Surgical Treatment of Cerebellar Metastases: Survival Benefits, Complications and Timing Issues
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Surgical Indications
2.3. Clinical and Radiological Data, Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Hydrocephalus Management and Surgical Treatment; Postoperative Radio- and Chemotherapy
3.3. Complications and Functional Outcomes
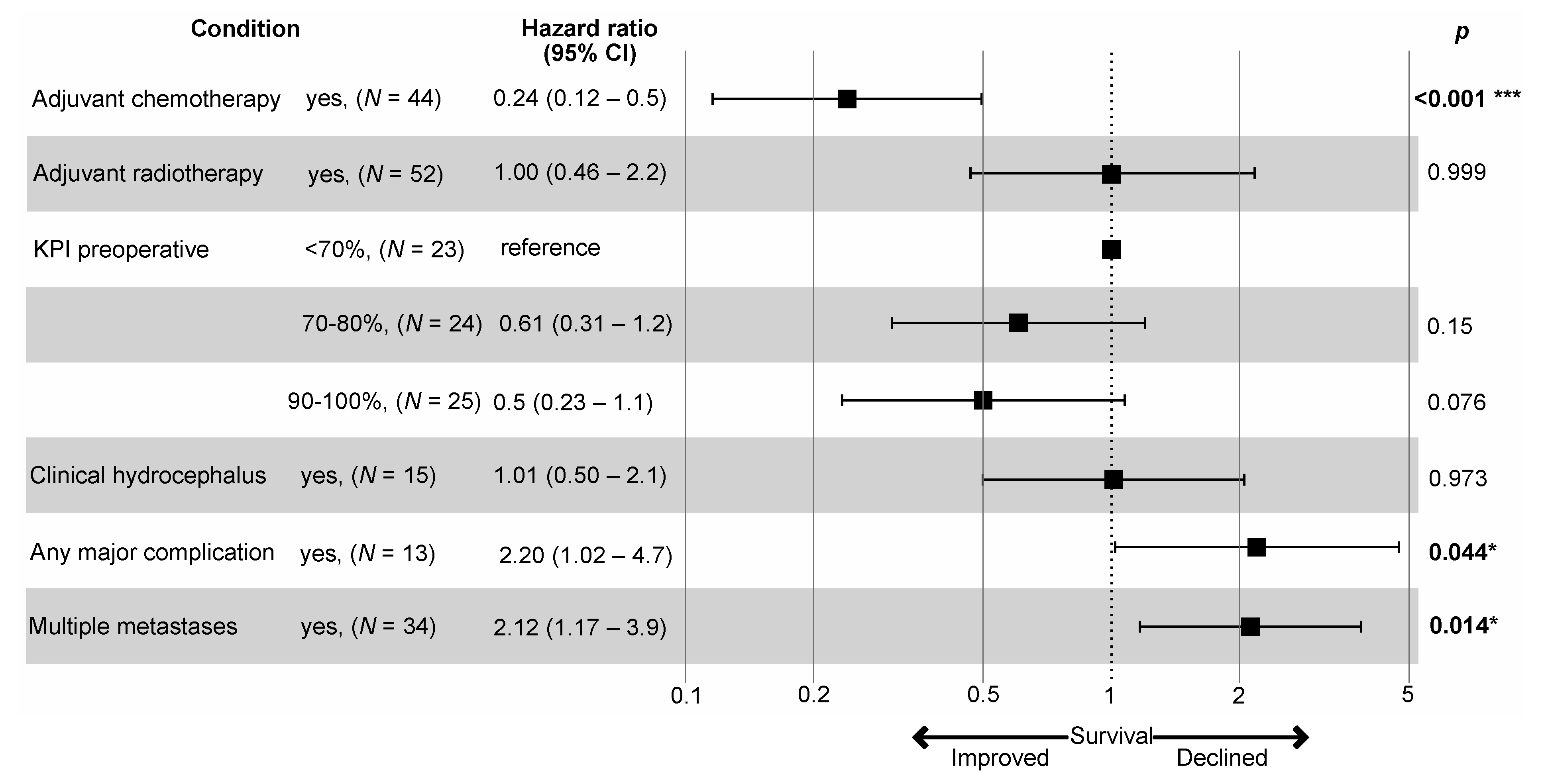
3.4. Patient Survival
3.5. Pre- vs. Postoperative Staging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Proescholdt, M.A.; Schödel, P.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.O.; Schebesch, K.-M. The Management of Brain Metastases-Systematic Review of Neurosurgical Aspects. Cancers 2021, 13, 1616. [Google Scholar] [CrossRef]
- Suh, J.H.; Kotecha, R.; Chao, S.T.; Ahluwalia, M.S.; Sahgal, A.; Chang, E.L. Current Approaches to the Management of Brain Metastases. Nat. Rev. Clin. Oncol. 2020, 17, 279–299. [Google Scholar] [CrossRef]
- Schackert, G.; Lindner, C.; Petschke, S.; Leimert, M.; Kirsch, M. Retrospective Study of 127 Surgically Treated Patients with Multiple Brain Metastases: Indication, Prognostic Factors, and Outcome. Acta Neurochir. (Wien) 2013, 155, 379–387. [Google Scholar] [CrossRef]
- Salvati, M.; Tropeano, M.P.; Maiola, V.; Lavalle, L.; Brogna, C.; Colonnese, C.; Frati, A.; D’Elia, A. Multiple Brain Metastases: A Surgical Series and Neurosurgical Perspective. Neurol. Sci. 2018, 39, 671–677. [Google Scholar] [CrossRef]
- Smith, T.R.; Lall, R.R.; Lall, R.R.; Abecassis, I.J.; Arnaout, O.M.; Marymont, M.H.; Swanson, K.R.; Chandler, J.P. Survival after Surgery and Stereotactic Radiosurgery for Patients with Multiple Intracranial Metastases: Results of a Single-Center Retrospective Study. J. Neurosurg. 2014, 121, 839–845. [Google Scholar] [CrossRef]
- Nahed, B.V.; Alvarez-Breckenridge, C.; Brastianos, P.K.; Shih, H.; Sloan, A.; Ammirati, M.; Kuo, J.S.; Ryken, T.C.; Kalkanis, S.N.; Olson, J.J. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on the Role of Surgery in the Management of Adults with Metastatic Brain Tumors. Neurosurgery 2019, 84, E152–E155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulsbergen, A.F.C.; Claes, A.; Kavouridis, V.K.; Ansaripour, A.; Nogarede, C.; Hughes, M.E.; Smith, T.R.; Brastianos, P.K.; Verhoeff, J.J.C.; Lin, N.U.; et al. Subtype Switching in Breast Cancer Brain Metastases: A Multicenter Analysis. Neuro Oncol. 