PARP Inhibitors in Combination with Radiotherapy: To Do or Not to Do?
Abstract
Simple Summary
Abstract
1. Introduction
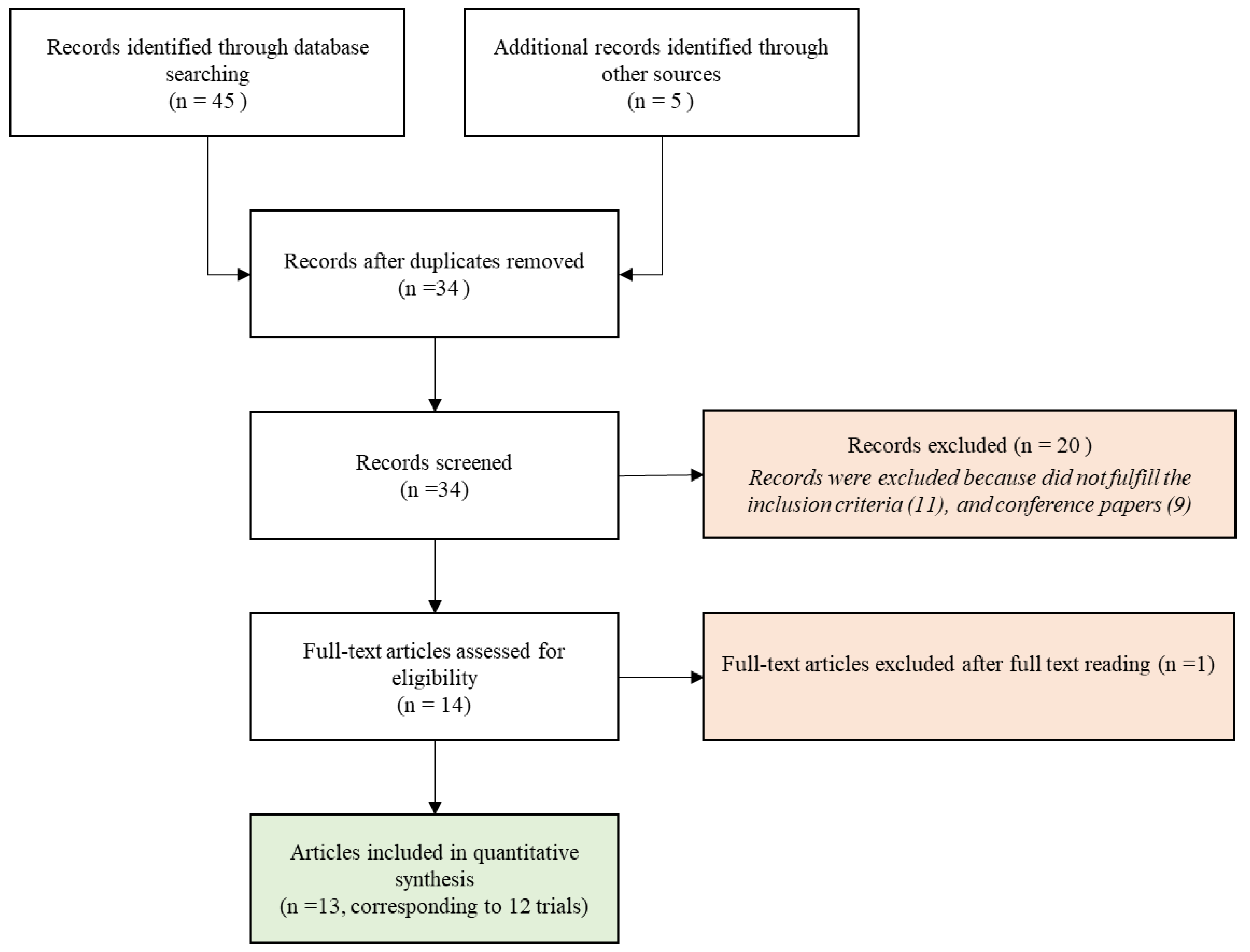
2. Material and Methods
2.1. Search Strategy
2.2. Selection Criteria for Full-Text Article Review
3. Results
3.1. Population
3.2. Intervention
3.3. Comparison
3.4. Outcomes
- -
- In the first trial by Chabot et al. [15] no differences in survival rates or toxicity across the arms were found;
- -
- In the phase I part of the trial by Argiris et al. [23], even if the early closure of the study did not allow to evaluate the full efficacy of the combination of Veliparib, the PFS and the OS from registration to consolidation were not statistically different between the ChemoRT arm and the ChemoRT + Veliparib arm.
