.; Meyermann, R.; Tatagiba, M.; Schittenhelm, J. Activated leukocyte cell adhesion molecule is expressed in neuroepithelial neoplasms and decreases with tumor malignancy, matrix metalloproteinase 2 expression, and absence of IDH1R132H mutation. Hum. Pathol. 2012, 43, 1289–1299. [Google Scholar] [CrossRef]
- Corrias, M.V.; Gambini, C.; Gregorio, A.; Croce, M.; Barisione, G.; Cossu, C.; Rossello, A.; Ferrini, S.; Fabbi, M. Different subcellular localization of ALCAM molecules in neuroblastoma: Association with relapse. Cell Oncol. 2010, 32, 77–86. [Google Scholar] [CrossRef]
- Chen, S.; Xu, W.; Jiao, J.; Jiang, D.; Liu, J.; Chen, T.; Wan, Z.; Xu, L.; Zhou, Z.; Xiao, J. Differential proteomic profiling of primary and recurrent chordomas. Oncol. Rep. 2015, 33, 2207–2218. [Google Scholar] [CrossRef] [Green Version]
- Berg, J.; Halvorsen, A.R.; Bengtson, M.B.; Tasken, K.A.; Maelandsmo, G.M.; Yndestad, A.; Halvorsen, B.; Brustugun, O.T.; Aukrust, P.; Ueland, T.; et al. Levels and prognostic impact of circulating markers of inflammation, endothelial activation and extracellular matrix remodelling in patients with lung cancer and chronic obstructive pulmonary disease. BMC Cancer 2018, 18, 739. [Google Scholar] [CrossRef] [Green Version]
- Van Kempen, L.C.; van den Oord, J.J.; van Muijen, G.N.; Weidle, U.H.; Bloemers, H.P.; Swart, G.W. Activated leukocyte cell adhesion molecule/CD166, a marker of tumor progression in primary malignant melanoma of the skin. Am. J. Pathol. 2000, 156, 769–774. [Google Scholar] [CrossRef] [Green Version]
- Djirackor, L.; Kalirai, H.; Coupland, S.E.; Petrovski, G. CD166high Uveal Melanoma Cells Represent a Subpopulation With Enhanced Migratory Capacity. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2696–2704. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Harrell, J.C.; Perou, C.M.; Dudley, A.C. Identification of a stable molecular signature in mammary tumor endothelial cells that persists in vitro. Angiogenesis 2014, 17, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Kulasingam, V.; Zheng, Y.; Soosaipillai, A.; Leon, A.E.; Gion, M.; Diamandis, E.P. Activated leukocyte cell adhesion molecule: A novel biomarker for breast cancer. Int. J. Cancer 2009, 125, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Ihnen, M.; Kohler, N.; Kersten, J.F.; Milde-Langosch, K.; Beck, K.; Holler, S.; Muller, V.; Witzel, I.; Janicke, F.; Kilic, E. Expression levels of Activated Leukocyte Cell Adhesion Molecule (ALCAM/CD166) in primary breast carcinoma and distant breast cancer metastases. Dis. Markers 2010, 28, 71–78. [Google Scholar] [CrossRef]
- Soto, M.S.; Serres, S.; Anthony, D.C.; Sibson, N.R. Functional role of endothelial adhesion molecules in the early stages of brain metastasis. Neuro Oncol. 2014, 16, 540–551. [Google Scholar] [CrossRef] [Green Version]
- Curis, C.; Percher, F.; Jeannin, P.; Montange, T.; Chevalier, S.A.; Seilhean, D.; Cartier, L.; Couraud, P.O.; Gout, O.; Gessain, A.; et al. Human T-Lymphotropic Virus Type 1-Induced Overexpression of Activated Leukocyte Cell Adhesion Molecule (ALCAM) Facilitates Trafficking of Infected Lymphocytes through the Blood-Brain Barrier. J. Virol. 2016, 90, 7303–7312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wrobel, J.K.; Wolff, G.; Xiao, R.; Power, R.F.; Toborek, M. Dietary Selenium Supplementation Modulates Growth of Brain Metastatic Tumors and Changes the Expression of Adhesion Molecules in Brain Microvessels. Biol. Trace Elem. Res. 2016, 172, 395–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willrodt, A.H.; Beffinger, M.; Vranova, M.; Protsyuk, D.; Schuler, K.; Jadhav, M.; Heikenwalder, M.; van den Broek, M.; Borsig, L.; Halin, C. Stromal Expression of Activated Leukocyte Cell Adhesion Molecule Promotes Lung Tumor Growth and Metastasis. Am. J. Pathol. 