Liquid Biopsy as a Diagnostic and Prognostic Tool for Women and Female Dogs with Breast Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
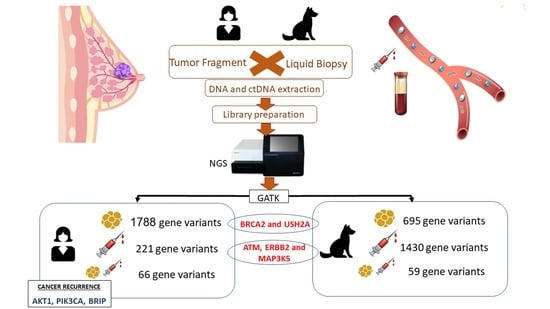
2. Materials and Methods
2.1. Ethics
2.2. Sample Collection
2.3. DNA and ctDNA Extraction
2.4. Preparation of DNA and ctDNA Sequencing Libraries for Illumina System
2.5. Next-Generation Sequencing
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
3.1. Characterizing Human and Canine Population with BC
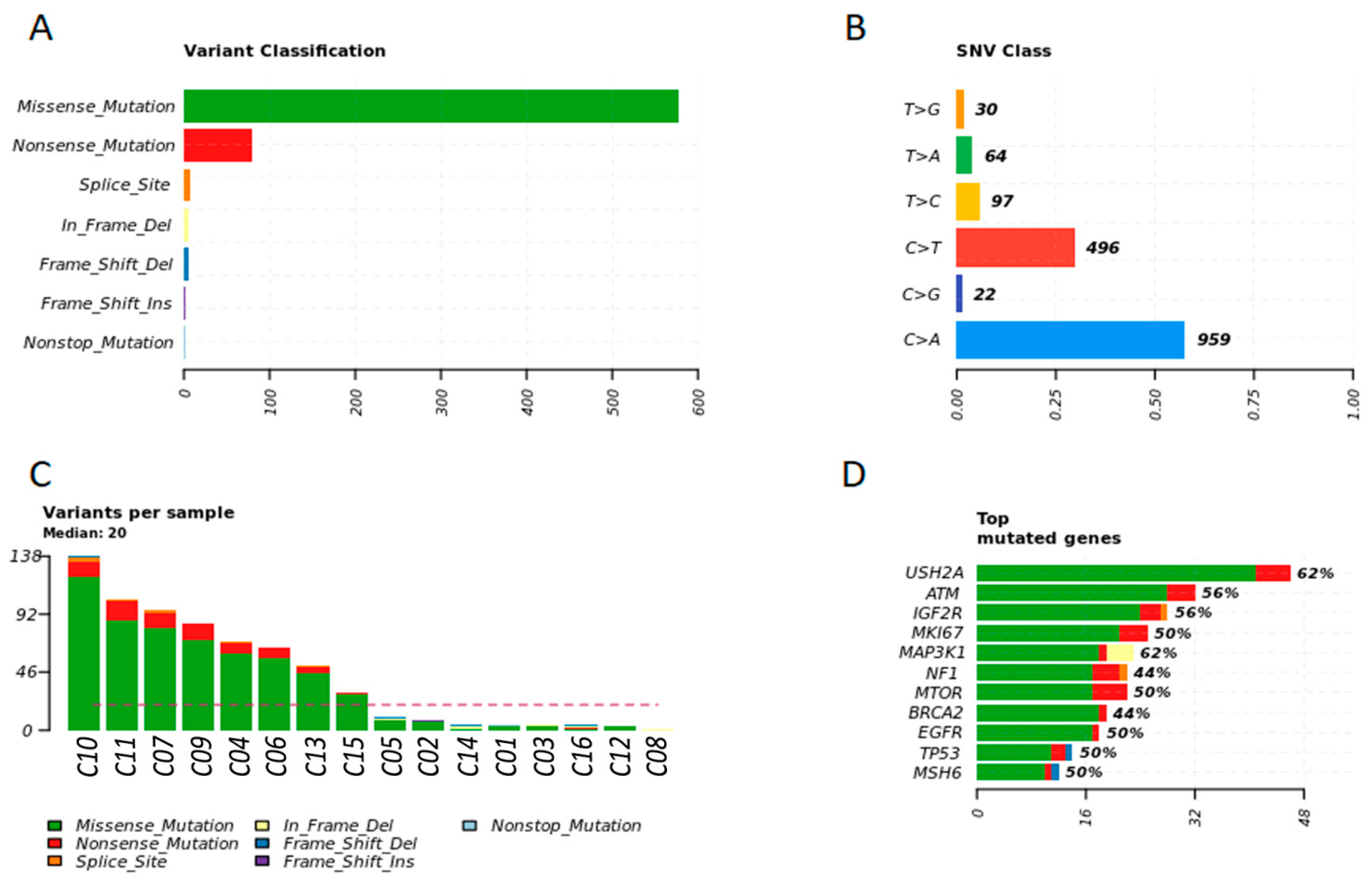
3.2. Detection of Individual Relevant Genetic Variants in Tumor Fragments and in Plasma of Women with BC
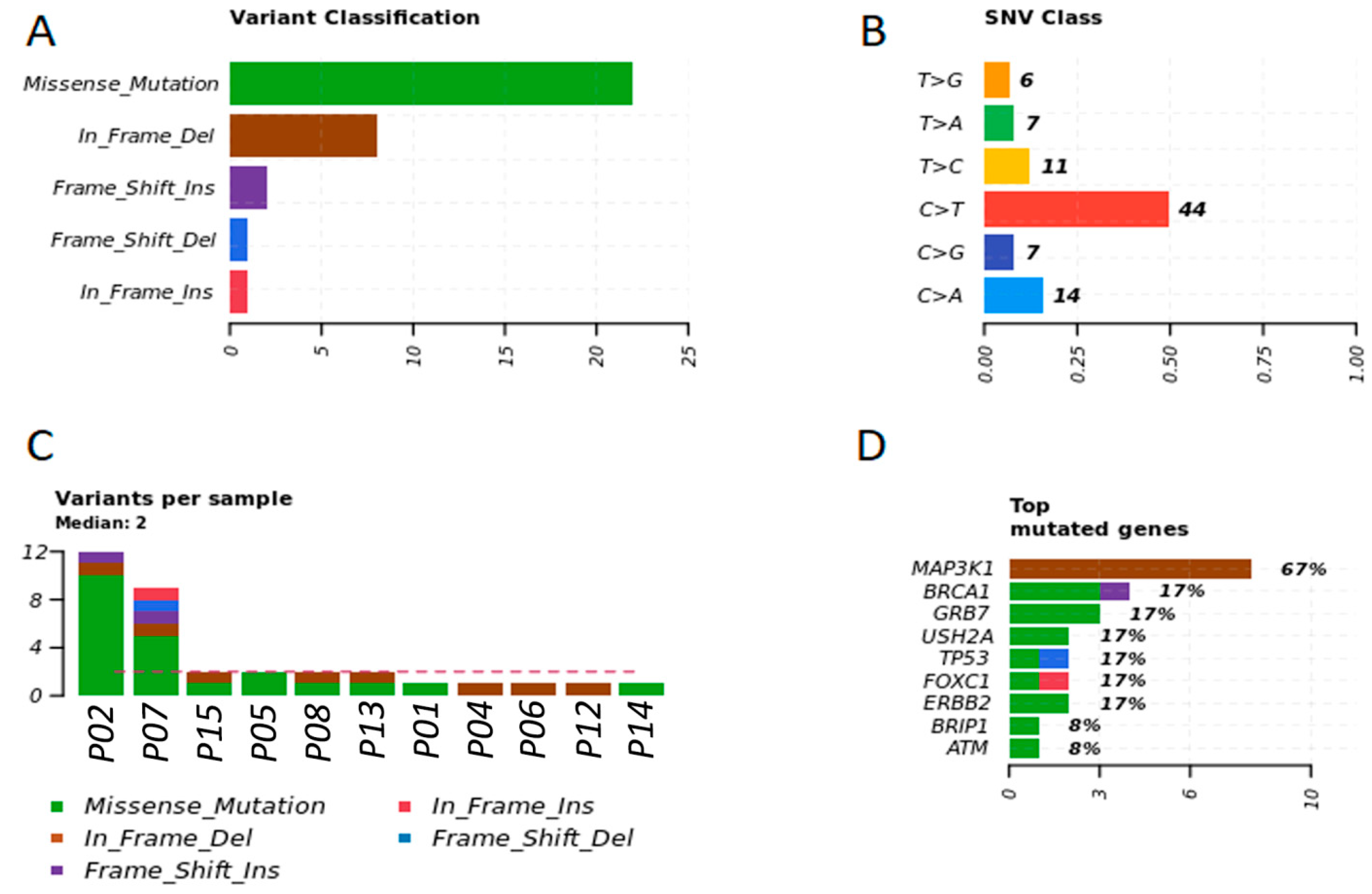
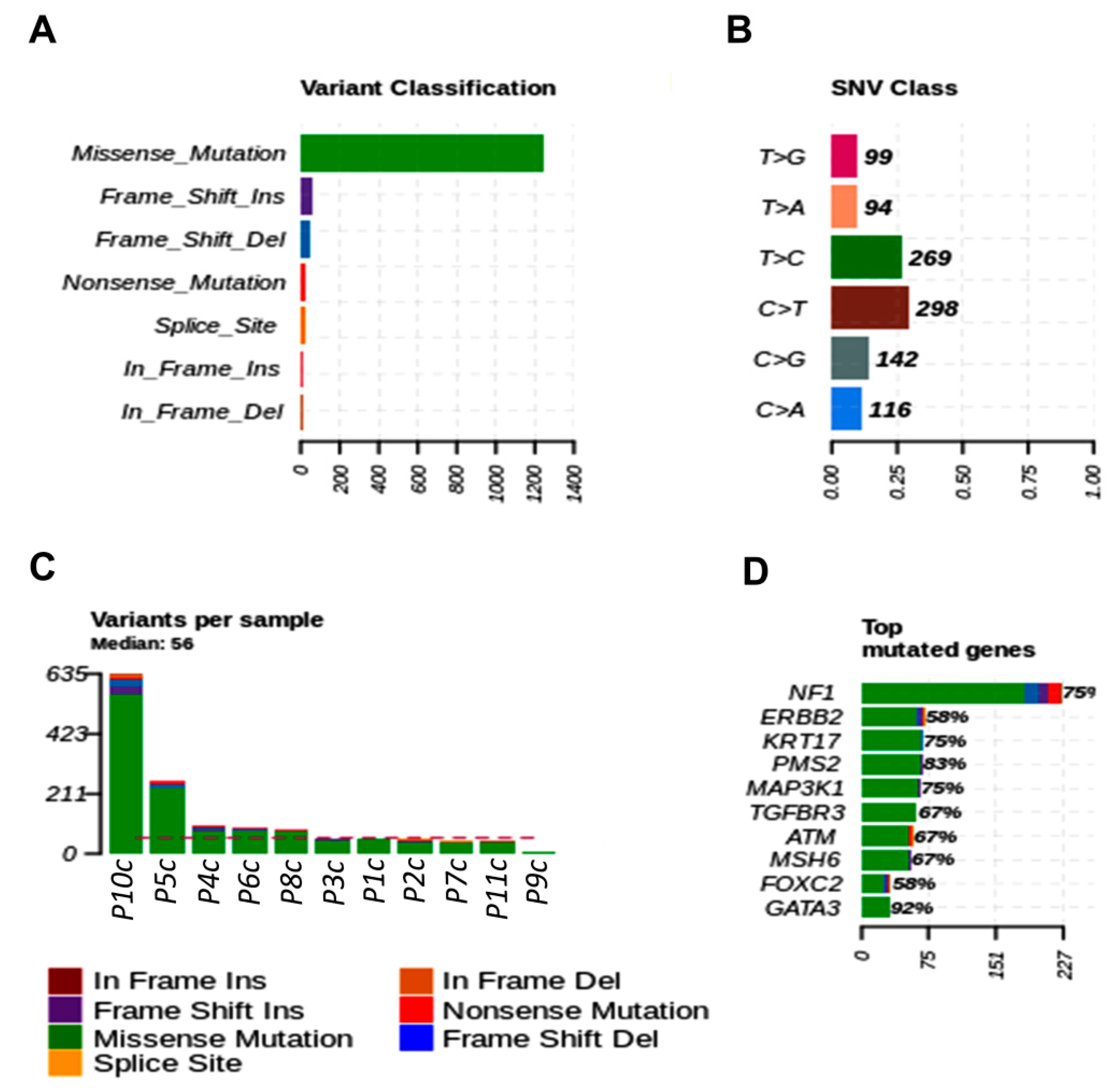
3.3. Detection of Individual Relevant Genetic Variants in Tumor Fragments and in Plasma of Dogs with BC
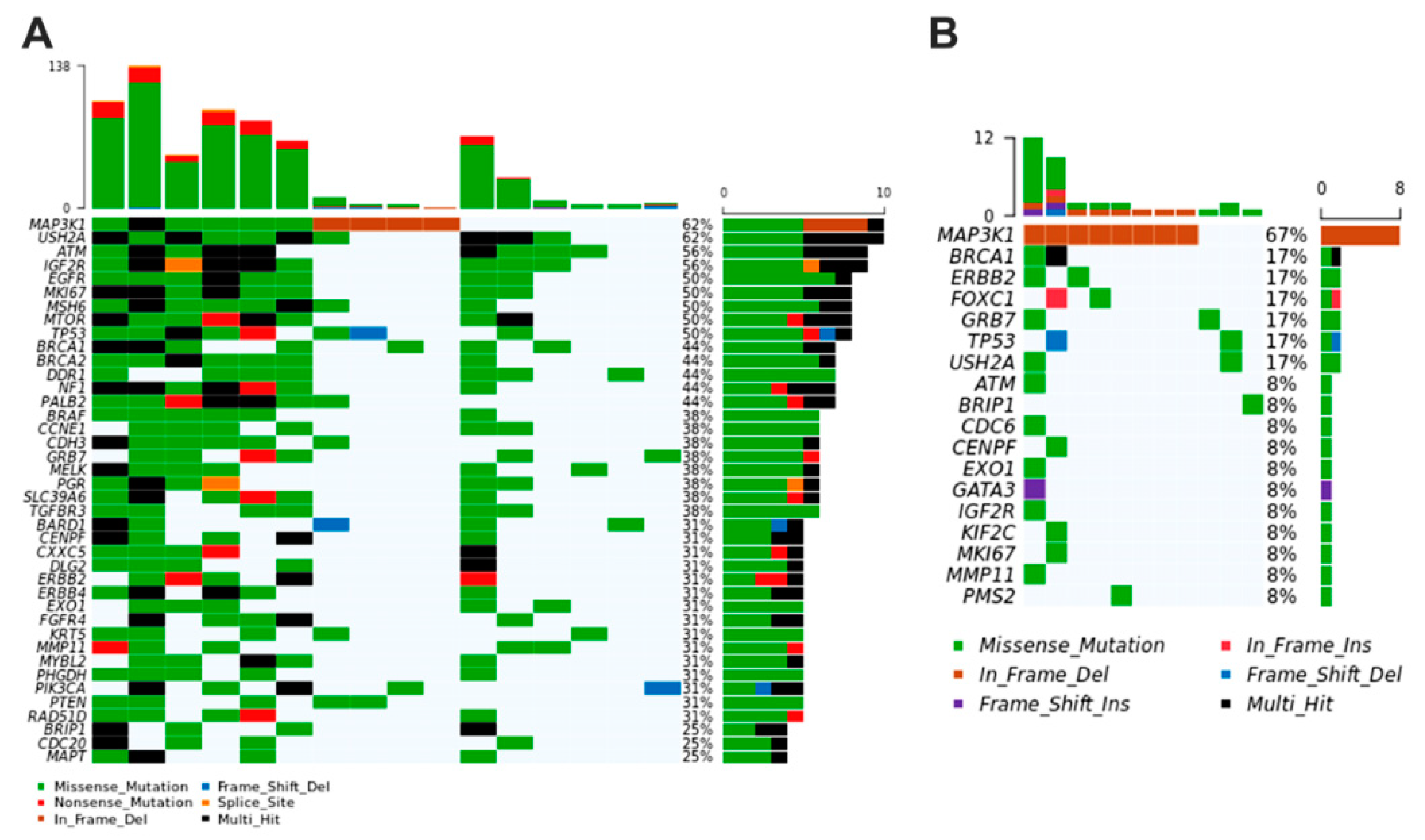
3.4. Breast Cancer Driver Genes in Women Share Similar Variant Mutations in Tumor Fragments and Plasma
3.5. Breast Cancer Driver Genes in Dogs Share Similar Variant Mutations in Tumor Fragments and Plasma
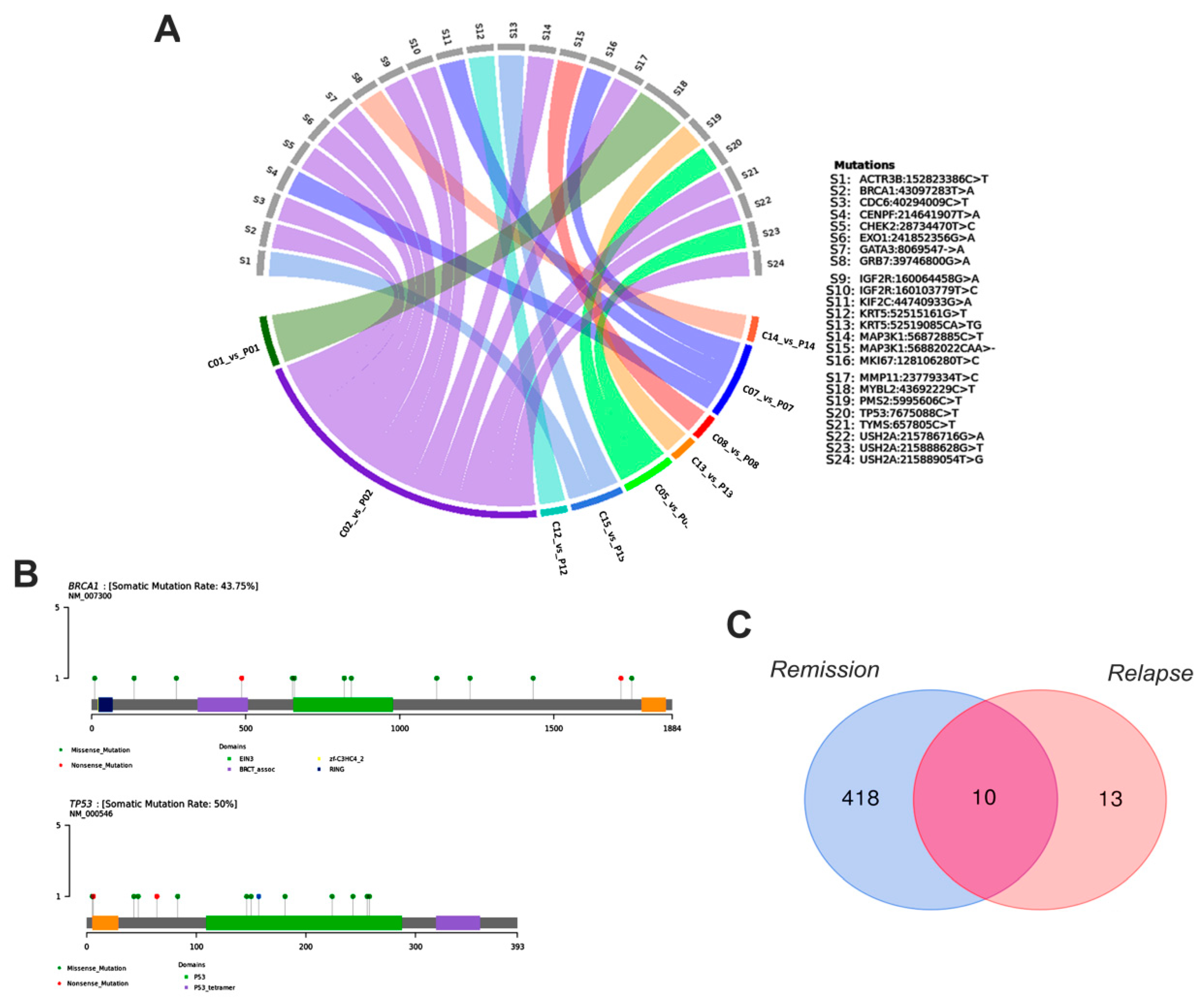
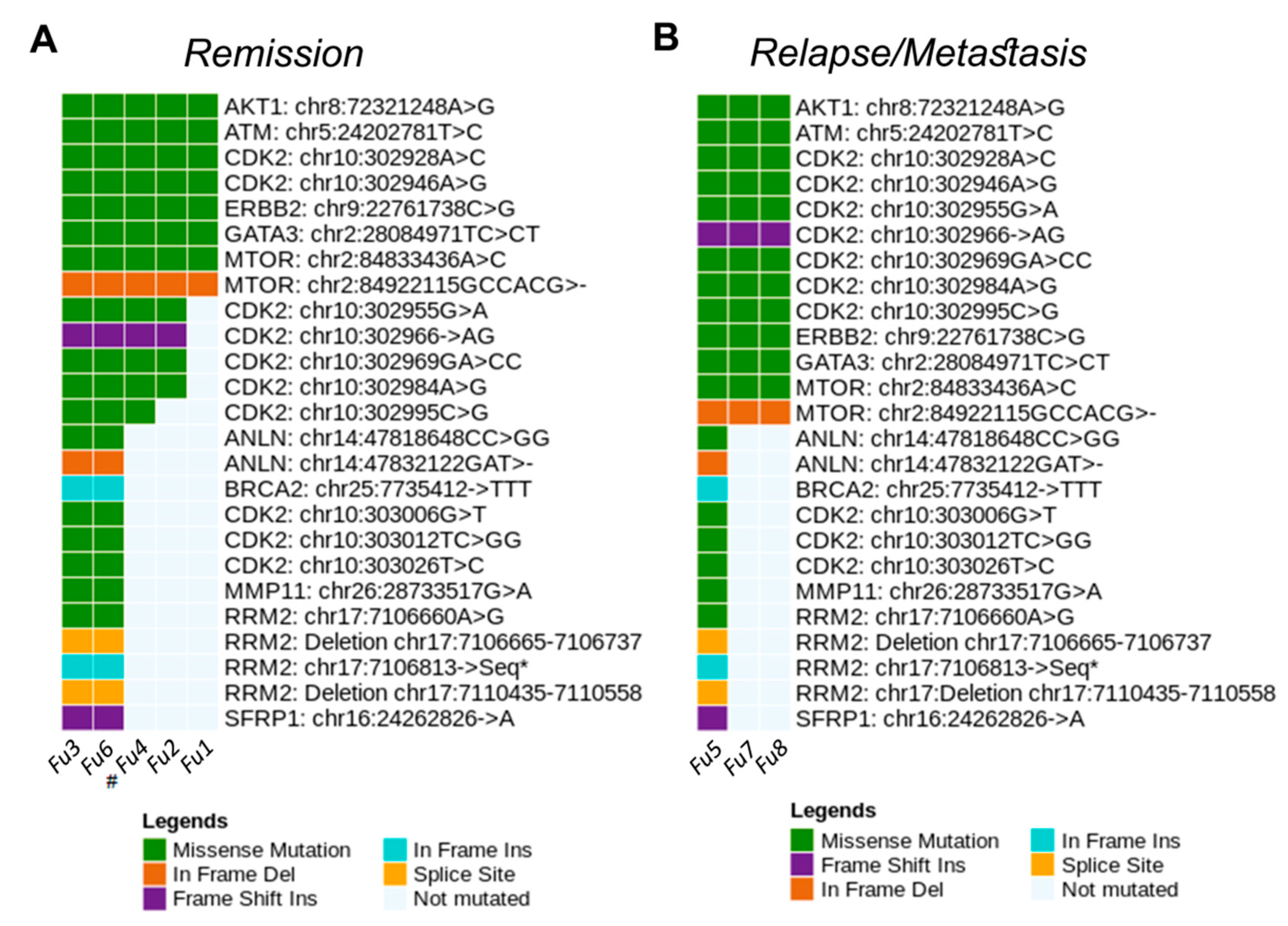
3.6. Main Variants Associated with Disease Progression Shared by Both Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelaleti, G.B.; Borin, T.F.; Maschio-Signorini, L.B.; Moschetta, M.G.; Hellmén, E.; Viloria-Petit, A.M.; Zuccari, D.A.P.C. Melatonin and IL-25 modulate apoptosis and angiogenesis mediators in metastatic (CF-41) and non-metastatic (CMT-U229) canine mammary tumour cells. Vet. Comp. Oncol. 2017, 15, 1572–1584. [Google Scholar] [CrossRef]
- Amirkhani Namagerdi, A.; d’Angelo, D.; Ciani, F.; Iannuzzi, C.A.; Napolitano, F.; Avallone, L.; De Laurentiis, M.; Giordano, A. Triple-Negative Breast Cancer Comparison With Canine Mammary Tumors From Light Microscopy to Molecular Pathology. Front. Oncol. 2020, 10, 2499. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Prim. 2019, 5, 66. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Quezada, S.A.; Larkin, J.; Swanton, C. Translational implications of tumor heterogeneity. Clin. Cancer Res. 2015, 21, 1258–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherdyntseva, N.; Litviakov, N.; Denisov, E.; Gervas, P.; Cherdyntsev, E. Circulating tumor cells in breast cancer: Functional heterogeneity, pathogenetic and clinical aspects. Exp. Oncol. 2017, 39, 2–11. [Google Scholar] [CrossRef]