2020, 22, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Priedigkeit, N.; Hartmaier, R.J.; Chen, Y.; Vareslija, D.; Basudan, A.; Watters, R.J.; Thomas, R.; Leone, J.P.; Lucas, P.C.; Bhargava, R.; et al. Intrinsic Subtype Switching and Acquired ERBB2/HER2 Amplifications and Mutations in Breast Cancer Brain Metastases. JAMA Oncol. 2017, 3, 666–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roux, A.; Botella, C.; Still, M.; Zanello, M.; Dhermain, F.; Metellus, P.; Pallud, J. Posterior Fossa Metastasis-Associated Obstructive Hydrocephalus in Adult Patients: Literature Review and Practical Considerations from the Neuro-Oncology Club of the French Society of Neurosurgery. World Neurosurg. 2018, 117, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, G.J.; Jenkinson, M.D.; Zakaria, R. Surgical Management of Posterior Fossa Metastases. J. Neurooncol. 2016, 130, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Calluaud, G.; Terrier, L.-M.; Mathon, B.; Destrieux, C.; Velut, S.; François, P.; Zemmoura, I.; Amelot, A. Peritumoral Edema/Tumor Volume Ratio: A Strong Survival Predictor for Posterior Fossa Metastases. Neurosurgery 2019, 85, 117–125. [Google Scholar] [CrossRef]
- Hadanny, A.; Rozovski, U.; Nossek, E.; Shapira, Y.; Strauss, I.; Kanner, A.A.; Sitt, R.; Ram, Z.; Shahar, T. Craniectomy Versus Craniotomy for Posterior Fossa Metastases: Complication Profile. World Neurosurg. 2016, 89, 193–198. [Google Scholar] [CrossRef]
- Cacho-Díaz, B.; Lorenzana-Mendoza, N.A.; Chávez-Hernandez, J.D.; González-Aguilar, A.; Reyes-Soto, G.; Herrera-Gómez, Á. Clinical Manifestations and Location of Brain Metastases as Prognostic Markers. Curr. Probl. Cancer 2019, 43, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, L.; Brown, D.; Lanzino, G.; Parney, I.F. Outcomes Following Cerebrospinal Fluid Shunting in High-Grade Glioma Patients. J. Neurosurg. 2018, 129, 984–996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosainey, S.A.M.; Hald, J.K.; Meling, T.R. Risk of Early Failure of VP Shunts Implanted for Hydrocephalus after Craniotomies for Brain Tumors in Adults. Neurosurg. Rev. 2021, 1–12. [Google Scholar] [CrossRef]
- Dubey, A.; Sung, W.-S.; Shaya, M.; Patwardhan, R.; Willis, B.; Smith, D.; Nanda, A. Complications of Posterior Cranial Fossa Surgery--an Institutional Experience of 500 Patients. Surg. Neurol. 2009, 72, 369–375. [Google Scholar] [CrossRef]