- -
- In the VERTU trial, no significant clinical benefit was found (Median PFS of 5.7 months (95% CI: 3.9–6.5 months) in the experimental arm vs. 4.2 months (95% CI: 2.4–5.7 months) in the standard arm) [24].
- -
- Chabot et al. [15] did not describe a significant difference in toxicity across the arms, even if a lower incidence for Grade 3/4 adverse events in the Veliparib arms (50 mg versus 200 mg; p < 0.05) was reported.
- -
- In the phase I part of the trial of Argiris et al. [23], 19% of patients underwent grade 4 toxicities, which included lymphopenia (3 cases) and neutropenia (1 case) and 57% showed grade 3 adverse events (AEs), which were mostly hematologic and not associated with dose levels. In the phase II part of the trial, the authors reported 18% of neutropenia and 3% of thrombocytopenia.
- -
- Veliparib-containing regimen was well tolerated in the VERTU trial [24], with thrombocytopenia and neutropenia, corresponding to the main grade ≥ 3 toxicities, being more frequent in the experimental arm (12% vs. 3% and 17% vs. 8%, respectively).
- -
- In the parallel-arm trial by de Haan et al. [22] grade ≥ 3 acute toxicities increased from 45% (Olaparib 25 mg once daily), 57% (Olaparib 25 mg twice daily) to 80% (CDDP + Olaparib), and hematologic grade ≥ 3 toxicities other than lymphocytopenia were only observed in patients treated with concomitant CDDP.
4. Bias and Limitation of the Analysis
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T.; Bryant, H.E.; Schultz, N. Poly(ADP-ribose) polymerase (PARP-1) in homologous recombination and as a target for cancer therapy. Cell Cycle 2005, 4, 1176–1178. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Nandi, S. Synthetic lethality in DNA repair network: A novel avenue in targeted cancer therapy and combination therapeutics. IUBMB Life 2017, 69, 929–937. [Google Scholar] [CrossRef]
- Turk, A.A.; Wisinski, K.B. PARP inhibitors in breast cancer: Bringing synthetic lethality to the bedside. Cancer 2018, 124, 2498–2506. [Google Scholar] [CrossRef] [PubMed]
- Pilié, P.G.; Gay, C.M.; Byers, L.A.; O’Connor, M.J.; Yap, T.A. PARP Inhibitors: Extending Benefit Beyond BRCA-Mutant Cancers. Clin. Cancer Res. 2019, 25, 3759–3771. [Google Scholar] [CrossRef]
- Sizemore, S.T.; Mohammad, R.; Sizemore, G.M.; Nowsheen, S.; Yu, H.; Ostrowski, M.C.; Chakravarti, A.; Xia, F. Synthetic Lethality of PARP Inhibition and Ionizing Radiation is p53-dependent. Mol. Cancer Res. 2018, 16, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Gadducci, A.; Guerrieri, M.E. PARP Inhibitors in Epithelial Ovarian Cancer: State of Art and Perspectives of Clinical Research. Anticancer Res. 2016, 36, 2055–2064. [Google Scholar] [PubMed]