- Palmero, E.I.; Carraro, D.M.; Alemar, B.; Moreira, M.A.M.; Ribeiro-Dos-Santos, Â.; Abe-Sandes, K.; Galvão, H.C.R.; Reis, R.M.; De Pádua Souza, C.; Campacci, N.; et al. The germline mutational landscape of BRCA1 and BRCA2 in Brazil. Sci. Rep. 2018, 8, 9188. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Shao, D.; Wu, H.; Zhu, C.; Guo, D.; Zhou, Y.; Chen, C.; Lin, Y.; Lu, T.; Zhao, B.; et al. Genomic Profiling Comparison of Germline BRCA and Non-BRCA Carriers Reveals CCNE1 Amplification as a Risk Factor for Non-BRCA Carriers in Patients With Triple-Negative Breast Cancer. Front. Oncol. 2020, 10, 2168. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Bottai, G.; Kelly, C.M.; Győrffy, B.; Székely, B.; Pusztai, L. Deciphering and Targeting Oncogenic Mutations and Pathways in Breast Cancer. Oncologist 2016, 21, 1063–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Redig, A.J.; Jänne, P.A. Basket trials and the evolution of clinical trial design in an era of genomic medicine. J. Clin. Oncol. 2015, 33, 975–977. [Google Scholar] [CrossRef] [Green Version]
- O’Leary, E.; Iacoboni, D.; Holle, J.; Michalski, S.T.; Esplin, E.D.; Yang, S.; Ouyang, K. Expanded Gene Panel Use for Women With Breast Cancer: Identification and Intervention Beyond Breast Cancer Risk. Ann. Surg. Oncol. 2017, 24, 3060–3066. [Google Scholar] [CrossRef] [Green Version]
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer genome landscapes. Science 2013, 340, 1546–1558. [Google Scholar] [CrossRef]
- de Figueiredo Barros, B.D.; Kupper, B.E.; Aguiar Junior, S.; de Mello, C.A.; Begnami, M.D.; Chojniak, R.; de Souza, S.J.; Torrezan, G.T.; Carraro, D.M. Mutation detection in tumor-derived cell free DNA anticipates progression in a patient with metastatic colorectal cancer. Front. Oncol. 2018, 8, 306. [Google Scholar] [CrossRef]
- Forbes, S.A.; Beare, D.; Gunasekaran, P.; Leung, K.; Bindal, N.; Boutselakis, H.; Ding, M.; Bamford, S.; Cole, C.; Ward, S.; et al. COSMIC: Exploring the world’s knowledge of somatic mutations in human cancer. Nucleic Acids Res. 2015, 43, D805–D811. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Law, S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int. J. Biochem. Cell Biol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Atashzar, M.R.; Baharlou, R.; Karami, J.; Abdollahi, H.; Rezaei, R.; Pourramezan, F.; Zoljalali Moghaddam, S.H. Cancer stem cells: A review from origin to therapeutic implications. J. Cell. Physiol. 2020, 235, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Olsson, E.; Winter, C.; George, A.; Chen, Y.; Howlin, J.; Tang, M.E.; Dahlgren, M.; Schulz, R.; Grabau, D.; Westen, D.; et al. Serial monitoring of circulating tumor DNA in patients with primary breast cancer for detection of occult metastatic disease. EMBO Mol. Med. 2015, 7, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Franken, A.; Honisch, E.; Reinhardt, F.; Meier-Stiegen, F.; Yang, L.; Jaschinski, S.; Esposito, I.; Alberter, B.; Polzer, B.; Huebner, H.; et al. Detection of ESR1 Mutations in Single Circulating Tumor Cells on Estrogen Deprivation Therapy but Not in Primary Tumors from Metastatic Luminal Breast Cancer Patients. J. Mol. Diagnost. 