- Brell, M.; Ibáñez, J.; Caral, L.; Ferrer, E. Factors Influencing Surgical Complications of Intra-Axial Brain Tumours. Acta Neurochir. (Wien) 2000, 142, 739–750. [Google Scholar] [CrossRef]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.M.; Wildrick, D.M. Neurosurgical Outcomes in a Modern Series of 400 Craniotomies for Treatment of Parenchymal Tumors. Neurosurgery 1998, 42, 1044–1055, discussion 1055–1056. [Google Scholar] [CrossRef]
- Olesrud, I.C.; Schulz, M.K.; Marcovic, L.; Kristensen, B.W.; Pedersen, C.B.; Kristiansen, C.; Poulsen, F.R. Early Postoperative MRI after Resection of Brain Metastases-Complete Tumour Resection Associated with Prolonged Survival. Acta Neurochir. (Wien) 2019, 161, 555–565. [Google Scholar] [CrossRef]
- Jünger, S.T.; Pennig, L.; Schödel, P.; Goldbrunner, R.; Friker, L.; Kocher, M.; Proescholdt, M.; Grau, S. The Debatable Benefit of Gross-Total Resection of Brain Metastases in a Comprehensive Treatment Setting. Cancers 2021, 13, 1435. [Google Scholar] [CrossRef]
- Proescholdt, M.; Jünger, S.; Schödel, P.; Schebesch, K.-M.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.-O.; Kocher, M.; Schulz, H.; et al. Brain Metastases in Elderly Patients—The Role of Surgery in the Context of Systemic Treatment. Brain Sci. 2021, 11, 123. [Google Scholar] [CrossRef]
- Yasin, H.; Hoff, H.-J.; Blümcke, I.; Simon, M. Experience with 102 Frameless Stereotactic Biopsies Using the Neuromate Robotic Device. World Neurosurg. 2019, 123, e450–e456. [Google Scholar] [CrossRef]
- Sperduto, P.W.; Berkey, B.; Gaspar, L.E.; Mehta, M.; Curran, W. A New Prognostic Index and Comparison to Three Other Indices for Patients with Brain Metastases: An Analysis of 1,960 Patients in the RTOG Database. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 510–514. [Google Scholar] [CrossRef] [PubMed]
- Ersoy, T.F.; Ridwan, S.; Grote, A.; Coras, R.; Simon, M. Early Postoperative Seizures (EPS) in Patients Undergoing Brain Tumour Surgery. Sci. Rep. 2020, 10, 13674. [Google Scholar] [CrossRef] [PubMed]
- Chaichana, K.L.; Rao, K.; Gadkaree, S.; Dangelmajer, S.; Bettegowda, C.; Rigamonti, D.; Weingart, J.; Olivi, A.; Gallia, G.L.; Brem, H.; et al. Factors Associated with Survival and Recurrence for Patients Undergoing Surgery of Cerebellar Metastases. Neurol. Res. 2014, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Kancharla, P.; Ivanov, A.; Chan, S.; Ashamalla, H.; Huang, R.Y.; Yanagihara, T.K. The Effect of Brain Metastasis Location on Clinical Outcomes: A Review of the Literature. Neurooncol. Adv. 2019, 1, vdz017. [Google Scholar] [CrossRef]