- Jannetti, S.A.; Zeglis, B.M.; Zalutsky, M.R.; Reiner, T. Poly(ADP-Ribose)Polymerase (PARP) Inhibitors and Radiation Therapy. Front. Pharmacol. 2020, 11, 170. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Emmanuel, P. Formulating a researchable question: A critical step for facilitating good clinical research. Indian J. Sex. Transm. Dis. AIDS 2010, 31, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Loap, P.; Loirat, D.; Berger, F.; Ricci, F.; Vincent-Salomon, A.; Ezzili, C.; Mosseri, V.; Fourquet, A.; Ezzalfani, M.; Kirova, Y. Combination of Olaparib and Radiation Therapy for Triple Negative Breast Cancer: Preliminary Results of the RADIOPARP Phase 1 Trial. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 436–440. [Google Scholar] [CrossRef]
- Loap, P.; Loirat, D.; Berger, F.; Cao, K.; Ricci, F.; Jochem, A.; Raizonville, L.; Mosseri, V.; Fourquet, A.; Kirova, Y. Combination of Olaparib with radiotherapy for triple-negative breast cancers: One-year toxicity report of the RADIOPARP Phase I trial. Int. J. Cancer 2021, 149, 1828–1832. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.P.; Wang, D.; Wang, F.; Kleinberg, L.; Brade, A.; Robins, H.I.; Turaka, A.; Leahy, T.; Medina, D.; Xiong, H.; et al. Veliparib in combination with whole brain radiation therapy in patients with brain metastases: Results of a phase 1 study. J. Neurooncol. 2015, 122, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Chabot, P.; Hsia, T.-C.; Ryu, J.-S.; Gorbunova, V.; Belda-Iniesta, C.; Ball, D.; Kio, E.; Mehta, M.; Papp, K.; Qin, Q.; et al. Veliparib in combination with whole-brain radiation therapy for patients with brain metastases from non-small cell lung cancer: Results of a randomized, global, placebo-controlled study. J. Neurooncol. 2017, 131, 105–115. [Google Scholar] [CrossRef]
- Czito, B.G.; Deming, D.A.; Jameson, G.S.; Mulcahy, M.F.; Vaghefi, H.; Dudley, M.W.; Holen, K.D.; DeLuca, A.; Mittapalli, R.K.; Munasinghe, W.; et al. Safety and tolerability of veliparib combined with capecitabine plus radiotherapy in patients with locally advanced rectal cancer: A phase 1b study. Lancet Gastroenterol. Hepatol. 2017, 2, 418–426. [Google Scholar] [CrossRef]
- Reiss, K.A.; Herman, J.M.; Armstrong, D.; Zahurak, M.; Fyles, A.; Brade, A.; Milosevic, M.; Dawson, L.A.; Scardina, A.; Fischer, P.; et al. A final report of a phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy (LDFWAR) in patients with advanced solid malignancies and peritoneal carcinomatosis with a dose escalation in ovarian and. Gynecol. Oncol. 2017, 144, 486–490. [Google Scholar] [CrossRef]
- Jagsi, R.; Griffith, K.A.; Bellon, J.R.; Woodward, W.A.; Horton, J.K.; Ho, A.; Feng, F.Y.; Speers, C.; Overmoyer, B.; Sabel, M.; et al. Concurrent Veliparib With Chest Wall and Nodal Radiotherapy in Patients With Inflammatory or Locoregionally Recurrent Breast Cancer: The TBCRC 024 Phase I Multicenter Study. J. Clin. Oncol. 2018, 36, 1317–1322. [Google Scholar] [CrossRef]