2017, 187, 2558–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, S.; Kobayashi, M.; Wang, J.; Habelhah, H.; Okada, F.; Hamada, J.; Moriuchi, T.; Totsuka, Y.; Hosokawa, M. Activated leukocyte cell adhesion molecule (ALCAM) and annexin II are involved in the metastatic progression of tumor cells after chemotherapy with Adriamycin. Clin. Exp. Metastasis 2000, 18, 45–50. [Google Scholar] [CrossRef]
- Ihnen, M.; Kilic, E.; Kohler, N.; Loning, T.; Witzel, I.; Hagel, C.; Holler, S.; Kersten, J.F.; Muller, V.; Janicke, F.; et al. Protein expression analysis of ALCAM and CEACAM6 in breast cancer metastases reveals significantly increased ALCAM expression in metastases of the skin. J. Clin. Pathol. 2011, 64, 146–152. [Google Scholar] [CrossRef]
- Orso, F.; Quirico, L.; Virga, F.; Penna, E.; Dettori, D.; Cimino, D.; Coppo, R.; Grassi, E.; Elia, A.R.; Brusa, D.; et al. miR-214 and miR-148b Targeting Inhibits Dissemination of Melanoma and Breast Cancer. Cancer Res. 2016, 76, 5151–5162. [Google Scholar] [CrossRef] [Green Version]
- Penna, E.; Orso, F.; Cimino, D.; Vercellino, I.; Grassi, E.; Quaglino, E.; Turco, E.; Taverna, D. miR-214 coordinates melanoma progression by upregulating ALCAM through TFAP2 and miR-148b downmodulation. Cancer Res. 2013, 73, 4098–4111. [Google Scholar] [CrossRef] [Green Version]
- Rosso, O.; Piazza, T.; Bongarzone, I.; Rossello, A.; Mezzanzanica, D.; Canevari, S.; Orengo, A.M.; Puppo, A.; Ferrini, S.; Fabbi, M. The ALCAM shedding by the metalloprotease ADAM17/TACE is involved in motility of ovarian carcinoma cells. Mol. Cancer Res. 2007, 5, 1246–1253. [Google Scholar] [CrossRef] [Green Version]
- Lunter, P.C.; van Kilsdonk, J.W.; van Beek, H.; Cornelissen, I.M.; Bergers, M.; Willems, P.H.; van Muijen, G.N.; Swart, G.W. Activated leukocyte cell adhesion molecule (ALCAM/CD166/MEMD), a novel actor in invasive growth, controls matrix metalloproteinase activity. Cancer Res. 2005, 65, 8801–8808. [Google Scholar] [CrossRef] [Green Version]
- van Kilsdonk, J.W.; Wilting, R.H.; Bergers, M.; van Muijen, G.N.; Schalkwijk, J.; van Kempen, L.C.; Swart, G.W. Attenuation of melanoma invasion by a secreted variant of activated leukocyte cell adhesion molecule. Cancer Res. 2008, 68, 3671–3679. [Google Scholar] [CrossRef] [Green Version]
- Carbotti, G.; Orengo, A.M.; Mezzanzanica, D.; Bagnoli, M.; Brizzolara, A.; Emionite, L.; Puppo, A.; Centurioni, M.G.; Bruzzone, M.; Marroni, P.; et al. Activated leukocyte cell adhesion molecule soluble form: A potential biomarker of epithelial ovarian cancer is increased in type II tumors. Int. J. Cancer 2013, 132, 2597–2605. [Google Scholar] [CrossRef]
- Chaker, S.; Kashat, L.; Voisin, S.; Kaur, J.; Kak, I.; MacMillan, C.; Ozcelik, H.; Siu, K.W.; Ralhan, R.; Walfish, P.G. Secretome proteins as candidate biomarkers for aggressive thyroid carcinomas. Proteomics 2013, 13, 771–787. [Google Scholar] [CrossRef] [Green Version]
- Erturk, K.; Tastekin, D.; Bilgin, E.; Serilmez, M.; Bozbey, H.U.; Sakar, B. Serum activated leukocyte cell adhesion molecule and intercellular adhesion molecule-1 in patients with gastric cancer: Can they be used as biomarkers? Biomed. Pharmacother. 2016, 77, 86–91. [Google Scholar] [CrossRef]
- Witzel, I.; Schroder, C.; Muller, V.; Zander, H.; Tachezy, M.; Ihnen, M.; Janicke, F.; Milde-Langosch, K. Detection of activated leukocyte cell adhesion molecule in the serum of breast cancer patients and implications for prognosis. Oncology 2012, 82, 305–312. [Google Scholar] [CrossRef]