2020, 22, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Maltoni, R.; Casadio, V.; Ravaioli, S.; Foca, F.; Tumedei, M.M.; Salvi, S.; Martignano, F.; Calistri, D.; Rocca, A.; Schirone, A.; et al. Cell-free DNA detected by “liquid biopsy” as a potential prognostic biomarker in early breast cancer. Oncotarget 2017, 8, 16642–16649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otandault, A.; Anker, P.; Al Amir Dache, Z.; Guillaumon, V.; Meddeb, R.; Pastor, B.; Pisareva, E.; Sanchez, C.; Tanos, R.; Tousch, G.; et al. Recent advances in circulating nucleic acids in oncology. Ann. Oncol. 2019, 30, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.K. Liquid Biopsy in Breast Cancer: Circulating Tumor Cells and Circulating Tumor DNA. Adv. Exp. Med. Bio. 2021, 1187, 337–361. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Yost, S.E.; Yuan, Y.C.; Solomon, N.M.; Mambetsariev, I.; Pal, S.; Frankel, P.; Salgia, R.; Neuhausen, S.L.; Mortimer, J. Genomic mutation-driven metastatic breast cancer therapy: A single center experience. Oncotarget 2017, 8, 26414–26423. [Google Scholar] [CrossRef] [Green Version]
- Rolfo, C.; Manca, P.; Salgado, R.; Van Dam, P.; Dendooven, A.; MacHado Coelho, A.; Ferri Gandia, J.; Rutten, A.; Lybaert, W.; Vermeij, J.; et al. Multidisciplinary molecular tumour board: A tool to improve clinical practice and selection accrual for clinical trials in patients with cancer. ESMO Open 2018, 3, e000398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaszak, I.; Ruszczak, A.; Kanafa, S.; Kacprzak, K.; Król, M.; Jurka, P. Current biomarkers of canine mammary tumors. Acta Vet. Scand. 2018, 60, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorenmo, K.U.; Rasotto, R.; Zappulli, V.; Goldschmidt, M.H. Development, anatomy, histology, lymphatic drainage, clinical features, and cell differentiation markers of canine mammary gland neoplasms. Vet. Pathol. 2011, 48, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Zhao, H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Lee, S.J.; Choi, S.H.; Ahn, S.H.; Son, B.H.; Lee, J.W.; Yu, J.H.; Kwon, N.J.; Lee, W.C.; Yang, K.S.; et al. Cancer panel analysis of circulating tumor cells in patients with breast cancer. Oncol. Lett. 2018, 16, 612–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.; Zou, J.; Chen, Q.; Zhu, T.; Sui, R.; Yang, J. Structural modeling, mutation analysis, and in vitro expression of usherin, a major protein in inherited retinal degeneration and hearing loss. Comput. Struct. Biotechnol. J. 2020, 18, 1363–1382. [Google Scholar] [CrossRef]