- Pojskic, M.; Bopp, M.H.A.; Schymalla, M.; Nimsky, C.; Carl, B. Retrospective Study of 229 Surgically Treated Patients with Brain Metastases: Prognostic Factors, Outcome and Comparison of Recursive Partitioning Analysis and Diagnosis-Specific Graded Prognostic Assessment. Surg. Neurol. Int. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Loh, D.; Hogg, F.; Edwards, P.; MacColl, J.; Brogna, C.; Bhangoo, R.; Ashkan, K.; Vergani, F. Two-Year Experience of Multi-Disciplinary Team (MDT) Outcomes for Brain Metastases in a Tertiary Neuro-Oncology Centre. Br. J. Neurosurg. 2018, 32, 53–60. [Google Scholar] [CrossRef]
- Patel, A.J.; Suki, D.; Hatiboglu, M.A.; Rao, V.Y.; Fox, B.D.; Sawaya, R. Impact of Surgical Methodology on the Complication Rate and Functional Outcome of Patients with a Single Brain Metastasis. J. Neurosurg. 2015, 122, 1132–1143. [Google Scholar] [CrossRef] [Green Version]
- Theodosopoulos, P.V.; Ringer, A.J.; McPherson, C.M.; Warnick, R.E.; Kuntz, C.; Zuccarello, M.; Tew, J.M. Measuring Surgical Outcomes in Neurosurgery: Implementation, Analysis, and Auditing a Prospective Series of More than 5000 Procedures. J. Neurosurg. 2012, 117, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Landriel Ibañez, F.A.; Hem, S.; Ajler, P.; Vecchi, E.; Ciraolo, C.; Baccanelli, M.; Tramontano, R.; Knezevich, F.; Carrizo, A. A New Classification of Complications in Neurosurgery. World Neurosurg. 2011, 75, 709–715, discussion 604–611. [Google Scholar] [CrossRef] [PubMed]

| Neurological Deficit ≥ 30 Days | Surgical Complication | Medical Complication | ||||||
|---|---|---|---|---|---|---|---|---|
| n | Yes | No | Yes | No | Yes | No | ||
| Age | ≥60 yrs. (median) | 37 (49.3%) | 4 (10.9%) | 33 (89.1%) | 6 (16.3%) | 31 (83.7%) | 4 (10.9%) | 33 (89.1%) |
| <60 yrs. | 36 (50.7%) | 2 (5.6%) | 34 (94.4%) | 4 (11.2%) | 32 (88.8%) | 3 (8.4%) | 33 (91.6%) | |
| p = 0.413 | p = 0.525 | p = 0.719 | ||||||
| Sex | Female | 44 (60.3%) | 7 (15.9%) | 37 (84.1%) | 8 (18.2%) | 36 (81.8%) | 7 (15.9%) | 37 (84.1%) |
| Male | 29 (39.7%) | 2 (6.9%) | 27 (93.1%) | 5 (17.2%) | 24 (82.8%) | 1 (3.4%) | 28 (96.6%) | |
| p = 0.303 | p = 1.000 | p = 0.135 | ||||||
| Preoperative KPI | 90–100% | 25 (34.2%) | 1 (4.0%) | 24 (96.0%) | 4 (16.0%) | 21 (84%) | 1 (4.0%) | 24 (96.0%) |
| 70–80% | 25 (34.2%) | 2 (8.0%) | 23 (92.0%) | 2 (8.0%) | 23 (92.0%) | 1 (4.0%) | 24 (96.0%) | |