- Tran, D.P.; Wolfrum, B.; Stockmann, R.; Pai, J.H.; Pourhassan-Moghaddam, M.; Offenhausser, A.; Thierry, B. Complementary metal oxide semiconductor compatible silicon nanowires-on-a-chip: Fabrication and preclinical validation for the detection of a cancer prognostic protein marker in serum. Anal. Chem. 2015, 87, 1662–1668. [Google Scholar] [CrossRef]
- Wiiger, M.T.; Gehrken, H.B.; Fodstad, O.; Maelandsmo, G.M.; Andersson, Y. A novel human recombinant single-chain antibody targeting CD166/ALCAM inhibits cancer cell invasion in vitro and in vivo tumour growth. Cancer Immunol. Immunother. 2010, 59, 1665–1674. [Google Scholar] [CrossRef] [Green Version]
- Zarghami, N.; Soto, M.S.; Perez-Balderas, F.; Khrapitchev, A.A.; Karali, C.S.; Johanssen, V.A.; Ansorge, O.; Larkin, J.R.; Sibson, N.R. A novel molecular magnetic resonance imaging agent targeting activated leukocyte cell adhesion molecule as demonstrated in mouse brain metastasis models. J. Cereb. Blood Flow Metab. 2021, 41, 1592–1607. [Google Scholar] [CrossRef]
- McCabe, K.E.; Liu, B.; Marks, J.D.; Tomlinson, J.S.; Wu, H.; Wu, A.M. An engineered cysteine-modified diabody for imaging activated leukocyte cell adhesion molecule (ALCAM)-positive tumors. Mol. Imaging Biol. 2012, 14, 336–347. [Google Scholar] [CrossRef] [Green Version]
- Tavare, R.; Wu, W.H.; Zettlitz, K.A.; Salazar, F.B.; McCabe, K.E.; Marks, J.D.; Wu, A.M. Enhanced immunoPET of ALCAM-positive colorectal carcinoma using site-specific (6)(4)Cu-DOTA conjugation. Protein Eng. Des. Sel. 2014, 27, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.K.; Su, Y.; Bidlingmaier, S.; Liu, B. Manipulation of Cell-Type Selective Antibody Internalization by a Guide-Effector Bispecific Design. Mol. Cancer Ther. 2019, 18, 1092–1103. [Google Scholar] [CrossRef] [Green Version]
- Darvishi, B.; Salehi, M.; Boroumandieh, S.; Majidzadeh, A.K.; Jalili, N.; Moradi-Kalbolandi, S.; Farahmand, L. Dual in vitro invasion/migration suppressing and tamoxifen response modulating effects of a recombinant anti-ALCAM scFv on breast cancer cells. Cell Biochem. Funct 2020, 38, 651–659. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, W.; Zhu, J.; Wang, J.; Xia, K.; Liang, C.; Tao, H. Anti-CD166/4-1BB chimeric antigen receptor T cell therapy for the treatment of osteosarcoma. J. Exp. Clin. Cancer Res. 2019, 38, 168. [Google Scholar] [CrossRef] [Green Version]
- Kudo-Saito, C.; Fuwa, T.; Kawakami, Y. Targeting ALCAM in the cryo-treated tumour microenvironment successfully induces systemic anti-tumour immunity. Eur. J. Cancer 2016, 62, 54–61. [Google Scholar] [CrossRef]
- Kinoshita, R.; Sato, H.; Yamauchi, A.; Takahashi, Y.; Inoue, Y.; Sumardika, I.W.; Chen, Y.; Tomonobu, N.; Araki, K.; Shien, K.; et al. exSSSRs (extracellular S100 soil sensor receptors)-Fc fusion proteins work as prominent decoys to S100A8/A9-induced lung tropic cancer metastasis. Int. J. Cancer 2019, 144, 3138–3145. [Google Scholar] [CrossRef]
- Nuti, E.; Casalini, F.; Avramova, S.I.; Santamaria, S.; Fabbi, M.; Ferrini, S.; Marinelli, L.; La Pietra, V.; Limongelli, V.; Novellino, E.; et al. Potent arylsulfonamide inhibitors of tumor necrosis factor-alpha converting enzyme able to reduce activated leukocyte cell adhesion molecule shedding in cancer cell models. J. Med. Chem. 2010, 53, 2622–2635. [Google Scholar] [CrossRef]