- Toualbi, L.; Toms, M.; Moosajee, M. USH2A-retinopathy: From genetics to therapeutics. Exp. Eye Res. 2020, 201, 108330. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Makunin, I.; Thompson, B.A.; Tao, K.; Young, E.L.; Lopez, J.; Camp, N.J.; Tavtigian, S.V.; John, E.M.; et al. Panel sequencing of 264 candidate susceptibility genes and segregation analysis in a cohort of non-BRCA1, non-BRCA2 breast cancer families. Breast Cancer Res. Treat. 2017, 166, 937–949. [Google Scholar] [CrossRef]
- Natrajan, R.; Wilkerson, P.M.; Marchiò, C.; Piscuoglio, S.; Ng, C.K.Y.; Wai, P.; Lambros, M.B.; Samartzis, E.P.; Dedes, K.J.; Frankum, J.; et al. Characterization of the genomic features and expressed fusion genes in micropapillary carcinomas of the breast. J. Pathol. 2014, 232, 553–565. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.H.; Ahn, S.; Suh, K.J.; Kim, Y.J.; Park, S.Y.; Kang, E.; Kim, E.K.; Kim, I.A.; Chae, S.; Choi, M.; et al. Identifying germline APOBEC3B deletion and immune phenotype in Korean patients with operable breast cancer. Breast Cancer Res. Treat. 2020, 183, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, F. LncRNA AC009022.1 enhances colorectal cancer cells proliferation, migration, and invasion by promoting ACTR3B expression via suppressing miR-497-5p. J. Cell. Biochem. 2020, 121, 1934–1944. [Google Scholar] [CrossRef]
- Korobeynikov, V.; Borakove, M.; Feng, Y.; Wuest, W.M.; Koval, A.B.; Nikonova, A.S.; Serebriiskii, I.; Chernoff, J.; Borges, V.F.; Golemis, E.A.; et al. Combined inhibition of Aurora A and p21-activated kinase 1 as a new treatment strategy in breast cancer. Breast Cancer Res. Treat. 2019, 177, 369–382. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.M.; Yost, S.E.; Hutchinson, K.E.; Li, S.M.; Yuan, Y.C.; Noorbakhsh, J.; Liu, Z.; Warden, C.; Johnson, R.M.; Wu, X.; et al. CCNE1 amplification is associated with poor prognosis in patients with triple negative breast cancer. BMC Cancer 2019, 19, 96. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Huang, W.; Yang, Y.; Qiu, R.; Zeng, Y.; Hou, Y.; Sun, G.; Shi, H.; Leng, S.; Feng, D.; et al. GATA3 recruits UTX for gene transcriptional activation to suppress metastasis of breast cancer. Cell Death Dis. 2019, 10, 832. [Google Scholar] [CrossRef] [PubMed]
