Trends in Tumor Site-Specific Survival of Bone Sarcomas from 1980 to 2018: A Surveillance, Epidemiology and End Results-Based Study
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Population Database
2.2. Parameters Extraction
2.3. Statistical Analysis
3. Results
3.1. Patients Characteristics
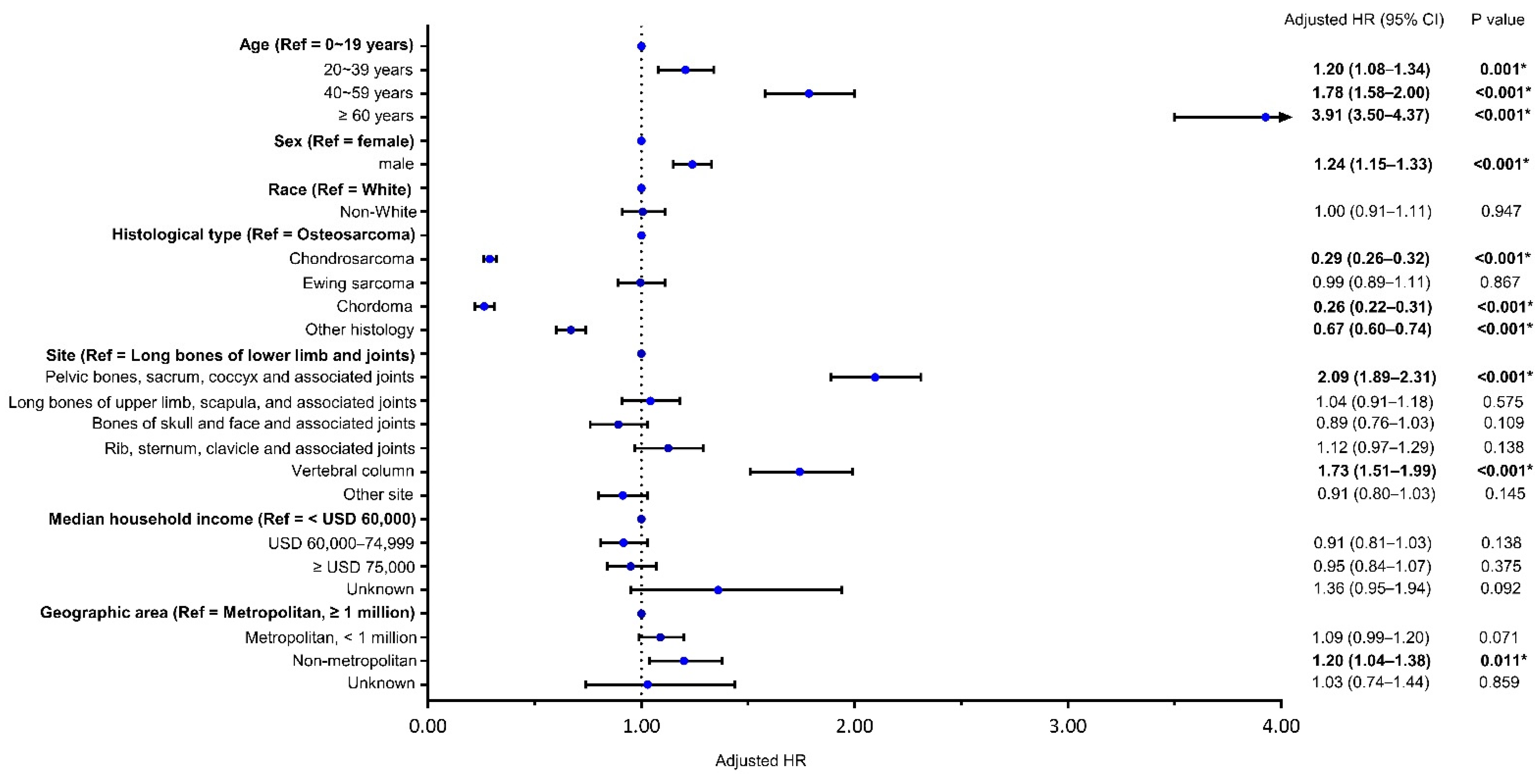
3.2. Risk Factors for Bone Sarcomas Mortality
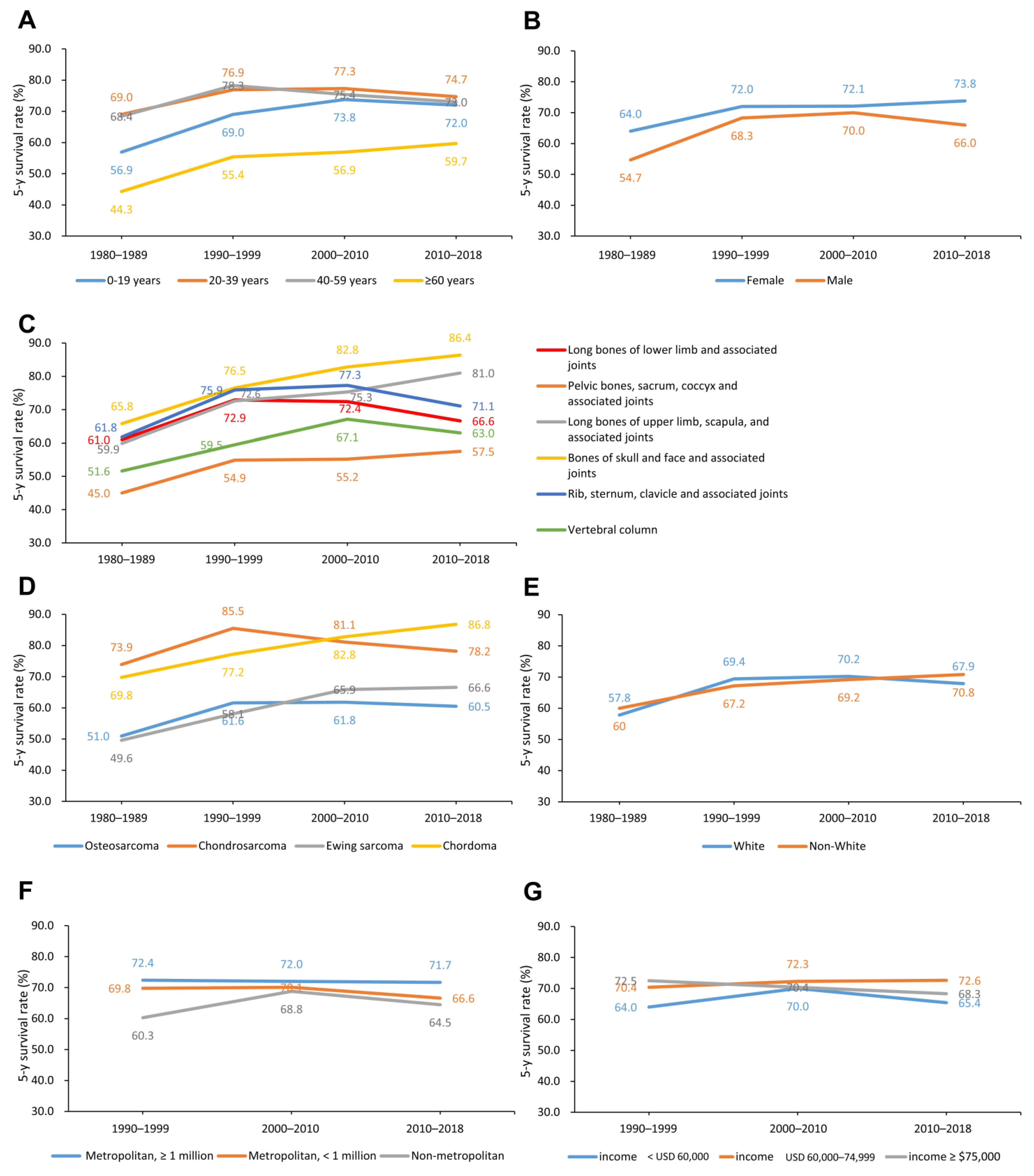
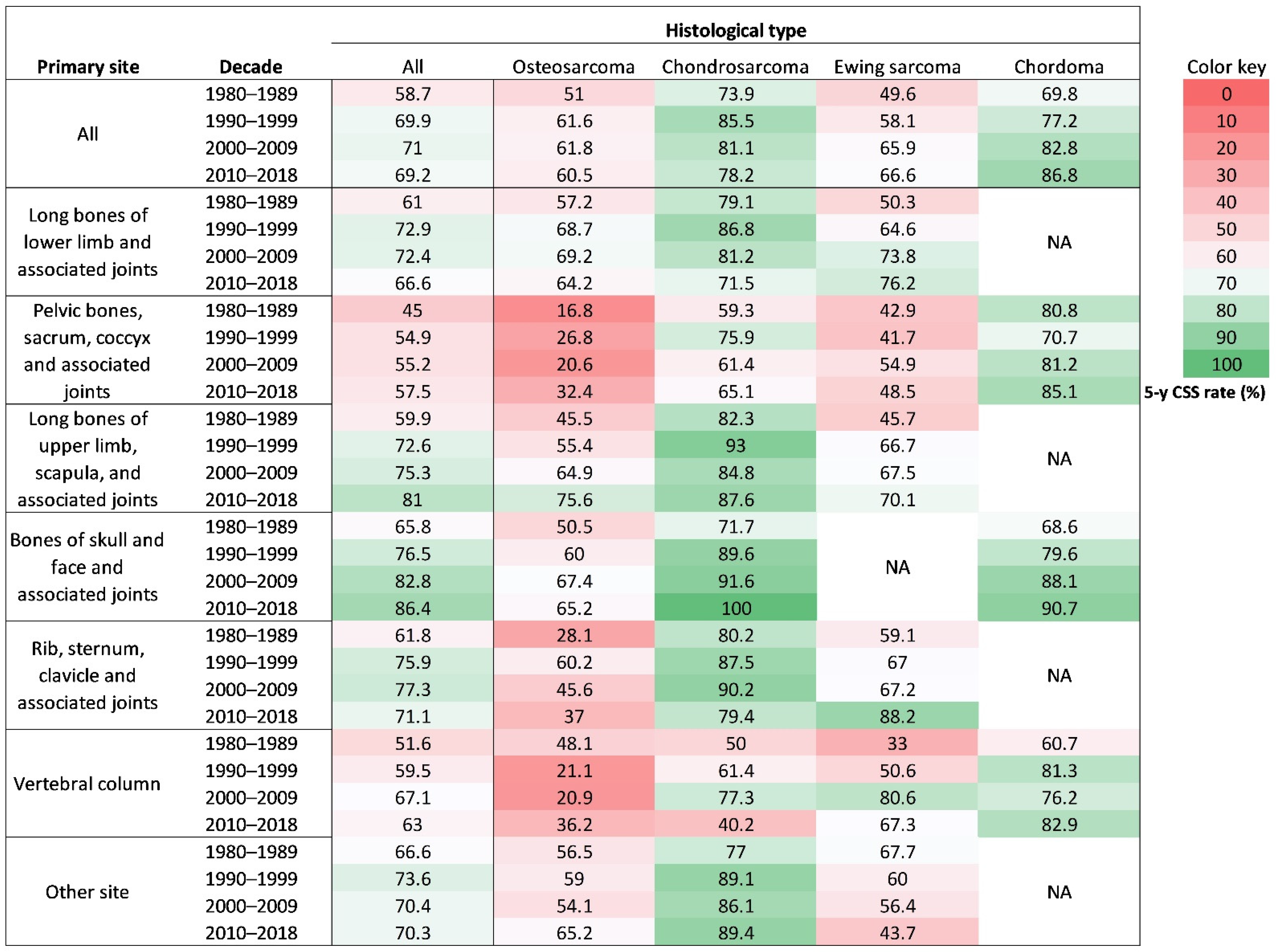
3.3. Changes in 5-Year CSS Rates by Age, Sex, Primary Site, Histological Type, Racial, Economic, and Geographic Categories