- Nuti, E.; Casalini, F.; Santamaria, S.; Fabbi, M.; Carbotti, G.; Ferrini, S.; Marinelli, L.; La Pietra, V.; Novellino, E.; Camodeca, C.; et al. Selective arylsulfonamide inhibitors of ADAM-17: Hit optimization and activity in ovarian cancer cell models. J. Med. Chem. 2013, 56, 8089–8103. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhang, J.; Chen, F.; Sun, Y. MiR-148b suppressed non-small cell lung cancer progression via inhibiting ALCAM through the NF-kappaB signaling pathway. Thorac. Cancer 2020, 11, 415–425. [Google Scholar] [CrossRef]
- Quirico, L.; Orso, F.; Esposito, C.L.; Bertone, S.; Coppo, R.; Conti, L.; Catuogno, S.; Cavallo, F.; de Franciscis, V.; Taverna, D. Axl-148b chimeric aptamers inhibit breast cancer and melanoma progression. Int. J. Biol. Sci. 2020, 16, 1238–1251. [Google Scholar] [CrossRef]
- Jin, U.H.; Karki, K.; Kim, S.B.; Safe, S. Inhibition of pancreatic cancer Panc1 cell migration by omeprazole is dependent on aryl hydrocarbon receptor activation of JNK. Biochem. Biophys. Res. Commun. 2018, 501, 751–757. [Google Scholar] [CrossRef]
- McRobb, L.S.; McKay, M.J.; Gauden, A.J.; Lee, V.S.; Subramanian, S.; Thomas, S.G.; Wiedmann, M.K.; Moutrie, V.; Grace, M.; Zhao, Z.; et al. Radiation-Stimulated Translocation of CD166 and CRYAB to the Endothelial Surface Provides Potential Vascular Targets on Irradiated Brain Arteriovenous Malformations. Int. J. Mol. Sci. 2019, 20, 5830. [Google Scholar] [CrossRef] [Green Version]
- Roth, A.; Drummond, D.C.; Conrad, F.; Hayes, M.E.; Kirpotin, D.B.; Benz, C.C.; Marks, J.D.; Liu, B. Anti-CD166 single chain antibody-mediated intracellular delivery of liposomal drugs to prostate cancer cells. Mol. Cancer Ther. 2007, 6, 2737–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Gu, Z.; Ni, P.; Qiao, Y.; Chen, C.; Liu, X.; Lin, J.; Chen, N.; Fan, Q. NF-kappaB P50/P65 hetero-dimer mediates differential regulation of CD166/ALCAM expression via interaction with micoRNA-9 after serum deprivation, providing evidence for a novel negative auto-regulatory loop. Nucleic Acids Res. 2011, 39, 6440–6455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.J.; Cheng, Y.M.; Chen, C.C.; Chen, Y.C.; Shen, C.J. MiR-148a and miR-152 reduce tamoxifen resistance in ER+ breast cancer via downregulating ALCAM. Biochem. Biophys. Res. Commun. 2017, 483, 840–846. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, F.; Corcos, L.; Durand, S.; Simon, B.; Le Jossic-Corcos, C. Acquisition of anticancer drug resistance is partially associated with cancer stemness in human colon cancer cells. Int. J. Oncol. 2016, 49, 2558–2568. [Google Scholar] [CrossRef]
- Amantini, C.; Morelli, M.B.; Nabissi, M.; Cardinali, C.; Santoni, M.; Gismondi, A.; Santoni, G. Capsaicin triggers autophagic cell survival which drives epithelial mesenchymal transition and chemoresistance in bladder cancer cells in an Hedgehog-dependent manner. Oncotarget 2016, 7, 50180–50194. [Google Scholar] [CrossRef] [Green Version]
- Su, J.; Wu, S.; Tang, W.; Qian, H.; Zhou, H.; Guo, T. Reduced SLC27A2 induces cisplatin resistance in lung cancer stem cells by negatively regulating Bmi1-ABCG2 signaling. Mol. Carcinog. 2016, 55, 1822–1832. [Google Scholar] [CrossRef]
- Chappell, N.P.; Teng, P.N.; Hood, B.L.; Wang, G.; Darcy, K.M.; Hamilton, C.A.; Maxwell, G.L.; Conrads, T.P. Mitochondrial proteomic analysis of cisplatin resistance in ovarian cancer. J. Proteome. Res. 2012, 11, 4605–4614. [Google Scholar] [CrossRef]


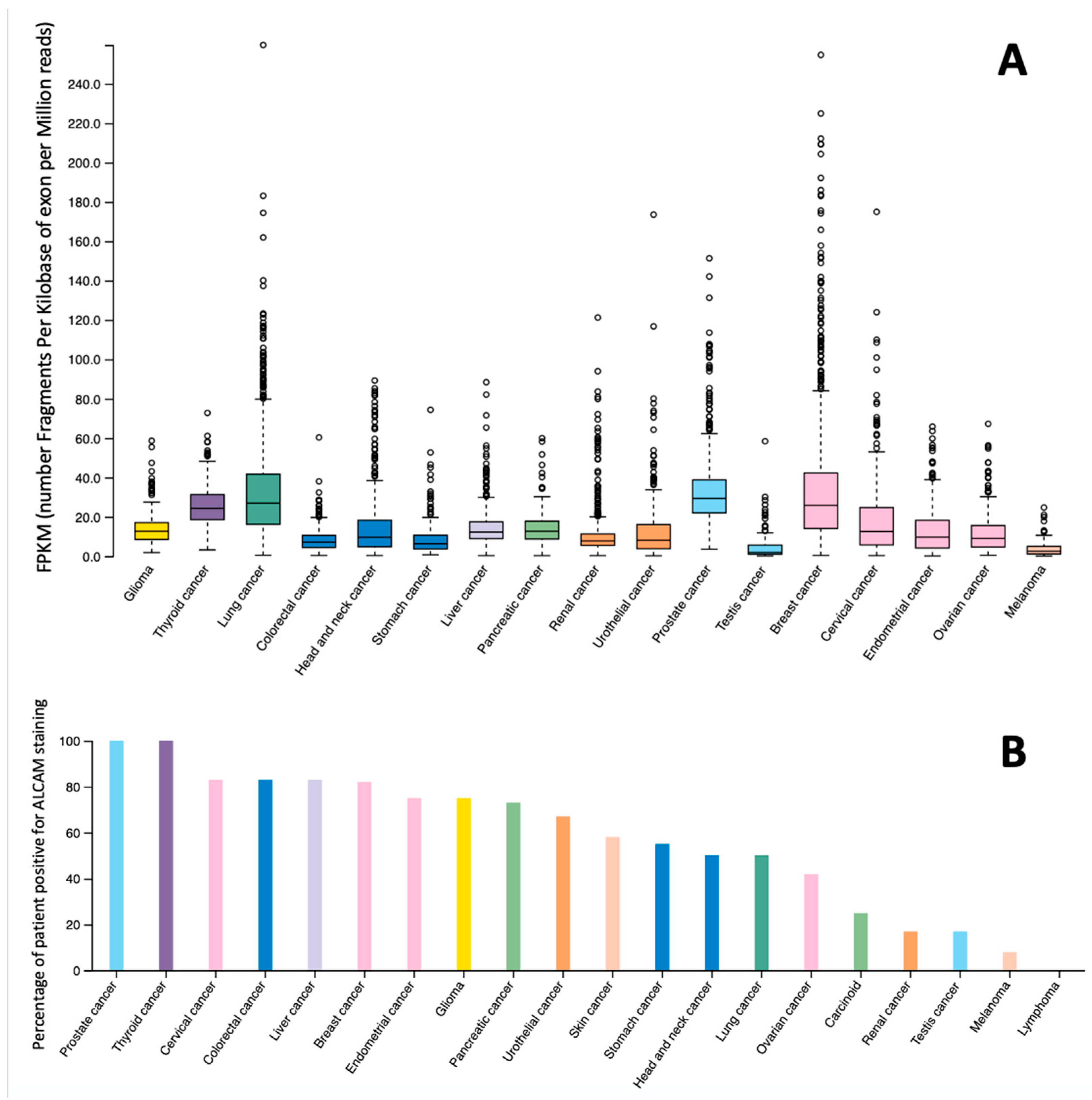
| Tumour Type | Study Methods | Findings | References |
|---|---|---|---|
| Colorectal cancer | IHC (n = 111) | Both membranous and cytoplasmic staining of ALCAM are seen in colon cancer tissues. However, membranous ALCAM is linked with shortened survival of the patients. | [36] |