| <70% | 23 (31.5%) | 6 (26.1%) | 17 (73.9%) | 7 (30.4%) | 16 (69.6%) | 6 (26.1%) | 17 (73.9%) | |
| p = 0.022 | p = 0.208 | p = 0.026 | ||||||
| Clinical hydrocephalus | Yes | 16 (21.9%) | 4 (25.0%) | 12 (75.0%) | 4 (25.0%) | 12 (75.0%) | 3 (18.8%) | 13 (81.3%) |
| No | 57 (78.1%) | 5 (8.8%) | 52 (91.2%) | 9 (15.8%) | 48 (84.2%) | 5 (8.8%) | 52 (91.2%) | |
| p = 0.099 | p = 0.463 | p = 0.361 | ||||||
| Radiological hydrocephalus | Yes | 29 (39.7%) | 3 (10.3%) | 26 (89.7%) | 7 (24.2%) | 22 (75.8%) | 4 (13.8%) | 25 (86.2%) |
| No | 44 (60.3%) | 3 (6.8%) | 41 (93.2%) | 3 (6.8%) | 41 (93.2%) | 3 (6.8%) | 41 (93.2%) | |
| p = 0.591 | p = 0.035 | p = 0.322 | ||||||
| Cerebellar tumor location | Hemispheres only | 62 (84.9%) | 3 (4.8%) | 59 (95.2%) | 5 (8.1%) | 57 (91.9%) | 3 (4.8%) | 59 (95.2%) |
| Vermis involved | 11 (15.5%) | 3 (27.3%) | 8 (72.7%) | 5 (45.5%) | 6 (54.5%) | 4 (36.4%) | 7 (63.6%) | |
| p = 0.04 | p = 0.005 | p = 0.008 | ||||||
| Extent of CNS disease | Single CM | 39 (53.4%) | 4 (10.3%) | 35 (89.7%) | 6 (15.4%) | 33 (84.6%) | 2 (5.1%) | 37 (94.9%) |
| Multiple metastases | 34 (46.6%) | 5 (14.7%) | 29 (85.3%) | 7 (20.6%) | 27 (79.4%) | 6 (17.6%) | 28 (82.4%) | |
| p = 0.725 | p = 0.760 | p = 0.135 | ||||||
| Supratentorial disease: yes | 26 (35.6%) | 3 (11.5%) | 23 (88.5%) | 3 (11.5%) | 23 (88.5%) | 3 (11.5%) | 23 (88.5%) | |
| ~: no | 47 (64.4%) | 3 (6.4%) | 44 (93.6%) | 7 (14.9%) | 40 (85.1%) | 4 (8.5%) | 43 (91.5%) | |
| p = 0.659 | p = 1.000 | p = 0.694 | ||||||
| Degree of resection (index tumor) | Gross total | 68 (93.2%) | 5 (7.4%) | 63 (92.6%) | 9 (13.2%) | 59 (86.8%) | 6 (8.8%) | 62 (91.2%) |
| Subtotal | 5 (6.8%) | 1 (20.0%) | 4 (80.0%) | 1 (20.0%) | 4 (80.0%) | 1 (20.0%) | 4 (80.0%) | |
| p = 0.357 | p = 0.532 | p = 0.405 | ||||||
| Postoperative tumor | Yes | 24 (32.9%) | 4 (16.7%) | 20 (83.3%) | 4 (16.7%) | 20 (83.3%) | 4 (16.7%) | 20 (83.3%) |
| No | 49 (67.1%) | 2 (4.1%) | 47 (95.9%) | 6 (12.2%) | 43 (87.8%) | 3 (6.1%) | 46 (93.9%) | |
| p = 0.086 | p = 0.720 | p = 0.208 | ||||||
| Volumetry 1 | Volume index tumor(s) ≥14.2 cm3 (median) | 35 (50.0%) | 3 (8.6%) | 32 (91.4%) | 6 (17.1%) | 29 (82.9%) | 4 (11.4%) | 31 (88.6%) |
| <14.2 cm3 | 35 (50.0%) | 3 (8.6%) | 32 (91.4%) | 4 (11.4%) | 31 (88.6%) | 3 (8.6%) | 32 (91.4%) | |
| p = 1.000 | p = 0.734 | p = 1.000 | ||||||