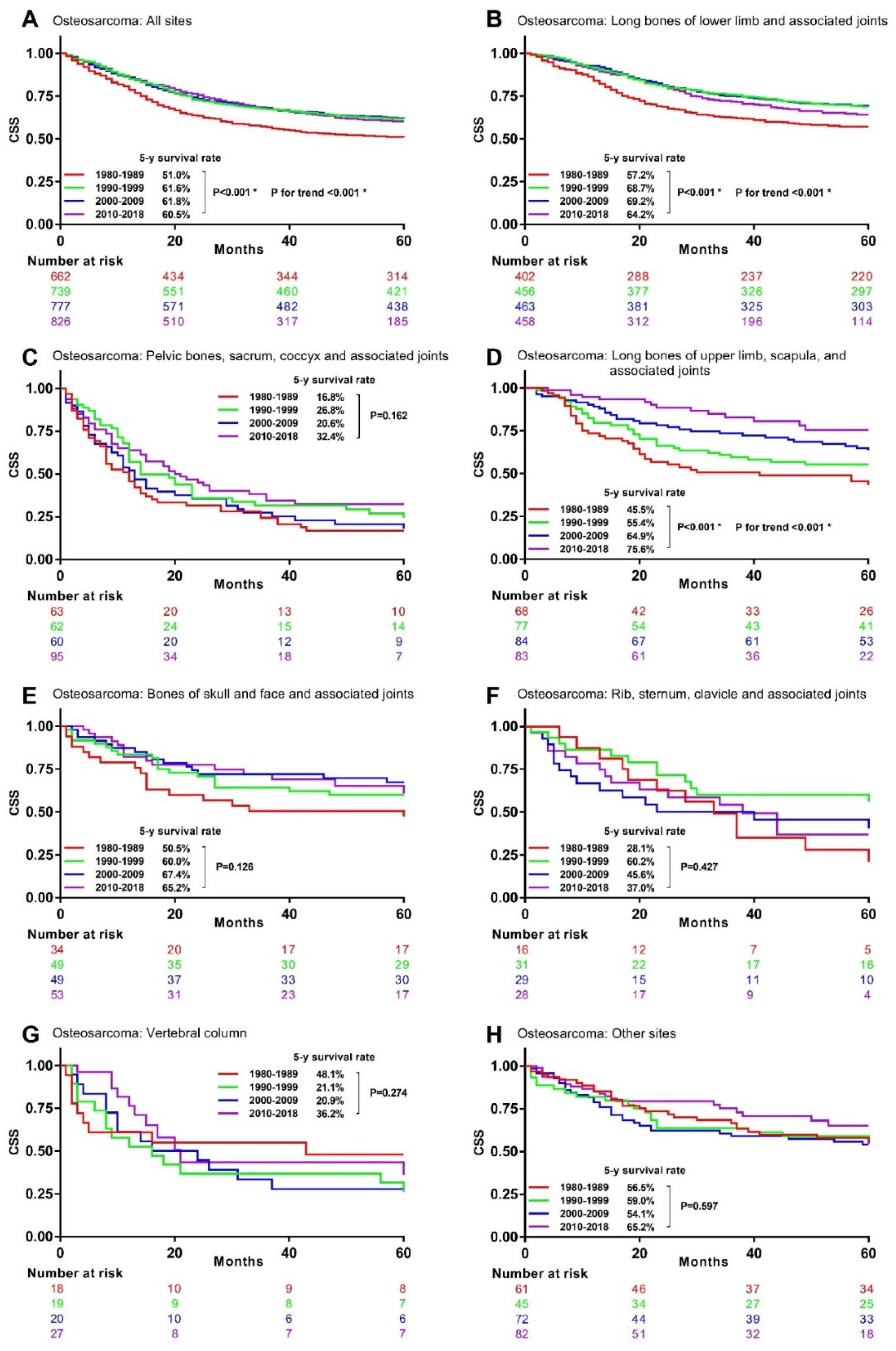
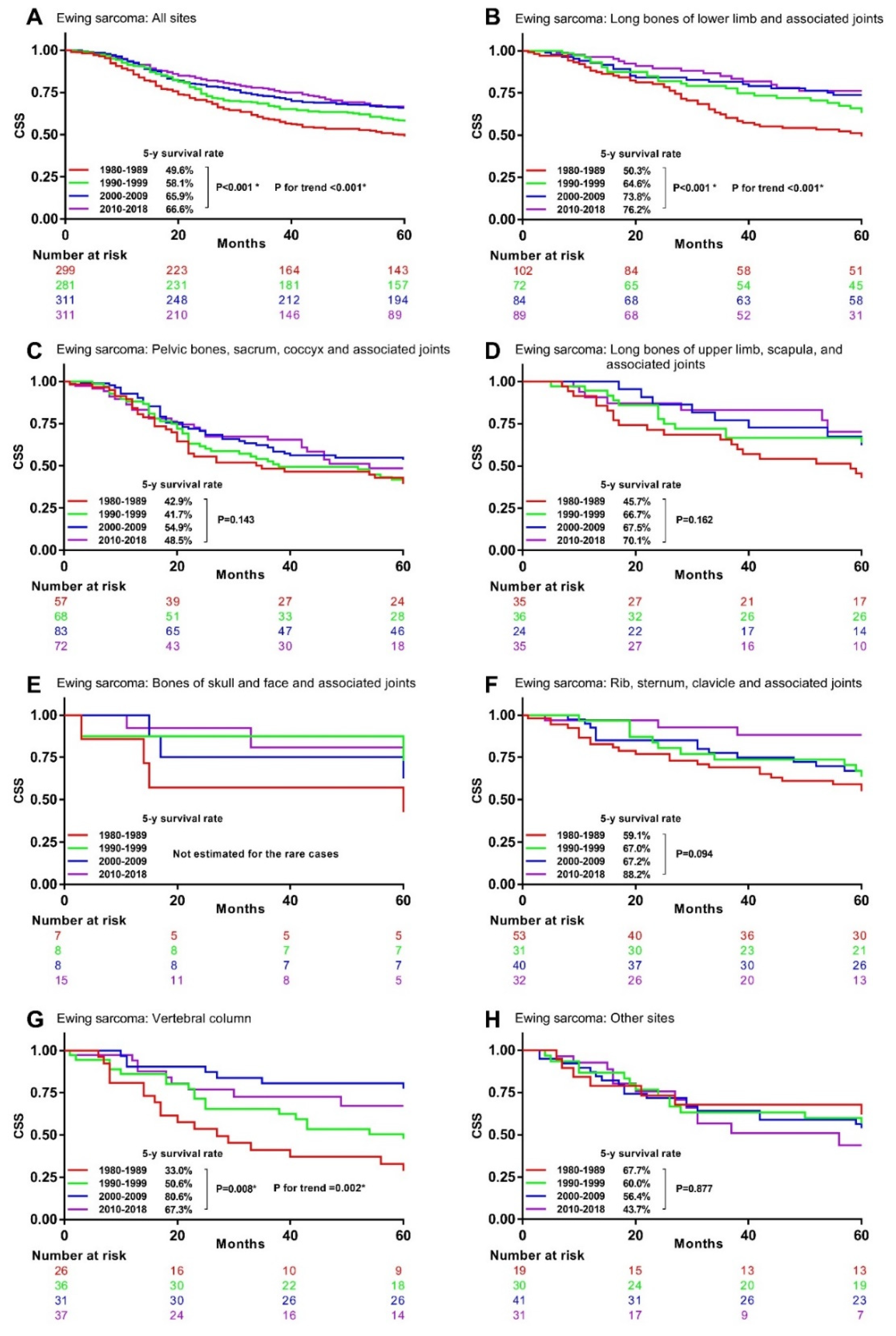
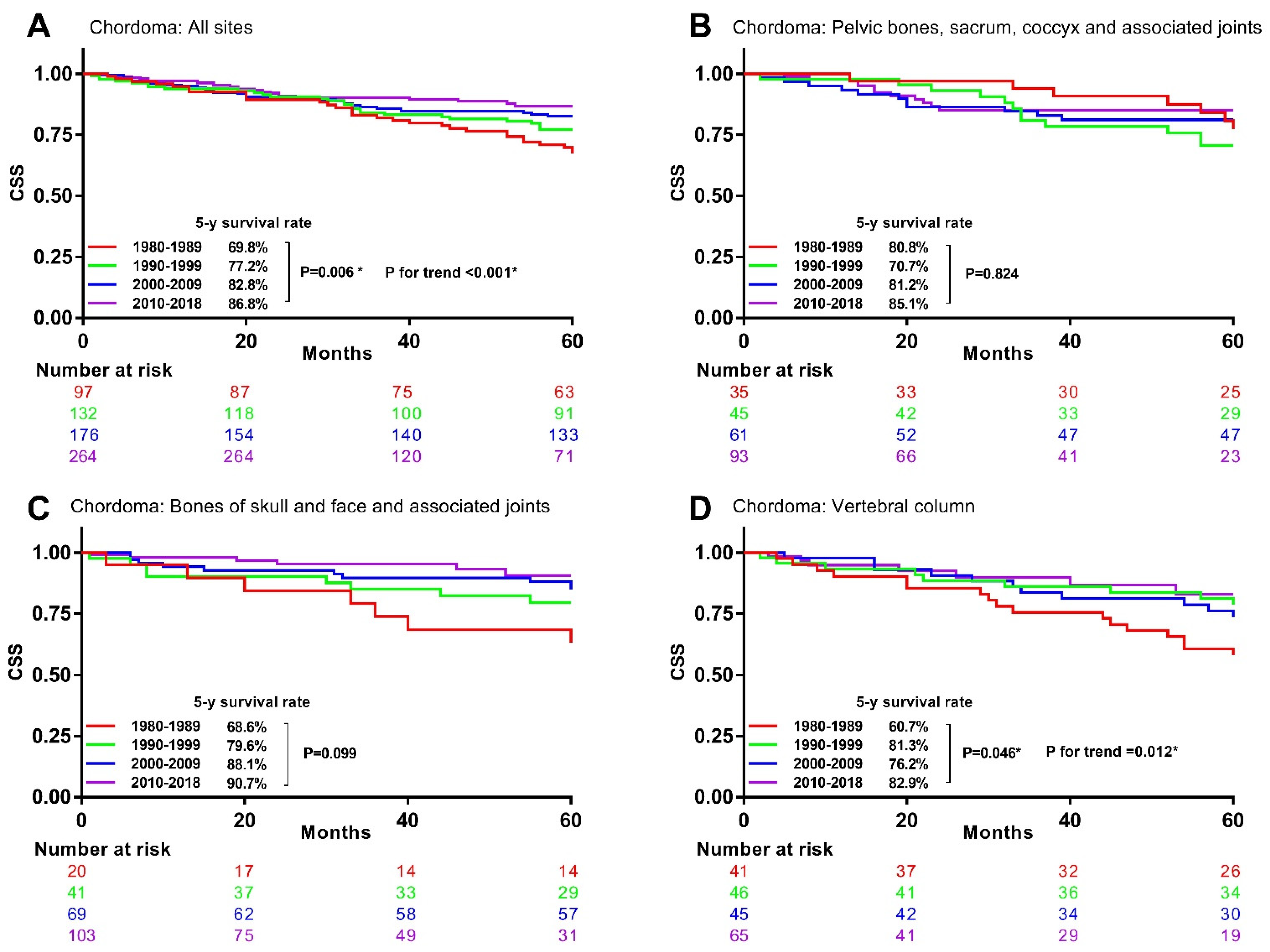
3.4. Associations between Time Periods and CSM in Specific Histological Type and Primary Site
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Complrehensive Cancer Network. Bone cancer. J. Natl. Compr. Cancer Netw. 2013, 11, 688–723. [Google Scholar] [CrossRef]
- SEER. Cancer Stat Facts: Bone and Joint Cancer; National Cancer Institute, Bethesda: Rockville, MD, USA. Available online: https://seer.cancer.gov/statfacts/html/bones.html (accessed on 25 August 2021).