| IHC (n = 9 pairs) | Tumour tissues showed highly stained ALCAM compared with normal tissues. | [37] | |
| IHC and PCR (n = 58) | ALCAM-positive staining is seen in 43%. ALCAM-negative tumours had greater incidence of lymph node metastasis, a link greatly increased when there is concurrent KRAS mutation. | [38] | |
| IHC (n = 112) | High levels of ALCAM were associated with longer recurrence-free survival. | [39] | |
| Gene expression (n = 250), IHC (n = 105) and ELISA (n = 91) | ALCAM is highly expression in tumour tissues and is linked to shorter survival of the patients. | [40] | |
| IHC (n = 299) | More than 70% and 60% of primary and secondary tumours stained positive for ALCAM, respectively. Positive ALCAM staining is a positive prognostic factor for the patients. | [41] | |
| Gene array (n = 64) | ALCAM transcripts were significantly raised in colorectal tissues compared with normal tissues. | [42] | |
| Germline polymorphism of peripheral blood of patients who resisted 5-FU therapies (n = 234) | Polymorphisms of ALCAM, along with LGR5, CD44, and ALDH1, form an independent signature in predicting the time to recurrence of patients with colon cancer who received 5-FU-based chemotherapies. | [43] | |
| Metastatic liver tumours from colorectal cancer | Paired primary and secondary (n = 9 pairs), IHC | Stepwise increase in ALCAM protein from normal colorectal tissues, primary colorectal cancer tissues to metastatic liver tumours. | [37] |
| Oesophageal SCC | IHC (n = 299) | High levels of ALCAM in primary tumours associated with recurrence-free and overall survival of the patients. | [44] |
| IHC (n = 65) | ALCAM staining was seen in 87.69% of tumours, compared with negative staining in control normal tissues. ALCAM linked to tumour grade, TNM staining, and survival. | [45] | |
| IHC and PCR (n = 65) | ALCAM expression is increased in SCC compared with control tissues, and the increase is seen with nodal metastasis and late clinical stages. | [46] | |
| Laryngeal, head, and neck SCC | IHC and RNAseq (n = 44) and gene array | Membranous staining of ALCAM. High levels of staining associated with shorter survival of the patients. | [47] |
| IHC (n = 400) | A total of 70% of HNSCC tumours stained positive for ALCAM, including 12.4% membranous, 40.1% cytoplasmic, and mixed membranous/cytoplasmic in 17.9%. | [48] | |
| IHC (n = 96) | Patients with high levels of ALCAM staining in HNSCC (n = 96) had shorter survival. Primary HNSCC (n = 68) had significantly lower ALCAM staining than the recurrent HNSCC (n = 36) tumours. | [49] | |
| Oral dysplasia and cancer | IHC (n = 105) | ALCAM total and cytoplasmic staining were correlated with loss of membranous E-cadherin and beta-catenin in oral squamous cell carcinoma. ALCAM expression together with cytoplasmic/nucleus beta-catenin is an indicator of nodal metastasis and late-stage tumours. | [50] |
| Oral melanoma | IHC (n = 35) | ALCAM-positive staining in oral melanoma is associated with vascular invasion. | [51] |
| Non-small cell lung cancer and brain metastasis | IHC (n = 143 comprised of 51 primary NSCLC, 15 metastatic nodes and 76 metastatic brain tumours) | Metastatic brain tumours and metastatic lymph nodes stained higher for ALCAM compared with primary NSCLC. High staining in NSCLC and in metastatic brain lesions associated with poor survival. | [11] |
| Breast cancer | IHC and QPCR (n = 120 tumour and 32 normal) | ALCAM transcript expression was lower in tumours with lymph node metastasis compared to those without. ALCAM levels were also lower in high-grade/TNM stage compared to lower stage/grade samples. ALCAM levels were lower in those with poorer outcomes. | [52] |