| Cerebellar tumor load ≥14.8 cm3 (median) | 35 (50.0%) | 3 (8.6%) | 32 (91.4%) | 6 (17.1%) | 29 (82.9%) | 4 (11.4%) | 31 (88.6%) | |
| <14.8 cm3 | 35 (50.0%) | 3 (8.6%) | 32 (91.4%) | 4 (11.4%) | 31 (88.6%) | 3 (8.6%) | 32 (91.4%) | |
| p = 1.000 | p = 0.734 | p = 1.000 | ||||||
| Overall tumor load ≥15.3 cm3 (median) | 35 (50.0%) | 3 (8.6%) | 32 (91.4%) | 6 (17.1%) | 29 (82.9%) | 4 (11.4%) | 31 (88.6%) | |
| <15.3 cm3 | 35 (50.0%) | 3 (8.6%) | 32 (91.4%) | 4 (11.4%) | 31 (88.6%) | 3 (8.6%) | 32 (91.4%) | |
| p = 1.000 | p = 0.734 | p = 1.000 | ||||||
| Presentation | Synchronous | 19 (26.0%) | 1 (5.3%) | 18 (94.7%) | 1 (5.3%) | 18 (94.7%) | 1 (5.3%) | 18 (94.7%) |
| Metachronous | 54 (74.0%) | 5 (9.0%) | 49 (91.0%) | 10 (19.0%) | 44 (81.0%) | 7 (13.0%) | 47 (87.0%) | |
| p = 0.585 | p = 0.164 | p = 0.355 | ||||||
| Primary tumor site | Lung | 37 (50.7%) | 3 (8.1%) | 34 (91.9%) | 4 (10.8%) | 33 (89.2%) | 3 (8.1%) | 34 (91.9%) |
| Breast | 19 (26.0%) | 5 (26.3%) | 14 (73.7%) | 7 (36.8%) | 12 (63.2%) | 5 (26.3%) | 14 (73.7%) | |
| Gastrointestinal tract | 9 (12.3%) | 1 (5.9%) | 16 (94.1%) | 2 (11.8%) | 15 (88.2%) | 0 | 17 (100%) | |
| Renal | 3 (4.1%) | |||||||
| Melanoma | 2 (2.7%) | |||||||
| Other | 3 (4.1%) | |||||||
| p = 0.840 | p = 0.561 | p = 0.712 | ||||||
| Extracranial metastases 2 | Yes | 52 (72.2%) | 8 (15.4%) | 44 (84.6%) | 11 (21.2%) | 41 (78.8%) | 7 (13.5%) | 45 (86.5%) |
| No | 20 (27.8%) | 1 (5.0%) | 19 (95.0%) | 2 (10.0%) | 18 (90.0%) | 1 (5.0%) | 19 (95.0%) | |
| p = 0.429 | p = 0.330 | p = 0.429 | ||||||
| Radiotherapy | Yes | 53 (72.6%) | 1 (1.9%) | 52 (98.1%) | 4 (7.5%) | 49 (92.5%) | 1 (1.9%) | 52 (98.1%) |
| No | 20 (27.4%) | 5 (25.0%) | 15 (75.0%) | 6 (30.0%) | 14 (70.0%) | 6 (30.0%) | 14 (70.0%) | |
| p = 0.005 | p = 0.021 | p = 0.001 | ||||||
| Chemo-/systemic therapy 2 | Yes | 44 (61.1%) | 1 (2.3%) | 43 (97.7%) | 4 (9.1%) | 40 (90.9%) | 1 (2.3%) | 43 (97.7%) |
| No | 28 (38.9%) | 5 (17.9%) | 23 (82.1%) | 6 (21.4%) | 22 (78.6%) | 6 (21.4%) | 22 (78.6%) | |
| p = 0.03 | p = 0.172 | p = 0.012 | ||||||
| GPA score 2 | 0–1.0 | 20 (27.8%) | 6 (30.0%) | 14 (70.0%) | 6 (30.0%) | 14 (70.0%) | 5 (25.0%) | 15 (75.0%) |
| 1.5–2.5 | 39 (54.2%) | 2 (5.1%) | 37 (94.9%) | 5 (12.8%) | 34 (87.2%) | 2 (5.1%) | 37 (94.9%) | |
| 3.0 | 8 (11.1%) | 1 (7.7%) | 12 (92.3%) | 2 (15.4%) | 11 (84.6%) | 1 (7.7%) | 12 (92.3%) | |
| 3.5–4.0 | 5 (6.9%) | |||||||
| p = 0.029 | p = 0.214 | p = 0.073 | ||||||
| n | OS (Months) | 95%CI (Months) | p (Log Rank Test) | ||