- Aran, V.; Devalle, S.; Meohas, W.; Heringer, M.; Caruso, A.C.; Aguiar, D.P.; Duarte, M.E.L.; Neto, V.M. Osteosarcoma, chondrosarcoma and Ewing sarcoma: Clinical aspects, biomarker discovery and liquid biopsy. Crit. Rev. Oncol. Hematol. 2021, 162, 103340. [Google Scholar] [CrossRef]
- Keil, L. Bone Tumors: Primary Bone Cancers. FP Essent. 2020, 493, 22–26. [Google Scholar] [PubMed]
- Strauss, S.J.; Whelan, J.S. Current questions in bone sarcomas. Curr. Opin. Oncol. 2018, 30, 252–259. [Google Scholar] [CrossRef]
- Zeng, C.; Wen, W.; Morgans, A.K.; Pao, W.; Shu, X.O.; Zheng, W. Disparities by Race, Age, and Sex in the Improvement of Survival for Major Cancers: Results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program in the United States, 1990 to 2010. JAMA Oncol. 2015, 1, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Snaebjornsson, P.; Jonasson, L.; Olafsdottir, E.J.; van Grieken, N.C.; Moller, P.H.; Theodors, A.; Jonsson, T.; Meijer, G.A.; Jonasson, J.A. Why is colon cancer survival improving by time? A nationwide survival analysis spanning 35 years. Int. J. Cancer 2017, 141, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, T.; Sun, H.; Li, X.; Li, J.; Zheng, X.; Mallampati, S.; Sun, H.; Zhou, X.; Zhou, C.; et al. Survival improvement in patients with non-small cell lung cancer between 1983 and 2012: Analysis of the Surveillance, Epidemiology, and End Results database. Tumour. Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Jabbour, E.; Short, N.J.; Jain, N.; Ravandi, F.; Pui, C.H.; Kantarjian, H. Acute lymphoblastic leukemia: A population-based study of outcome in the United States based on the surveillance, epidemiology, and end results (SEER) database, 1980–2017. Am. J. Hematol. 2021, 96, 650–658. [Google Scholar] [CrossRef]
- Sasaki, K.; Ravandi, F.; Kadia, T.M.; DiNardo, C.D.; Short, N.J.; Borthakur, G.; Jabbour, E.; Kantarjian, H.M. De novo acute myeloid leukemia: A population-based study of outcome in the United States based on the Surveillance, Epidemiology, and End Results (SEER) database, 1980 to 2017. Cancer 2021, 127, 2049–2061. [Google Scholar] [CrossRef]
- Biermann, J.S.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Butrynski, J.E.; Sundar, H. National Comprehensive Cancer Network. Bone cancer. J. Natl. Compr. Cancer Netw. 2007, 5, 420–437. [Google Scholar] [CrossRef]
- Biermann, J.S.; Adkins, D.R.; Benjamin, R.S.; Brigman, B.; Chow, W.; Conrad, E.U., III; Frassica, E.U.; Frassica, F.J.; George, S.; Hande, K.R. National Comprehensive Cancer Network Bone Cancer Panel. Bone cancer. J. Natl. Compr. Cancer Netw. 2010, 8, 688–712. [Google Scholar] [CrossRef][Green Version]
- Biermann, J.S.; Chow, W.; Reed, D.; Lucas, D.; Adkins, D.R.; Agulnik, M.; Benjamin, R.S.; Brigman, B.; Budd, G.T.; Curry, W.T.; et al. NCCN Guidelines Insights: Bone Cancer, Version 2.2017. J. Natl. Compr. Cancer Netw. 2017, 15, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Weir, H.K.; Carreira, H.; Harewood, R.; Spika, D.; Wang, X.S.; CONCORD Working Group. Global surveillance of cancer survival 1995-2009: Analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 2015, 385, 977–1010. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Hood, M.; CONCORD Working Group. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. Osteosarcoma incidence and survival rates from 1973 to 2004: Data from the Surveillance, Epidemiology, and End Results Program. Cancer 2009, 115, 1531–1543. [Google Scholar] [CrossRef]
- Schneiderman, B.A.; Kliethermes, S.A.; Nystrom, L.M. Survival in Mesenchymal Chondrosarcoma Varies Based on Age and Tumor Location: A Survival Analysis of the SEER Database. Clin. Orthop. Relat. Res. 2017, 475, 799–805. [Google Scholar] [CrossRef]
- Evans, D.R.; Lazarides, A.L.; Visgauss, J.D.; Somarelli, J.A.; Blazer, D.G.; Brigman, B.E.; Eward, W.C. Limb salvage versus amputation in patients with osteosarcoma of the extremities: An update in the modern era using the National Cancer Database. BMC Cancer 2020, 20, 995. [Google Scholar] [CrossRef] [PubMed]
- Traven, S.A.; Brinton, D.L.; Walton, Z.J.; Leddy, L.R. A propensity-score matched analysis of limb salvage vs amputation for osteosarcoma. J. Surg. Oncol. 2019, 120, 1252–1258. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Bi, W.Z.; Xu, M.; Jia, J.-P.; Wang, Y. Amputation Versus Limb-Salvage Surgery in Patients with Osteosarcoma: A Meta-analysis. World J. Surg. 2016, 40, 2016–2027. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.H.; Lu, E.W.; Lin, C.W.; Yang, J.S.; Yang, S.F. New insights into molecular and cellular mechanisms of zoledronate in human osteosarcoma. Pharm. Ther. 2020, 214, 107611. [Google Scholar] [CrossRef]
- Piperno-Neumann, S.; Le Deley, M.C.; Rédini, F.; Pacquement, H.; Marec-Bérard, P.; Petit, P.; Brugières, L. Sarcoma Group of UNICANCER.; French Society of Pediatric Oncology (SFCE); French Sarcoma Group (GSF-GETO). Zoledronate in combination with chemotherapy and surgery to treat osteosarcoma (OS2006): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2016, 17, 1070–1080. [Google Scholar] [CrossRef]