| IHC (n = 162) | Cytoplasmic staining of ALCAM is associated with shorten survival, nodal status, and early recurrence. | [53] | |
| Protein blotting (n = 160) and gene microarray | Neither protein nor mRNA expressions are linked to pathological factors. Protein ALCAM is seen more in ER-positive tumours. High levels of ALCAM mRNA, when receiving chemotherapy, have better clinical survival, but those who did not receive chemotherapy had worse survival. | [54] | |
| Gene microarray(n = 481) | Low ALCAM expression, together with the status of ER, Her2, and osteopontin, identified a set of patients with markedly shorter survival from three separate cohorts with combined number of 481 patients. | [55] | |
| GWS (6,669) | ALCAM is a new foci associated with breast cancer (Japan), and together with CLIC6-RUNX1, it makes susceptibility SNPs of breast cancer. | [56] | |
| IHC (n = 153) | ALCAM is largely membranous when present. Seventy out of 153 stained positive for ALCAM and the remaining 83 negatives, and the staining has an intimate relationship with the levels of Wnt5a. | [15] | |
| IHC and microarray (n = 110) | Patients with high levels of ALCAM transcript had longer disease-free survival. The correlation is more significant in patients with high levels of membranous ALCAM and high levels of mannonidase (MAN1A). | [57] | |
| Transcript analysis and IHC (n = 47) | Breast tumour tissues had higher levels of ALCAM gene methylation than normal tissues, and the raised ALCAM gene methylation was seen with lower levels of ALCAM transcript. | [58] | |
| IHC (n = 161) | Patients had highly raised circulating levels of soluble ALCAM and had a potential diagnostic value of breast cancer amongst the particular ethnic population (Saudi patients). | [59] | |
| IHC (n = 2,197) | Reduced/loss of ALCAM staining was seen in most tumour types and was linked to high tumour grade and poor OS and RFS. | [60] | |
| Pancreatic cancer | Circulating cancer cells (CTC) (n = 20) | Patients with circulating cancer cells showing high levels of ALCAM had shorter survival. | [61] |
| IHC (n = 264) and ELISA (n = 116) | At the tissue level, there was no significant correlation between tumour grade and staging. However, the circulating levels of ALCAM were significantly higher than the control and those with pancreatitis. | [62] | |
| IHC (n = 97) | Patients who died of pancreatic cancer had high levels of ALCAM staining in pancreatic cancer cells. | [14] | |
| IHC (n = 98) | A total of 12% of pancreatic cancers were positive for ALCAM staining, compared with none in normal pancreatic tissues. | [63] | |
| Ampulla of Vater | IHC | In a rather large series of this uncommon cancer, there was a progressive increase in ALCAM staining from normal mucosa (n = 152) to adenoma (n = 111) to carcinoma of ampulla of Vater (n = 175). | [64] |
| Lung cancer | IHC (n = 147 NSCLC) | Membrane ALCAM staining is seen in 44.9% of NSCLC tumours and is an independent prognostic factor for shorter overall survival of the patients. | [65] |
| Small intestinal adenocarcinoma | IHC (n = 191) | A total of 42% of the tumours stained positive. | [66] |
| Ovarian cancer | IHC (n = 204) | Concurrent high levels of ALCAM and mannosidase MAN1A1 linked to shorter relapse-free survival of the patients, yet low levels of MAN1A1 and high levels of ALCAM linked to better survival of the patients. | [13] |
| IHC (n = 109) | Cytoplasmic staining or loss of membrane staining of ALCAM is a prognostic factor for patients with ovarian cancer. | [67] | |