|---|---|---|---|---|---|
| Age | ≥60 yrs. (median) | 37 (49.3%) | 7.9 | 6.2–9.7 | 0.727 |
| <60 yrs. | 36 (50.7%) | 10.7 | 3.9–17.5 | ||
| Sex | Female | 44 (60.3%) | 11.5 | 3.4–19.7 | 0.063 |
| Male | 29 (39.7%) | 8.1 | 4.3–11.8 | ||
| Preoperative KPI | 90–100% | 25 (34.2%) | 14.2 | 9.1–19.2 | 0.007 |
| 70–80% | 25 (34.2%) | 10.7 | 2.4–19.0 | ||
| <70% | 23 (31.5%) | 2.7 | 0.4–5.1 | ||
| Clinical hydrocephalus | Yes | 16 (21.9%) | 5.2 | 1.6–8.8 | 0.037 |
| No | 57 (78.1%) | 11.3 | 4.6–18.0 | ||
| Radiological hydrocephalus | Yes | 29 (39.7%) | 8.1 | 6.6–9.5 | 0.071 |
| No | 44 (60.3%) | 14.0 | 7.3–20.7 | ||
| Cerebellar tumor location | Hemispheres only | 62 (84.9%) | 9.2 | 5.6–12.8 | 0.988 |
| Vermis involved | 11 (15.5%) | 66.9 | 0.6–13.3 | ||
| Extent of CNS disease | Single CM | 39 (53.4%) | 14.0 | 9.4–18.6 | 0.047 |
| Multiple metastases | 34 (46.6%) | 7.3 | 3.5–11.1 | ||
| Supratentorial disease: yes | 26 (35.6%) | 7.3 | 2.0–12.6 | 0.095 | |
| ~: no | 47 (64.4%) | 11.5 | 5.3–17.7 | ||
| Degree of resection (index tumor/~s) | Gross total | 68 (93.2%) | 8.1 | 4.5–11.8 | 0.314 |
| Subtotal | 5 (6.8%) | 14.0 | 0–37.7 | ||
| Any postoperative CNS tumor | Yes | 24 (32.9%) | 7.3 | 0.5–14.1 | 0.136 |
| No | 49 (67.1%) | 11.3 | 4.8–17.7 | ||
| Volumetry 1 | Volume index tumor(s) ≥14.2 cm3 (median) | 35 (50.0%) | 11.3 | 2.8–19.8 | 0.983 |
| <14.2 cm3 | 35 (50.0%) | 9.2 | 6.0–12.4 | ||
| Cerebellar tumor load ≥14.8 cm3 (median) | 35 (50.0%) | 11.3 | 3.7–18.8 | 0.902 | |
| <14.8 cm3 | 35 (50.0%) | 7.9 | 4.5–11.3 | ||
| Overall tumor load ≥15.3 cm3 (median) | 35 (50.0%) | 11.5 | 3.2–19.7 | 0.558 | |
| <15.3 cm3 | 35 (50.0%) | 7.9 | 6.5–9.3 | ||
| Presentation | Synchronous | 19 (26.0%) | 7.4 | 2.2–12.5 | 0.419 |
| Metachronous | 54 (74.0%) | 9.7 | 6.3–13.1 | ||
| Primary tumor site | Lung | 37 (50.7%) | 7.4 | 3.0–11.7 | 0.088 |
| Breast | 19 (26.0%) | 6.9 | 0–14.0 | ||
| Gastrointestinal tract | 9 (12.3%) | 16.1 | 11.7–20.6 | ||
| Renal | 3 (4.1%) | ||||
| Melanoma | 2 (2.7%) | ||||
| Other | 3 (4.1%) | ||||
| Extracranial metastases 2 | Yes | 52 (72.2%) | 7.9 | 2.6–13.2 | 0.321 |
| No | 20 (27.8%) | 10.7 | 2.0–19.3 | ||
| Radiotherapy | Yes | 53 (72.6%) | 11.3 | 5.9–16.6 | 0.005 |
| No | 20 (27.4%) | 2.3 | 2.2–2.4 | ||
| Chemo-/systemic therapy 2 | Yes | 44 (61.1%) | 15.5 | 10.2–20.9 | <0.0001 |
| No | 28 (38.9%) | 3.2 | 1.2–5.2 | ||
| GPA score 2 | 0–1.0 | 20 (27.8%) | 2.3 | 0–5.4 | 0.059 |