- Marina, N.M.; Smeland, S.; Bielack, S.S.; Bernstein, M.; Jovic, G.; Krailo, M.D.; Whelan, J.S. Comparison of MAPIE versus MAP in patients with a poor response to preoperative chemotherapy for newly diagnosed high-grade osteosarcoma (EURAMOS-1): An open-label, international, randomised controlled trial. Lancet Oncol. 2016, 17, 1396–1408. [Google Scholar] [CrossRef]
- Spałek, M.J.; Poleszczuk, J.; Czarnecka, A.M.; Dudzisz-Śledź, M.; Napieralska, A.; Matysiakiewicz, J.; Rutkowski, P. Radiotherapy in the Management of Pediatric and Adult Osteosarcomas: A Multi-Institutional Cohort Analysis. Cells 2021, 10, 366. [Google Scholar] [CrossRef]
- Subbiah, V.; Anderson, P.; Rohren, E. Alpha Emitter Radium 223 in High-Risk Osteosarcoma: First Clinical Evidence of Response and Blood-Brain Barrier Penetration. JAMA Oncol. 2015, 1, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.M.; Scott, J.; Parsai, S.; Zahler, S.; Worley, S.; Shrikanthan, S.; Murphy, E. 223-Radium for metastatic osteosarcoma: Combination therapy with other agents and external beam radiotherapy. ESMO Open 2020, 5, e000635. [Google Scholar] [CrossRef]
- Kansara, M.; Teng, M.W.; Smyth, M.J.; Thomas, D.M. Translational biology of osteosarcoma. Nat. Rev. Cancer 2014, 14, 722–735. [Google Scholar] [CrossRef]
- Chen, C.; Xie, L.; Ren, T.; Huang, Y.; Xu, J.; Guo, W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021, 500, 1–10. [Google Scholar] [CrossRef]
- Wedekind, M.F.; Wagner, L.M.; Cripe, T.P. Immunotherapy for osteosarcoma: Where do we go from here? Pediatr. Blood Cancer 2018, 65, e27227. [Google Scholar] [CrossRef]
- Nabergoj, S.; Mlinarič-Raščan, I.; Jakopin, Ž. Harnessing the untapped potential of nucleotide-binding oligomerization domain ligands for cancer immunotherapy. Med. Res. Rev. 2019, 39, 1447–1484. [Google Scholar] [CrossRef]
- Meazza, C.; Asaftei, S.D. State-of-the-art, approved therapeutics for the pharmacological management of osteosarcoma. Expert Opin. Pharm. 2021, 22, 1995–2006. [Google Scholar] [CrossRef]
- Boehme, K.A.; Schleicher, S.B.; Traub, F.; Rolauffs, B. Chondrosarcoma: A Rare Misfortune in Aging Human Cartilage? The Role of Stem and Progenitor Cells in Proliferation, Malignant Degeneration and Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Houdek, M.T.; Witten, B.G.; Hevesi, M.; Griffin, A.M.; Salduz, A.; Wenger, D.E.; Sim, F.H.; Ferguson, P.C.; Rose, P.D.; Wunder, J.S. Advancing patient age is associated with worse outcomes in low- and intermediate-grade primary chondrosarcoma of the pelvis. J. Surg. Oncol. 2020, 121, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Song, J.; Chen, F.; Lin, K.; Ma, X.; Jiang, J. Does Resection of the Primary Tumor Improve Survival in Patients With Metastatic Chondrosarcoma? Clin. Orthop. Relat. Res. 2019, 477, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Lin, K.; Kang, H.; Dong, Y.; Guan, H.; Li, F. Primary Tumor Resection Prolongs Survival in Spinal Chondrosarcoma Patients With Distant Metastasis. Spine 2020, 45, E1661–E1668. [Google Scholar] [CrossRef]
- Imai, R.; Kamada, T.; Araki, N.; WORKING GROUP FOR BONE and SOFT-TISSUE SARCOMAS. Clinical Efficacy of Carbon Ion Radiotherapy for Unresectable Chondrosarcomas. Anticancer Res. 2017, 37, 6959–6964. [Google Scholar]
- Wu, S.; Li, P.; Cai, X.; Hong, Z.; Yu, Z.; Zhang, Q.; Fu, S. Carbon Ion Radiotherapy for Patients with Extracranial Chordoma or Chondrosarcoma—Initial Experience from Shanghai Proton and Heavy Ion Center. J. Cancer 2019, 10, 3315–3322. [Google Scholar] [CrossRef]
- Amer, K.M.; Munn, M.; Congiusta, D.; Abraham, J.A.; Mallick, A.B. Survival and Prognosis of Chondrosarcoma Subtypes: SEER Database Analysis. J. Orthop. Res. 2020, 38, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Speetjens, F.M.; de Jong, Y.; Gelderblom, H.; Bovée, J.V. Molecular oncogenesis of chondrosarcoma: Impact for targeted treatment. Curr. Opin. Oncol. 2016, 28, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Polychronidou, G.; Karavasilis, V.; Pollack, S.M.; Huang, P.H.; Lee, A.; Jones, R.L. Novel therapeutic approaches in chondrosarcoma. Future Oncol. 2017, 13, 637–648. [Google Scholar] [CrossRef]
- Balamuth, N.J.; Womer, R.B. Ewing’s sarcoma. Lancet Oncol. 2010, 11, 184–192. [Google Scholar] [CrossRef]
- Gargallo, P.; Juan, A.; Yáñez, Y.; Dolz, S.; Segura, V.; Castel, V.; Cañete, A. Precision medicine in Ewing sarcoma: A translational point of view. Clin. Transl. Oncol. 2020, 22, 1440–1454. [Google Scholar] [CrossRef]