- Karam, S.D.; Reddy, K.; Blatchford, P.J.; Waxweiler, T.; DeLouize, A.M.; Oweida, A.; Somerset, H.; Marshall, C.; Young, C.; Davies, K.D.; et al. Final Report of a Phase I Trial of Olaparib with Cetuximab and Radiation for Heavy Smoker Patients with Locally Advanced Head and Neck Cancer. Clin. Cancer Res. 2018, 24, 4949–4959. [Google Scholar] [CrossRef]
- Tuli, R.; Shiao, S.L.; Nissen, N.; Tighiouart, M.; Kim, S.; Osipov, A.; Bryant, M.; Ristow, L.; Placencio-Hickok, V.; Hoffman, D.; et al. A phase 1 study of veliparib, a PARP-1/2 inhibitor, with gemcitabine and radiotherapy in locally advanced pancreatic cancer. EBioMedicine 2019, 40, 375–381. [Google Scholar] [CrossRef]
- Baxter, P.A.; Su, J.M.; Onar-Thomas, A.; Billups, C.A.; Li, X.-N.; Poussaint, T.Y.; Smith, E.R.; Thompson, P.; Adesina, A.; Ansell, P.; et al. A phase I/II study of veliparib (ABT-888) with radiation and temozolomide in newly diagnosed diffuse pontine glioma: A Pediatric Brain Tumor Consortium study. Neuro. Oncol. 2020, 22, 875–885. [Google Scholar] [CrossRef] [PubMed]
- de Haan, R.; van den Heuvel, M.M.; van Diessen, J.; Peulen, H.M.U.; van Werkhoven, E.; de Langen, A.J.; Lalezari, F.; Pluim, D.; Verwijs-Janssen, M.; Vens, C.; et al. Phase I and Pharmacologic Study of Olaparib in Combination with High-dose Radiotherapy with and without Concurrent Cisplatin for Non-Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 1256–1266. [Google Scholar] [CrossRef]
- Argiris, A.; Miao, J.; Cristea, M.C.; Chen, A.M.; Sands, J.M.; Decker, R.H.; Gettinger, S.N.; Daly, M.E.; Faller, B.A.; Albain, K.S.; et al. A Dose-finding Study Followed by a Phase II Randomized, Placebo-controlled Trial of Chemoradiotherapy With or Without Veliparib in Stage III Non-small-cell Lung Cancer: SWOG 1206 (8811). Clin. Lung Cancer 2021, 22, 313–323.e1. [Google Scholar] [CrossRef] [PubMed]
- Sim, H.-W.; McDonald, K.L.; Lwin, Z.; Barnes, E.H.; Rosenthal, M.; Foote, M.C.; Koh, E.-S.; Back, M.; Wheeler, H.; Sulman, E.P.; et al. A randomized phase II trial of veliparib, radiotherapy and temozolomide in patients with unmethylated MGMT glioblastoma: The VERTU study. Neuro-Oncology 2021, 23, 1736–1749. [Google Scholar] [CrossRef] [PubMed]
- Boussios, S.; Karihtala, P.; Moschetta, M.; Abson, C.; Karathanasi, A.; Zakynthinakis-Kyriakou, N.; Ryan, J.E.; Sheriff, M.; Rassy, E.; Pavlidis, N. Veliparib in ovarian cancer: A new synthetically lethal therapeutic approach. Investig. New Drugs 2020, 38, 181–193. [Google Scholar] [CrossRef]
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Lakomy, D.S.; Urbauer, D.L.; Westin, S.N.; Lin, L.L. Phase I study of the PARP inhibitor talazoparib with radiation therapy for locally recurrent gynecologic cancers. Clin. Transl. Radiat. Oncol. 2019, 21, 56–61. [Google Scholar] [CrossRef]
- Noël, G.; Godon, C.; Fernet, M.; Giocanti, N.; Mégnin-Chanet, F.; Favaudon, V. Radiosensitization by the poly(ADP-ribose) polymerase inhibitor 4-amino-1,8-naphthalimide is specific of the S phase of the cell cycle and involves arrest of DNA synthesis. Mol. Cancer Ther. 2006, 5, 564–574. [Google Scholar] [CrossRef][Green Version]