| Endometrial cancer | IHC | For early endometrioid endometrial cancer (n = 174), positive ALCAM (76.2%) is seen in patients with short recurrence-free survival. Of all the tumours (n = 116), 67.4% stained positive for ALCAM with remaining negative. Weak or negative staining was seen at the invading front of cancer tissues. | [68,69] |
| Oral squamous cell carcinoma (OSCC) | IHC (n = 41) | Staining of ALCAM on the membrane of the leading front of the SCC cells was seen in tumours with node involvement and high tumour grade. | [70] |
| IHC (n = 101) | Less than half (47.5%) of the oral SCC tumours stained positive for ALCAM. | [16] | |
| IHC (n = 107) | Membranous and cytoplasmic staining was seen in OSCC. Cytoplasmic staining was associated with clinical outcomes and survival of the patients. | [32] | |
| Giant cell bone tumours | IHC (n = 64) | Patients with high levels of ALCAM staining in the giant cell tumour had shorter disease-free survival. | [71] |
| Bladder cancer | TCGA analysis and cell work | Bladder cancer cells and bladder tumour tissues expressed high levels of an ALCAM variant (ALCAM-iso2), which is subject to easy shedding in response to MMP14. | [72] |
| IHC (n = 198) and ELISA (n = 120) | Bladder tumours had reduced ALCAM staining with increased staging. Both circulating and urinary soluble ALCAM seen to markedly increase in patients with bladder cancer. | [73] | |
| Thyroid cancer | IHC (n = 158) | Total ALCAM in poorly differential thyroid tumours was markedly lower than those with well/moderately differentiated tumours, and the reduction was associated with distant metastasis and shortened survival. | [74] |
| Prostate cancer | IHC (n = 54 pairs) | Over 80% of tumours have raised ALCAM staining, which is largely seen in low grade and low Gleason scores. | [75] |
| IHC (n = 2,390) | Approximate 70% had membrane ALCAM staining. High ALCAM expression was associated with less aggressive tumour phenotypes, pre-operative PSA (prostate-specific antigen) levels, and a reduced risk of biochemical recurrence. | [76] | |
| Gene microarray and IHC (n = 42 pairs) | A total of 86% of prostate tumours are positive for ALCAM and are a prognostic marker for prostate cancer. | [77] | |
| Public microarray analysis | ALCAM mRNA enhanced in metastatic disease. High mRNA expression of ALCAM corresponded with poor outcomes. | [78] | |
| IHC (n = 48) and ELISA (n = 229) | Patients with metastatic disease, nodal-positive tumours, and who died from prostate cancer had high ALCAM staining. Circulating ALCAM has a prognostic value on the survival of the patients. | [79] | |
| Nasopharyngeal carcinoma | ELISA (n = 60) | Patients with radioresistant response had high levels of circulating ALCAM. High staining of ALCAM is linked to favourable clinical and pathological features and with low risk of biochemical recurrence of the patients. | [80] |
| Mesothelioma of the pleural cavity | IHC (n = 175) | ALCAM-positive mesotheliomas had a significantly shorter survival of the patients. | [81] |
| Intracranial meningioma | IHC (n = 20) | Meningioma tissues had significantly higher levels of ALCAM compared with normal tissues. | [82] |
| Neuroblastoma | IHC (n = 66) | Weak ALCAM staining is linked to a short RFS and OS. | [83] |
| Glioblastoma | IHC (n = 39) | Tumours rich in ALCAM had shorter overall and disease-free survival. | [84] |
| Medulloblastoma | IHC (n = 45) | Majority of the tumours (67%) were negative for ALCAM staining. The positive stained tumours (18) were seen in the WNT group and SHH group. The presence of ALCAM is associated with CTNNB1 and nuclear β-catenin expression. | [85] |