- Andreou, D.; Ranft, A.; Gosheger, G.; Timmermann, B.; Ladenstein, R.; Hartmann, W.; Dirksen, U.; GPOH-Euro-EWING99 Consortium. Which Factors Are Associated with Local Control and Survival of Patients with Localized Pelvic Ewing’s Sarcoma? A Retrospective Analysis of Data from the Euro-EWING99 Trial. Clin. Orthop. Relat. Res. 2020, 478, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Robinson, S.I.; Arndt, C.A.S.; Petersen, I.A.; Haddock, M.G.; Rose, P.S.; Laack, N.N.I. Pelvis Ewing sarcoma: Local control and survival in the modern era. Pediatr. Blood Cancer 2017, 64. [Google Scholar] [CrossRef] [PubMed]
- Nesbit, M.E., Jr.; Gehan, E.A.; Burgert, E.O., Jr.; Vietti, T.J.; Cangir, A.; Tefft, M.; Kissane, J.M. Multimodal therapy for the management of primary, nonmetastatic Ewing’s sarcoma of bone: A long-term follow-up of the First Intergroup study. J. Clin. Oncol. 1990, 8, 1664–1674. [Google Scholar] [CrossRef] [PubMed]
- Womer, R.B.; West, D.C.; Krailo, M.D.; Dickman, P.S.; Pawel, B.R.; Grier, H.E.; Weiss, A.R. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: A report from the Children’s Oncology Group. J. Clin. Oncol. 2012, 30, 4148–4154. [Google Scholar] [CrossRef]
- Dirksen, U.; Brennan, B.; Le Deley, M.C.; Cozic, N.; Van Den Berg, H.; Bhadri, V.; Brichard, B.; Claude, L.; Craft, A.; Euro-Ewing 99 and Ewing 2008 Investigators; et al. High-Dose Chemotherapy Compared with Standard Chemotherapy and Lung Radiation in Ewing Sarcoma with Pulmonary Metastases: Results of the European Ewing Tumour Working Initiative of National Groups, 99 Trial and EWING 2008. J. Clin. Oncol. 2019, 37, 3192–3202. [Google Scholar] [CrossRef]
- Heinemann, M.; Ranft, A.; Langer, T.; Jürgens, H.; Kreyer, J.; Vieth, V.; Schäfers, M.; Weckesser, M.; Simon, T.; Hassenpflug, W.; et al. Recurrence of Ewing sarcoma: Is detection by imaging follow-up protocol associated with survival advantage? Pediatr. Blood Cancer 2018, 65, e27011. [Google Scholar] [CrossRef] [PubMed]
- Frezza, A.M.; Botta, L.; Trama, A.; Dei Tos, A.P.; Stacchiotti, S. Chordoma: Update on disease, epidemiology, biology and medical therapies. Curr. Opin. Oncol. 2019, 31, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Obid, P.; Fekete, T.; Drees, P.; Haschtmann, D.; Kleinstück, F.; Loibl, M.; Jeszenszky, D. Revision surgery for incomplete resection or recurrence of cervical spine chordoma: A consecutive case series of 24 patients. Eur. Spine J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Jin, J.; Jiang, C.; Huang, R.; Yin, H.; Song, D.; Cheng, L. Molecular Targeted Therapy in the Treatment of Chordoma: A Systematic Review. Front. Oncol. 2019, 9, 30. [Google Scholar] [CrossRef]
- Akinduro, O.O.; Suarez-Meade, P.; Garcia, D.; Brown, D.A.; Sarabia-Estrada, R.; Attia, S.; Gokaslan, Z.L.; Quiñones-Hinojosa, A. Targeted Therapy for Chordoma: Key Molecular Signaling Pathways and the Role of Multimodal Therapy. Target. Oncol. 2021, 16, 325–337. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Overall (n = 9178) | Osteosarcoma (n = 3049) | Chondrosarcoma (n = 2707) | Ewing sarcoma (n = 1208) | Chordoma (n = 679) | Other (n = 1535) |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| Median (quartiles) | 39 (18–61) | 22 (14–49) | 52 (38–66) | 16 (12–23) | 57 (42–70) | 54 (32–71) |
| 0–19 years | 2522 (27.5%) | 1390 (45.6%) | 115 (4.2%) | 813 (67.3%) | 24 (3.5%) | 180 (11.7%) |
| 20–39 years | 2070 (22.6%) | 682 (22.4%) | 625 (23.1%) | 308 (25.5%) | 125 (18.4%) | 330 (21.5%) |
| 40–59 years | 2058 (22.4%) | 431 (14.1%) | 962 (35.5%) | 65 (5.4%) | 222 (32.7%) | 378 (24.6%) |
| ≥60 years | 2528 (27.5%) | 546 (17.9%) | 1005 (37.1%) | 22 (1.8%) | 308 (45.4%) | 647 (42.1%) |
| Sex | ||||||
| Female | 4033 (43.9%) | 1351 (44.3%) | 1259 (46.5%) | 463 (38.3%) | 282 (41.5%) | 678 (44.2%) |
| Male | 5145 (56.1%) | 1698 (55.7%) | 1448 (53.5%) | 745 (61.7%) | 397 (58.5%) | 857 (55.8%) |
| Race | ||||||
| White | 7571 (82.5%) | 2300 (75.4%) | 2374 (87.7%) | 1094 (90.6%) | 578 (85.1%) | 1225 (79.8%) |
| Non-white | 1607 (17.5%) | 749 (24.6%) | 333 (12.3%) | 114 (9.4%) | 101 (14.9%) | 310 (20.2%) |
| Tumor primary site | ||||||
| Long bones of lower limb and associated joints | 3456 (37.7%) | 1793 (58.8%) | 842 (31.1%) | 348 (28.8%) | 1 (0.1%) | 472 (30.7%) |
| Pelvic bones, sacrum, coccyx and associated joints | 1489 (16.2%) | 292 (9.6%) | 448 (16.5%) | 280 (23.2%) | 240 (35.3%) | 229 (14.9%) |
| Long bones of upper limb, scapula, and associated joints | 995 (10.8%) | 313 (10.3%) | 425 (15.7%) | 130 (10.8%) | 0 (0.0%) | 127 (8.3%) |
| Bones of skull and face and associated joints | 857 (9.3%) | 187 (6.1%) | 227 (8.4%) | 39 (3.2%) | 236 (34.8%) | 168 (10.9%) |
| Rib, sternum, clavicle and associated joints | 714 (7.8%) | 107 (3.5%) | 393 (14.5%) | 158 (13.1%) | 1 (0.1%) | 55 (3.6%) |
| Vertebral column | 670 (7.3%) | 86 (2.8%) | 124 (4.6%) | 131 (10.8%) | 198 (29.2%) | 131 (8.5%) |
| Other site | 997 (10.9%) | 271 (8.9%) | 248 (9.2%) | 122 (10.1%) | 3 (0.4%) | 353 (23.0%) |
| Year of diagnosis | ||||||
| 1980–1989 | 1841 (20.1%) | 674 22.1%) | 469 (17.3%) | 301 (24.9%) | 100 (14.7%) | 297 (19.3%) |