- Dungey, F.A.; Löser, D.A.; Chalmers, A.J. Replication-dependent radiosensitization of human glioma cells by inhibition of poly(ADP-Ribose) polymerase: Mechanisms and therapeutic potential. Int. J. Radiat. Oncol. Biol. Phys. 2008, 72, 1188–1197. [Google Scholar] [CrossRef]
- Lourenco, L.M.; Jiang, Y.; Drobnitzky, N.; Green, M.; Cahill, F.; Patel, A.; Shanneik, Y.; Moore, J.; Ryan, A.J. PARP Inhibition Combined With Thoracic Irradiation Exacerbates Esophageal and Skin Toxicity in C57BL6 Mice. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 767–775. [Google Scholar] [CrossRef]
- Césaire, M.; Thariat, J.; Candéias, S.M.; Stefan, D.; Saintigny, Y.; Chevalier, F. Combining PARP inhibition, radiation, and immunotherapy: A possible strategy to improve the treatment of cancer? Int. J. Mol. Sci. 2018, 19, 3793. [Google Scholar] [CrossRef]
- Lesueur, P.; Chevalier, F.; Austry, J.-B.; Waissi, W.; Burckel, H.; Noël, G.; Habrand, J.-L.; Saintigny, Y.; Joly, F. Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: A systematic review of pre-clinical and clinical human studies. Oncotarget 2017, 8, 69105–69124. [Google Scholar] [CrossRef] [PubMed]
- Tinganelli, W.; Durante, M. Carbon Ion Radiobiology. Cancers 2020, 12, 3022. [Google Scholar] [CrossRef] [PubMed]
- Hirai, T.; Shirai, H.; Fujimori, H.; Okayasu, R.; Sasai, K.; Masutani, M. Radiosensitization effect of poly(ADP-ribose) polymerase inhibition in cells exposed to low and high liner energy transfer radiation. Cancer Sci. 2012, 103, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Césaire, M.; Ghosh, U.; Austry, J.-B.; Muller, E.; Cammarata, F.P.; Guillamin, M.; Caruso, M.; Castéra, L.; Petringa, G.; Cirrone, G.A.P.; et al. Sensitization of chondrosarcoma cells with PARP inhibitor and high-LET radiation. J. Bone Oncol. 2019, 17, 100246. [Google Scholar] [CrossRef]
| Query | Population | Intervention | Comparison | Outcomes |
|---|---|---|---|---|
| 1 | Patient with new diagnosis or recurrent tumor | Radiotherapy and concomitant Parp-I | Radiotherapy alone or with chemotherapy or standard of care (if available) | Locoregional control Disease free survival Overall survival |
| 2 | Patient with new diagnosis or recurrent tumor | Radiotherapy and concomitant Parp-I | Radiotherapy alone or with chemotherapy or standard of care (if available) | Acute and late toxicity with grade ≥ 3 |
| Study | Population | Intervention | ||||||
|---|---|---|---|---|---|---|---|---|
| Author | Type of Study | Number of Patients (pts) | Age Median [Range] | Localization | Radiotherapy (Total Dose/Fraction) with or without Chemotherapy | Aim of Radiotherapy | Parp-INHIBITORS | Follow Up |
| Mehta (2015) [14] | Single-arm dose-escalation phase I | 81 | 58 (31–84) | Brain metastases | 30 Gy/10 fr or 37.5 Gy/15 fr | Definitive | Veliparib | NA |