| Melanoma | IHC | There is a progressively increased staining of ALCAM from nevi (15%, n = 71), primary melanoma (53%, n = 71) to metastatic melanoma (69%, n = 84). | [86] |
| IHC (n = 104) | High levels of ALCAM staining seen in patients with shorter overall and disease-free survivals. In addition, low ALCAM staining in metastatic lymph nodes is also seen with shorter overall survival of the patients. | [87] | |
| IHC (n = 110) | A total of 65% melanoma were positive for ALCAM compared with 74% nevi. | [88] | |
| Hepatocellular carcinoma (HCC) | IHC and ELISA | HCC tumours stained more strongly for ALCAM than normal liver tissues, and HCC patients had markedly high levels of circulating HCC. | [89] |
| IHC (n = 129) | In recurrent hepatocellular carcinoma (RHCC), positive ALCAM was associated with time to recurrence and microvascular invasion. | [22] | |
| Salivary gland tumours | IHC (n = 45) | Adenoid cystic carcinoma and mucoepidermoid carcinoma ALCAM staining were markedly higher than benign pleomorphic adenomas and normal tissues. High-grade and late-stage malignant tumours had higher staining than early stages. | [90] |
| Gastric cancer | IHC (n = 142) | Both membrane and cytoplasmic staining are present and linked to nodal metastasis and vascular invasion. | [91] |
| IHC and PCR (n = 66), ELISA (n = 72) | Both protein (IHC staining) and transcript expression of ALCAM were highly raised in gastric cancer compared with control tissues. The patients also had significantly higher levels of circulating ALCAM than controls and those with non-cancerous conditions. | [92] | |
| Ewing’s sarcoma | ChIP-seq and gene profiling (n = 98) | Most sarcomas stained positively for membranous ALCAM, and high levels of staining seen in patients with suitable MFS survival. Furthermore, high levels of HDGF (an ALCAM transcription suppressor) and low levels of ALCAM collectively present in patients with the worse prognosis. | [93] |
| Osteosarcoma | IHC (n = 10) | Membrane and cytoplasmic staining are seen in both primary and metastatic tumours. | [94] |
| Malignant mesothelioma | IHC (n = 55) | A total of 55% of the 47 malignant mesotheliomas stained positive for ALCAM. Overexpression is linked to shorter survival. | [95] |
| Cervical cancer | IHC (n = 233) | Over half (58.4%) of tumours were strongly positive for ALCAM. | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Sanders, A.J.; Dou, Q.P.; Jiang, D.G.; Li, A.X.; Jiang, W.G. The Clinical and Theranostic Values of Activated Leukocyte Cell Adhesion Molecule (ALCAM)/CD166 in Human Solid Cancers. Cancers 2021, 13, 5187. https://doi.org/10.3390/cancers13205187
Yang Y, Sanders AJ, Dou QP, Jiang DG, Li AX, Jiang WG. The Clinical and Theranostic Values of Activated Leukocyte Cell Adhesion Molecule (ALCAM)/CD166 in Human Solid Cancers. Cancers. 2021; 13(20):5187. https://doi.org/10.3390/cancers13205187
Chicago/Turabian StyleYang, Yiming, Andrew J. Sanders, Q. Ping Dou, David G. Jiang, Amber Xinyu Li, and Wen G. Jiang. 2021. "The Clinical and Theranostic Values of Activated Leukocyte Cell Adhesion Molecule (ALCAM)/CD166 in Human Solid Cancers" Cancers 13, no. 20: 5187. https://doi.org/10.3390/cancers13205187
APA StyleYang, Y., Sanders, A. J., Dou, Q. P., Jiang, D. G., Li, A. X., & Jiang, W. G. (2021). The Clinical and Theranostic Values of Activated Leukocyte Cell Adhesion Molecule (ALCAM)/CD166 in Human Solid Cancers. Cancers, 13(20), 5187. https://doi.org/10.3390/cancers13205187