| 1990–1999 | 2225 (24.2%) | 750 (24.6%) | 681 (25.2%) | 281 (23.3%) | 132 (19.4%) | 381 (24.8%) |
| 2000–2009 | 2525 (27.5%) | 786 (25.8%) | 806 (29.8%) | 314 (26.0%) | 178 (26.2%) | 441 (28.7%) |
| 2010–2018 | 2587 (28.2%) | 839 (27.5%) | 751 (27.7%) | 312 (25.8%) | 269 (39.6%) | 416 (27.1%) |
| Decade | 1980–1989 | 1990–1999 | 2000–2009 | 2010–2018 | ||||
|---|---|---|---|---|---|---|---|---|
| Model | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | p for Trend | |
| Multivariable model 1 | 1.00 | 0.74 (0.68–0.82) | <0.001 * | 0.71 (0.65–0.78) | <0.001 * | 0.68 (0.62–0.76) | <0.001 * | <0.001 * |
| Multivariable model 2 | - | 1.00 | 0.96 (0.87–1.05) | 0.365 | 0.93 (0.84–1.04) | 0.190 | 0.184 | |
| Decade | 1980–1989 | 1990–1999 | 2000–2009 | 2010–2018 | ||||
|---|---|---|---|---|---|---|---|---|
| Histological Type | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | p for Trend | |
| Multivariable model 1 | ||||||||
| Osteosarcoma | 1.00 | 0.75 (0.64–0.87) | <0.001 * | 0.74 (0.63–0.86) | <0.001 * | 0.65 (0.56–0.77) | <0.001 * | <0.001 * |
| Chondrosarcoma | 1.00 | 0.68 (0.55–0.85) | 0.001 * | 0.77 (0.62–0.95) | 0.016 * | 0.88 (0.70–1.12) | 0.300 | 0.478 |
| Ewing sarcoma | 1.00 | 0.63 (0.50–0.79) | <0.001 * | 0.53 (0.42–0.67) | <0.001 * | 0.48 (0.37–0.63) | <0.001 * | <0.001 * |
| Chordoma | 1.00 | 0.85 (0.59–1.22) | 0.366 | 0.65 (0.45–0.95) | 0.026 * | 0.58 (0.37–0.90) | 0.015 * | 0.005 * |
| Multivariable model 2 | ||||||||
| Osteosarcoma | _ | 1.00 | 0.97 (0.83–1.14) | 0.712 | 0.88 (0.74–1.05) | 0.149 | 0.153 | |
| Chondrosarcoma | _ | 1.00 | 1.13 (0.91–1.40) | 0.287 | 1.34 (1.05–1.71) | 0.019 * | 0.021 * | |
| Ewing sarcoma | _ | 1.00 | 0.83 (0.65–1.06) | 0.140 | 0.74 (0.56–0.99) | 0.041 * | 0.035 * | |
| Chordoma | _ | 1.00 | 0.85 (0.58–1.25) | 0.409 | 0.75 (0.47–1.19) | 0.220 | 0.208 | |
| Decade | 1980–1989 | 1990–1999 | 2000–2009 | 2010–2018 | ||||
|---|---|---|---|---|---|---|---|---|
| Primary Site | Adjusted HR | p | Adjusted HR | p | Adjusted HR | p | p for Trend | |
| Multivariable model 1 | ||||||||
| Long bones of lower limb and associated joints | 1.00 | 0.68 (0.58–0.79) | <0.001 * | 0.70 (0.60–0.82) | <0.001 * | 0.80 (0.68–0.95) | 0.01 * | 0.006 * |
| Pelvic bones, sacrum, coccyx and associated joints | 1.00 | 0.76 (0.62–0.94) | 0.011 * | 0.81 (0.66–0.99) | 0.043 * | 0.65 (0.52–0.81) | <0.001 * | 0.001* |
| Long bones of upper limb, scapula, and associated joints | 1.00 | 0.80 (0.59–1.07) | 0.128 | 0.68 (0.50–0.93) | 0.016 * | 0.51 (0.35–0.74) | <0.001 * | <0.001 * |
| Bones of skull and face and associated joints | 1.00 | 0.65 (0.45–0.94) | 0.021 * | 0.43 (0.29–0.62) | <0.001 * | 0.32 (0.21–0.51) | <0.001 * | <0.001 * |
| Rib, sternum, clavicle and associated joints | 1.00 | 0.68 (0.48–0.97) | 0.033 * | 0.59 (0.41–0.86) | 0.006 * | 0.79 (0.54–1.16) | 0.226 | 0.122 |
| Vertebral column | 1.00 | 0.87 (0.64–1.19) | 0.393 | 0.68 (0.49–0.94) | 0.019 * | 0.66 (0.46–0.94) | 0.022 * | 0.006 * |
| Multivariable model 2 | ||||||||
| Long bones of lower limb and associated joints | _ | 1.00 | 1.05 (0.89–1.24) | 0.561 | 1.22 (1.02–1.46) | 0.032 * | 0.036 * | |
| Pelvic bones, sacrum, coccyx and associated joints | _ | 1.00 | 1.10 (0.90–1.36) | 0.351 | 0.88 (0.70–1.11) | 0.282 | 0.298 | |
| Long bones of upper limb, scapula, and associated joints | _ | 1.00 | 0.87 (0.63–1.19) | 0.384 | 0.65 (0.44–0.95) | 0.027 * | 0.029 * | |
| Bones of skull and face and associated joints | _ | 1.00 | 0.63 (0.44–0.90) | 0.012 * | 0.48 (0.31–0.75) | 0.001 * | 0.001 * | |
| Rib, sternum, clavicle and associated joints | _ | 1.00 | 0.84 (0.57–1.25) | 0.396 | 1.16 (0.77–1.74) | 0.483 | 0.534 | |
| Vertebral column | _ | 1.00 | 0.78 (0.56–1.09) | 0.147 | 0.76 (0.53–1.09) | 0.137 | 0.121 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Deng, K.; Ye, H.; Sun, Z.; Huang, W.; Sun, Y.; Yan, W. Trends in Tumor Site-Specific Survival of Bone Sarcomas from 1980 to 2018: A Surveillance, Epidemiology and End Results-Based Study. Cancers 2021, 13, 5381. https://doi.org/10.3390/cancers13215381
Hu X, Deng K, Ye H, Sun Z, Huang W, Sun Y, Yan W. Trends in Tumor Site-Specific Survival of Bone Sarcomas from 1980 to 2018: A Surveillance, Epidemiology and End Results-Based Study. Cancers. 2021; 13(21):5381. https://doi.org/10.3390/cancers13215381
Chicago/Turabian StyleHu, Xianglin, Kai Deng, Hui Ye, Zhengwang Sun, Wending Huang, Yangbai Sun, and Wangjun Yan. 2021. "Trends in Tumor Site-Specific Survival of Bone Sarcomas from 1980 to 2018: A Surveillance, Epidemiology and End Results-Based Study" Cancers 13, no. 21: 5381. https://doi.org/10.3390/cancers13215381
APA StyleHu, X., Deng, K., Ye, H., Sun, Z., Huang, W., Sun, Y., & Yan, W. (2021). Trends in Tumor Site-Specific Survival of Bone Sarcomas from 1980 to 2018: A Surveillance, Epidemiology and End Results-Based Study. Cancers, 13(21), 5381. https://doi.org/10.3390/cancers13215381