| Chabot (2017) [15] | Three-arm phase II controlled trial; placebo (102 pts) vs. Veliparib 50 mg (103 pts) vs. Veliparib 200 mg (102 pts). | 307 | 60 (41–86) (placebo arm) vs. 60 (33–83) (50 mg BID arm) vs. 62 (39–81) (200 mg BID arm) | Brain metastases | 30 Gy/10 fr | Definitive | Veliparib | NA |
| Czito (2017) [16] | Single-arm dose-escalation phase I | 32 | 57 (37–75) | Rectum | 50.4 Gy/28 fr + capecitabine 825 mg/m2 twice daily | Neoadjuvant | Veliparib | NA |
| Reiss (2017) [17] | Single-arm dose-escalation phase I | 32 | 58 (55–65) | Peritoneal carcinomatosis (ovarian and fallopian cancer) | 21.6 Gy/36 fr (BID) | Radical | Veliparib | 45 months |
| Jagsi (2018) [18] | Single-arm dose-escalation phase I | 30 | 50.5 (41–40) | Breast (inflammatory or locoregionally recurrent) | 50 Gy + 10 Gy (boost)/25 fr | Adjuvant | Veliparib | 3 years |
| Karam (2018) [19] | Single-arm dose-escalation phase I | 16 | 61 (46–75) | Head and Neck (locally advanced) | 69.3 Gy/33 fr + Cetuximab | Radical | Olaparib | 26 months |
| Tuli (2019) [20] | Single-arm dose-escalation phase I | 30 | 68 (60–77) | Pancreas (locally advanced) | 36 Gy/15 fr + gemcitabine 400 mg/m2 | Radical | Veliparib | NA |
| Baxter (2020) [21] | Phase I/II trial: single-arm dose-escalation trial with comparison to historical series | 66 | 6.6 (2.2–15.8) | Diffuse intrinsic pontine glioma | 54 Gy/30 fr + Temozolomide (135 mg/m2 d1–5/28 d) | Radical | Veliparib | 6.3 months (stopped early for futility) |
| de Haan (2020) [22] | Phase I: two parallel arms dose-escalation trial | 28 | 58 (55–65) (Arm CDDP+ Olaparib) 62 (58–68) (Arm Olaparib) | Lung (NSCLC) | 66 Gy/24 fr (1 pts s stopped at 52 Gy) CDDP 6 mg/m2/daily in the CDDP + Olaparib arm | Radical | Olaparib | 14 months |
| Argiris (2021) [23] | Single-arm dose-escalation phase I trial follow by a two-arm controlled phase II trial placebo (13 pts) vs. Veliparib (18 pts) | 21 (phase I) and 31 (phase II) | phase I: 70 (53–81). Phase II: 64.7 (47–78.9) (arm Veliparib), 65 (56.6–75.6) (arm placebo) | Lung (stage III NSCLC) | 60 Gy/30 fr + paclitaxel 45 mg/m2/carboplatin AUC2 (weekly concomitant and in consolidation) | Radical | Veliparib (concomitant and consolidation) | Phase I: 40.6 months; phase II: 26.9 months |
| Sim (2021) [24] | Two-arm controlled phase II trial; standard arm(41 pts) vs. Veliparib 200 mg (84 pts) | 125 | 60 (22–78) (Veliparib arm) vs. 62 (24–73) (Standard Arm) | Glioblastoma (unmethylated MGMT promoter) | 60 Gy/30 fr + Temozolomide (75 mg/m2 OD concomitant and 150–200 mg/m2 d1–5/28 d) | Radical | Veliparib | 27.2 months |
| Loap (2020 and 2021) [12,13] | Single-arm dose-escalation phase I | 24 | 46 (25–74) | Breast (triple negative) | 50 Gy/25 fr; 50.4 Gy/28 fr ± SIB tumor boost (63 Gy) | Adjuvant | Olaparib | 12 months |
| a. Double Combination Outcomes | |||||
| Study | Toxicity ≥ Grade 3 | Main ≥ Grade 3 Hematological Toxicities | Main ≥ Grade 3 Non-Hematological Toxicities | Clinical Outcome | |