- Takaku, M.; Grimm, S.A.; Wade, P.A. GATA3 in breast cancer: Tumor suppressor or oncogene? Gene Expr. 2015, 16, 163–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Amico, P.; Corvaja, C.; Gerratana, L.; Reduzzi, C.; Curigliano, G.; Cristofanilli, M. The use of liquid biopsy in early breast cancer: Clinical evidence and future perspectives. J. Cancer Metastasis Treat. 2021, 7, 1–16. [Google Scholar] [CrossRef]
- Ellis, H.; Ma, C.X. PI3K Inhibitors in Breast Cancer Therapy. Curr. Oncol. Rep. 2019, 21, 110. [Google Scholar] [CrossRef]
- Verret, B.; Cortes, J.; Bachelot, T.; Andre, F.; Arnedos, M. Efficacy of PI3K inhibitors in advanced breast cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, x12–x20. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Hwang, H.J.; Noh, H.J.; Shin, T.J.; Cho, J.Y. Somatic mutation of PIK3ca (H1047R) is a common driver mutation hotspot in canine mammary tumors as well as human breast cancers. Cancers 2019, 11, 2006. [Google Scholar] [CrossRef] [Green Version]
- Sobhani, N.; Roviello, G.; Corona, S.P.; Scaltriti, M.; Ianza, A.; Bortul, M.; Zanconati, F.; Generali, D. The prognostic value of PI3K mutational status in breast cancer: A meta-analysis. J. Cell. Biochem. 2018, 119, 4287–4292. [Google Scholar] [CrossRef]
- Kim, J.H. PIK3CA mutations matter for cancer in dogs. Res. Vet. Sci. 2020, 133, 39–41. [Google Scholar] [CrossRef] [PubMed]
- Alsaihati, B.A.; Ho, K.-L.; Watson, J.; Feng, Y.; Wang, T.; Zhao, S. Canine tumor mutation rate is positively correlated with TP53 mutation across cancer types and breeds. Biorxiv 2020, 7, 1–23. [Google Scholar] [CrossRef]
- Maués, T.; El-Jaick, K.B.; Costa, F.B.; Araujo, G.E.F.; Soares, M.V.G.; Moreira, A.S.; Ferreira, M.L.G.; Ferreira, A.M.R. Common germline haplotypes and genotypes identified in BRCA2 exon 11 of dogs with mammary tumours and histopathological analyses. Vet. Comp. Oncol. 2018, 16, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.R.; Colombo, J.; Gonçalves, F.M.; Carvalho, L.A.L.; Costa, D.S.; Henrique, T.; Novais, A.A.; Moscheta-Pinheiro, M.G.; Chuffa, L.G.A.; Coutinho, L.L.; et al. Liquid biopsy can detect BRCA2 gene variants in female dogs with mammary neoplasia short title: Liquid biopsy detects malignant gene variants. Vet. Comp. Oncol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, Y.; Morimatsu, M.; Ochiai, K.; Nagano, M.; Tomioka, Y.; Sasaki, N.; Hashizume, K.; Iwanaga, T. Novel variations and loss of heterozygosity of BRCA2 identified in a dog with mammary tumors. Am. J. Vet. Res. 2008, 69, 1323–1328. [Google Scholar] [CrossRef]
- Yoshikawa, Y.; Morimatsu, M.; Ochiai, K.; Ishiguro-Oonuma, T.; Wada, S.; Orino, K.; Watanabe, K. Reduced canine BRCA2 expression levels in mammary gland tumors. BMC Vet. Res. 2015, 11, 159. [Google Scholar] [CrossRef] [Green Version]