| 1.5–2.5 | 39 (54.2%) | 9.2 | 5.2–13.1 | ||
| 3.0 | 8 (11.1%) | 15.3 | 5.9–24.6 | ||
| 3.5–4.0 | 5 (6.9%) | ||||
| Complications (CTCAE grades III–V) | Surgical: yes | 10 (13.7%) | 2.5 | 0–11.3 | 0.085 |
| ~: no | 63 (86.3%) | 10.7 | 3.2–18.1 | ||
| Neurological (≥30 days): yes | 6 (8.2%) | 1.1 | 0–3.1 | <0.0001 | |
| ~: no | 67 (91.8%) | 10.7 | 4.3–17.1 | ||
| Medical: yes | 7 (9.6%) | 1.1 | 0–2.6 | 0.002 | |
| ~: no | 66 (90.4%) | 10.7 | 4.4–16.9 | ||
| Any | 13 (17.8%) | 2.5 | 0–7.9 | 0.020 | |
| None | 60 (82.2%) | 10.7 | 3.7–17.6 |
| Complications | CTCAE | ||||
|---|---|---|---|---|---|
| III | IV | V | III–V | ||
| Neurological 1 | Confusion | 2 | 2 | ||
| Depressed level of consciousness | 2 | 2 | |||
| Dizziness | 2 | 2 | |||
| Dysphagia | 2 | 2 | |||
| Patients | 6 | ||||
| Surgical | Intracranial hemorrhage | 1 | 4 | 5 | |
| Hydrocephalus | 1 | 9 | 10 | ||
| Meningitis | 1 | 1 | 2 | ||
| Wound infection | 1 | 1 | |||
| Pneumocephalus | 1 | 1 | |||
| Patients | 10 | ||||
| Medical | Acute kidney injury | 1 | 1 | ||
| Anemia | 1 | 1 | |||
| Asystole | 1 | 1 | |||
| Atelectasis | 1 | 1 | |||
| Laryngeal edema | 1 | 1 | |||
| Lung infection | 1 | 2 | 3 | ||
| Pneumothorax | 1 | 1 | |||
| Patients | 7 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersoy, T.F.; Mokhtari, N.; Brainman, D.; Berger, B.; Salay, A.; Schütt, P.; Weissinger, F.; Grote, A.; Simon, M. Surgical Treatment of Cerebellar Metastases: Survival Benefits, Complications and Timing Issues. Cancers 2021, 13, 5263. https://doi.org/10.3390/cancers13215263
Ersoy TF, Mokhtari N, Brainman D, Berger B, Salay A, Schütt P, Weissinger F, Grote A, Simon M. Surgical Treatment of Cerebellar Metastases: Survival Benefits, Complications and Timing Issues. Cancers. 2021; 13(21):5263. https://doi.org/10.3390/cancers13215263
Chicago/Turabian StyleErsoy, Tunc Faik, Neda Mokhtari, Daniel Brainman, Björn Berger, Attila Salay, Philipp Schütt, Florian Weissinger, Alexander Grote, and Matthias Simon. 2021. "Surgical Treatment of Cerebellar Metastases: Survival Benefits, Complications and Timing Issues" Cancers 13, no. 21: 5263. https://doi.org/10.3390/cancers13215263
APA StyleErsoy, T. F., Mokhtari, N., Brainman, D., Berger, B., Salay, A., Schütt, P., Weissinger, F., Grote, A., & Simon, M. (2021). Surgical Treatment of Cerebellar Metastases: Survival Benefits, Complications and Timing Issues. Cancers, 13(21), 5263. https://doi.org/10.3390/cancers13215263