| Mehta (2015) [14] | 29.6% (acute) | lymphopenia (5%), anemia (4%) | Fatigue (7%) hyponatremia (6%) dehydration (5%) hyperglycemia (4%) | 6-month OS: 54% | |
| Chabot (2017) [15] | 43% (acute, placebo arm) vs. 28% (50 mg BID arm) vs. 25% (200 mg BID arm) | anemia (3% vs. 1% vs. 2%, for placebo, 50 mg BID and 200 mg BID, resp.); thrombocytopenia (1% vs. 3% vs. 2%). | pulmonary embolism (1% vs. 4% vs. 2% for placebo, 50 mg BID and 200 mg BID resp.); pneumonia (8% vs. 3% vs. 2%) fatigue (4% vs. 2%. vs. 2%) hyperglycemia (1% vs. 2%. vs. 2%) | OS: 185 d. (placebo) vs. 209 d. (50 mg) vs. 209 d. (200 mg) ORR: 41.2% (placebo) vs. 36.9% (50 mg) vs. 42.2% (200 mg) | |
| Reiss (2017) [17] | ≥59% (acute) | lymphopenia (59%), thrombocytopenia (12%), anemia (9%), neutropenia (6%) | nausea (6%) diarrhea (6%) anorexia (6%) vomiting (6%) fatigue (6%) | Median PFS: 3.6 months. Median OS: 9.1 months | |
| Jagsi (2018) [18] | 46.7% (late; at year 3) | no late G3 hematological toxicity | fibrosis (40% at 3 years) lymphoedema (20%) skin induration (13%) chest wall pain (7%) atrial clot (7%) | 3-year disease control: 50% (15 failures) 3-year OS: 56.7% (13/30 deaths). | |
| Loap (2020 and 2021) [12,13] | Acute toxicity ≥50% Late (1-year) toxicity: 0% | Acute: lymphopenia (50%) Late: 0% treatment related | Acute: Radiodermatitis (8%) pain (4%) Late 0% treatment related | 1-year OS 96% (88%-100%) | |
| b. Triple combination outcomes | |||||
| Study | Drug | Toxicity ≥ Grade 3 | Main ≥ Grade 3 Hematological Toxicities | Main ≥ Grade 3 Non-Hematological Toxicities | Clinical Outcome |
| Czito (2017) [16] | capecitabine 825 mg/m2 twice daily | 25% (acute) | anemia (3%), lymphopenia (3%) | diarrhea (9%) radiation skin injury (3%) hyperglycemia (3%) pulmonary embolism (3%) syncope (3%) vaginal stricture (3%) radiation enteritis (3%) | pCR: 29%. Tumor downstaging: 71%. CEA response rate: 68% |
| Karam (2018) [19] | Cetuximab 400 mg/m2 (5–7 day before RT) and 250 mg/m2 weekly | ≥69% (acute) | lymphopenia (19%) | mucositis (69%) dermatitis (38%) dysphagia (31%) nausea (13%) dehydration (13%) hypomagnesemia (13%) malnutrition (13%) vomiting (13%) oral pain (6%) weight loss (6%) | 2-year OS: 72% 2-year PFS: 63% 2-year LC: 72% 2-year DC: 79% |
| Tuli (2021) [20] | Gemcitabine 400 mg/m2 | >96% (acute) | lymphopenia (96%) anemia (38%) thrombocytopenia (19%) neutropenia (4%) febrile neutropenia (4%) | anorexia (19%) abdominal pain (12%) nausea (12%) vomiting (8%) diarrhea (4%) colitis (4%) fatigue (4%) | median PFS: 9.8 months; median OS: 14.6 months |