- Huskey, A.L.W.; Goebel, K.; Lloveras-Fuentes, C.; McNeely, I.; Merner, N.D. Whole genome sequencing for the investigation of canine mammary tumor inheritance—An initial assessment of high-risk breast cancer genes reveal BRCA2 and STK11 variants potentially associated with risk in purebred dogs. Canine Med. Genet. 2020, 7, 8. [Google Scholar] [CrossRef]
- Amoroso, V.; Generali, D.; Buchholz, T.; Cristofanilli, M.; Pedersini, R.; Curigliano, G.; Daidone, M.G.; Di Cosimo, S.; Dowsett, M.; Fox, S.; et al. International expert consensus on primary systemic therapy in the management of early breast cancer: Highlights of the Fifth Symposium on Primary Systemic Therapy in the Management of Operable Breast Cancer, Cremona, Italy (2013). J. Natl. Cancer Inst. -Monogr. 2015, 2015, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Poulet, G.; Massias, J.; Taly, V. Liquid Biopsy: General Concepts. Acta Cytol. 2019, 63, 449–455. [Google Scholar] [CrossRef] [PubMed]




| Core | Molecular | ||||||
|---|---|---|---|---|---|---|---|
| Plasma | Biopsy | Age | Pathology | TNM | Subtype | Immunohistochemistry | |
| P01 | C01 | 60 | Invasive ductal carcinoma | T2N0M0 | Luminal B | C.D.I Grade 2 (ER+/PR+/HER2−/Ki-67+ (20–25%)/E-cadherin+/Cytokeratin 5/6)− | |
| P02 | C02 | 57 | Invasive ductal carcinoma | TNM1 | Luminal Hybrid | C.D.I. Grade 2 (ER+/PR−/HER2+/Ki-67+ (40%)/E- cadherin+/Cytokeratin 5/6−) | |
| P03 | C03 | 42 | Invasive ductal carcinoma | T2N0M0 | Luminal Hybrid | C.D.I. Grade 2 (ER+/PR+/HER2+/K-67+ (25%)/E- cadherin+/Cytokeratin 5/6−) | |
| P04 | C04 | 44 | Invasive ductal carcinoma | T2N0M0 | Luminal B | C.D.I. Grade 2 (ER+/PR+/HER− /KI-67+(90%)/E- cadherin+/Cytokeratin 5/6−) | |
| P05 | C05 | 43 | Invasive ductal carcinoma | T4bN3M0 | Triple Negative | C.D.I. Grade 2 (ER−/PR−/Her2− /Ki-67+(95%)/E- cadherin+/Cytokeratin 5/6+) | |
| P06 | C06 | 61 | Invasive ductal carcinoma | T2N0M0 | Luminal B | C.D.I. Grade 2 (ER+/PR+/HER2+/KI-67+/E- cadherin+/Cytokeratin 5/6−) | |
| P07 | C07 | 61 | Invasive ductal carcinoma | T3N3M0 | Triple Negative | C.D.I. Grade 2 (ER−/PR−/Her2−/Ki-67+(70%)/E- cadherin+/Cytokeratin 5/6+) | |
| P08 | C08 | 88 | Invasive ductal carcinoma | T4N1MX | Triple Negative | C.D.I. Grade 2 (ER-/PR-/HER2-/KI-67+(40%)/E- cadherin+/Cytokeratin 5/6+) | |
| P09 | C09 | 53 | Invasive ductal carcinoma | T4N1M 0-IIIB | Triple Negative | C.D.I. Grade 2 (ER−/PR−/HER2−/Ki-67+(50%)/ E-cadherin +/Cytokeratin5/6−) | |
| P10 | C10 | 42 | Invasive ductal carcinoma | T2N0M0 | Luminal B | C.D.I. Grade 2 (ER+/PR+/HER+/Ki-67+(50%)/ E-cadherin+/Cytokeratin 5/6+) | |