| de Haan (2020) [22] | CDDP 6 mg/m2/daily | 80% in CDDP + Olaparib arm vs. 57% Olaparib BID vs. 45% Olaparib once/day | CDDP + Olaparib arm: Neutropenia (30%, Lymphocytopenia (70% G3, 30% G4 with 1% G3 late), Thrombocytopenia G4 (10%) Olaparib BID Lymphocytopenia (23% G3, 14% G4 with 17% G3 late) Olaparib once daily: Lymphocytopenia (18% G3, 18% G4 with 11% G3 late) | CDDP + Olaparib arm: Gastro-intestinal (33%) G4 fibrosis (11%), G5 fibrosis (11%) Olaparib BID: gastrointestinal (29% acute,17% late) Pneumonitis (17%) Lung hemorrhage G4 (17%) Olaparib once daily: gastrointestinal (9% acute only). Lung infection (9%), dyspnea (9%), pneumonitis G5 (9%), lung hemorrhage G5 (11%) and fibrosis G5 (11%); spinal fracture (11%) | 2-year LC:84% (with a 95% confidence interval of 58–95%; 89% with cisplatin and 83% without cisplatin Median PFS: 6.5 months (oligometastatic) and 12 months (Locally advanced) Median OS: 23 months (oligometastatic) and 28 months (locally advanced) |
| Baxter (2020) [21] | Temozolomide (135 mg/m2 d1–5/28 d) | ≥50% (acute) | lymphopenia (50%) neutropenia (32.7%) thrombocytopenia (23.1%) | maculopapular rash (3%) neurological deterioration (2%) | 1-year OS: 37.2% 2-year OS: 5.3% PR: 14% |
| Argiris (2021) [23] | Paclitaxel 45 mg/m2/carboplatin AUC2 (weekly concomitant and in consolidation) | Phase I: 81% (acute), including one treatment-related G5 esophageal perforation. Phase II: 47% (arm Veliparib); 69% (arm placebo) | Phase I: lymphopenia (57%); neutropenia (38%); Phase II: neutropenia (18%), thrombocytopenia (3%) | Phase I: esophagitis (19%), fatigue (10%); esophageal perforation (5%) Phase II: anorexia (6%), esophageal pain (6%), fatigue (6%), hyperglycemia (6%), oral mucositis (1%) | 1-year PFS: 43% (Veliparib) vs. 40% (placebo); 1-year OS: 76% (Veliparib) vs. 50% (placebo) |
| Sim (2021) [24] | Temozolomide (75 mg/m2 OD concomitant and 150–200 mg/m2 d1–5/28 d) | 55% (both Veliparib and standard arm) | Veliparib arm: thrombocytopenia (17%); neutropenia (12%). Standard arm: thrombocytopenia (8%), neutropenia (3%) | Veliparib arm: seizures (11%), fatigue (7%). Standard arm: seizures (5%), hyperglycemia (5%), diarrhea (5%) | median PFS: 5.7 months (Veliparib) vs. 4.2 months (standard); median OS: 12.7 months (Veliparib) vs. 12.8 months (standard) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barcellini, A.; Loap, P.; Murata, K.; Villa, R.; Kirova, Y.; Okonogi, N.; Orlandi, E. PARP Inhibitors in Combination with Radiotherapy: To Do or Not to Do? Cancers 2021, 13, 5380. https://doi.org/10.3390/cancers13215380
Barcellini A, Loap P, Murata K, Villa R, Kirova Y, Okonogi N, Orlandi E. PARP Inhibitors in Combination with Radiotherapy: To Do or Not to Do? Cancers. 2021; 13(21):5380. https://doi.org/10.3390/cancers13215380
Chicago/Turabian StyleBarcellini, Amelia, Pierre Loap, Kazutoshi Murata, Riccardo Villa, Youlia Kirova, Noriyuki Okonogi, and Ester Orlandi. 2021. "PARP Inhibitors in Combination with Radiotherapy: To Do or Not to Do?" Cancers 13, no. 21: 5380. https://doi.org/10.3390/cancers13215380
APA StyleBarcellini, A., Loap, P., Murata, K., Villa, R., Kirova, Y., Okonogi, N., & Orlandi, E. (2021). PARP Inhibitors in Combination with Radiotherapy: To Do or Not to Do? Cancers, 13(21), 5380. https://doi.org/10.3390/cancers13215380