| P11 | C11 | 62 | Invasive ductal carcinoma | T4N3 M0-IIIC | Luminal B | C.D.I. Grade 2 (ER+/PR+ /HER2−Ki-67+(40%)/E- cadherin+/Cytokeratin 5/6−) | |
| P12 | C12 | 46 | Fibroadenoma | Not applicable | Not applicable | C.DI. Grade 2 ER+/Ki67+ (50% stroma and 50% epithelium) | |
| P13 | C13 | 68 | Invasive ductal carcinoma | T2N0M0 | Luminal A | C.D.I. Grade 1 (ER+/PR+/Her2−/Ki- 67+(<10%)/E- cadherin+/Cytokeratin 5/6−) | |
| P14 | C14 | 90 | Invasive ductal carcinoma | T3N1M0 | Luminal A | C.D.I. Grade 1 (RE+/RP+/HER2−/Ki-67+(10%)/ E-cadherin+/Cytokeratin 5/6−) | |
| P15 | C15 | 51 | Invasive ductal carcinoma | T3N1M0 | Triple Negative | C.D.I. Grade 1 (RE−/RP−/HER2−/Ki-67+(25%)/E- cadherin+/Cytokeratin 5/6−) | |
| P16 | C16 | 63 | Invasive ductal carcinoma | T4BN1M0 | Triple Negative | C.D.I. Grade 1 (RE−/RP−/HER2−/Ki-67+(>30%)/E- cadherin+/Cytokeratin 5/6−) |
| Molecular | |||||
|---|---|---|---|---|---|
| Plasma | Age | Pathology | TNM | Subtype | Immunohistochemistry |
| Rec1 | 60 | Invasive Breast carcinoma | T2N2MX | Luminal B | C.D.I. Grade 2 (ER+/PR+/HER2−/Ki-67+(40- 50%)/E- cadherin+/Cytokeratin 5/6−) |
| Rec2 | 47 | Invasive ductal carcinoma | T4N0M 0 | Luminal B | C.D.I. Grade 2 (ER+/PR−/HER2−/ Ki-67+(70%)/E-cadherin+/Cytokeratin 5/6−) |
| Rec3 | 51 | Invasive ductal carcinoma | T3N1M0 | Triple Negative | C.D.I. Grade 1 (RE−/RP−/HER2−/Ki-67+(25%)/E- cadherin+/Cytokeratin 5/6−) |
| Rec4 | 51 | Metastatic ductal breast cancer | T2N1Mx | HER2 positive | C.D.I. Grade 2 (ER+/PR+/HER2+/Ki-67+(40- 50%)/E- cadherin+/Cytokeratin 5/6−) |
| Plasma | Age | Remission | Treatment |
|---|---|---|---|
| Rem1 | 83 | 6 years + | Chemotherapy + Radiotherapy |
| Rem2 | 58 | 5 years + | Chemotherapy + Radiotherapy |
| Rem3 | 55 | 5 years + | Chemotherapy |
| Rem4 | 46 | 5 years + | Mastectomy |
| Rem5 | 47 | 5 years + | Chemotherapy |
| Rem6 | 47 | 5 years + | Hormone therapy |
| Rem7 | 65 | 3 years + | Radiotherapy + Hormone therapy |
| Rem8 | 63 | 5 years + | Radiotherapy |
| Rem9 | 53 | 4 months + | Chemotherapy + Radiotherapy |
| Rem10 | 74 | 5 years + | Chemotherapy + Radiotherapy + Mastectomy |
| Rem11 | 79 | 3 years + | Chemotherapy |
| Plasma | Age | Information |
|---|---|---|
| Control1 | 63 | Healthy women without a family history of breast cancer and without any other disease |
| Control2 | 78 | |
| Control3 | 41 | |
| Control4 | 70 | |
| Control5 | 56 | |
| Control6 | 73 | |
| Control7 | 70 | |
| Control8 | 62 | |
| Control9 | 32 | |
| Control10 | 50 |
| Plasma | Age (years) | TNM | Pathology | Castrated | Breed | |
|---|---|---|---|---|---|---|
| F1 | P1c | 10 | T2N0M0 | Mixed Carcinoma, grade I | Yes | MBD |
| F2 | P2c | 15 | T2N0M1 | Osteosarcoma | Yes | Pekinese |
| F3 | P3c | 7 | T2N0M0 | Complex carcinoma, grade II | No | MBD |
| F4 | P4c | 10 | T1N0M0 | Complex adenoma | No | MBD |
| F5 | P5c | 10 | T1N0M0 | Osteoma | No | Poodle |
| F6 | P6c | 7 | T3N0M0 | Mast cell tumor | Yes | Beagle |
| F7 | P7c | 12 | T3N0M0 | Ductal apocrine carcinoma | No | Dachshund |
| F8 | P8c | 16 | T2N0M0 | Mixed carcinoma, grade I | Yes | MBD |
| F9 | P9c | 13 | T1N1M0 | Tubular carcinoma, grade I | Yes | Yorkshire |
| F10 | P10c | 16 | T3N0M0 | Mixed carcinoma, grade I | Yes | MBD |
| F11 | P11c | 13 | T2N0M0 | Complex carcinoma, grade II | Yes | Belgian Shepherd |
| Plasma | Age (years) | TNM | Pathology | Castrated | Breed |
|---|---|---|---|---|---|
| Fu1 | 13 | * | * | Yes | Poodle |
| Fu2 | 12 | * | * | No | Maltese |
| Fu3 | 10 | T2N0M1 | Papillary carcinoma, grade II | Yes | Labrador |
| Fu4 | 10 | T2N1M1 | Tubular carcinoma, grade III | Yes | SRD |
| Fu5 | 8 | T2N1M0 | Carcinosarcoma | Yes | Beagle |
| Fu6 | 9 | T3N1M0 | Mixed carcinoma, grade II | Yes | Poodle |
| Fu7 | 13 | T3N0M1 | Osteosarcoma | Yes | Cocker sp |
| Fu8 | 10 | T2N2M1 | Complex Carcinoma, grade I | No | MDB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colombo, J.; Moschetta-Pinheiro, M.G.; Novais, A.A.; Stoppe, B.R.; Bonini, E.D.; Gonçalves, F.M.; Fukumasu, H.; Coutinho, L.L.; Chuffa, L.G.d.A.; Zuccari, D.A.P.d.C. Liquid Biopsy as a Diagnostic and Prognostic Tool for Women and Female Dogs with Breast Cancer. Cancers 2021, 13, 5233. https://doi.org/10.3390/cancers13205233
Colombo J, Moschetta-Pinheiro MG, Novais AA, Stoppe BR, Bonini ED, Gonçalves FM, Fukumasu H, Coutinho LL, Chuffa LGdA, Zuccari DAPdC. Liquid Biopsy as a Diagnostic and Prognostic Tool for Women and Female Dogs with Breast Cancer. Cancers. 2021; 13(20):5233. https://doi.org/10.3390/cancers13205233
Chicago/Turabian StyleColombo, Jucimara, Marina Gobbe Moschetta-Pinheiro, Adriana Alonso Novais, Bruna Ribeiro Stoppe, Enrico Dumbra Bonini, Francine Moraes Gonçalves, Heidge Fukumasu, Luiz Lehmann Coutinho, Luiz Gustavo de Almeida Chuffa, and Debora Aparecida Pires de Campos Zuccari. 2021. "Liquid Biopsy as a Diagnostic and Prognostic Tool for Women and Female Dogs with Breast Cancer" Cancers 13, no. 20: 5233. https://doi.org/10.3390/cancers13205233
APA StyleColombo, J., Moschetta-Pinheiro, M. G., Novais, A. A., Stoppe, B. R., Bonini, E. D., Gonçalves, F. M., Fukumasu, H., Coutinho, L. L., Chuffa, L. G. d. A., & Zuccari, D. A. P. d. C. (2021). Liquid Biopsy as a Diagnostic and Prognostic Tool for Women and Female Dogs with Breast Cancer. Cancers, 13(20), 5233. https://doi.org/10.3390/cancers13205233