Systemic and Local Silk-Based Drug Delivery Systems for Cancer Therapy
Abstract
Simple Summary
Abstract
1. Introduction
2. Silk Fibroin
3. Silk Sericins
4. Spider Silk
5. Advantages of Silk Proteins for Controlled Drug Delivery
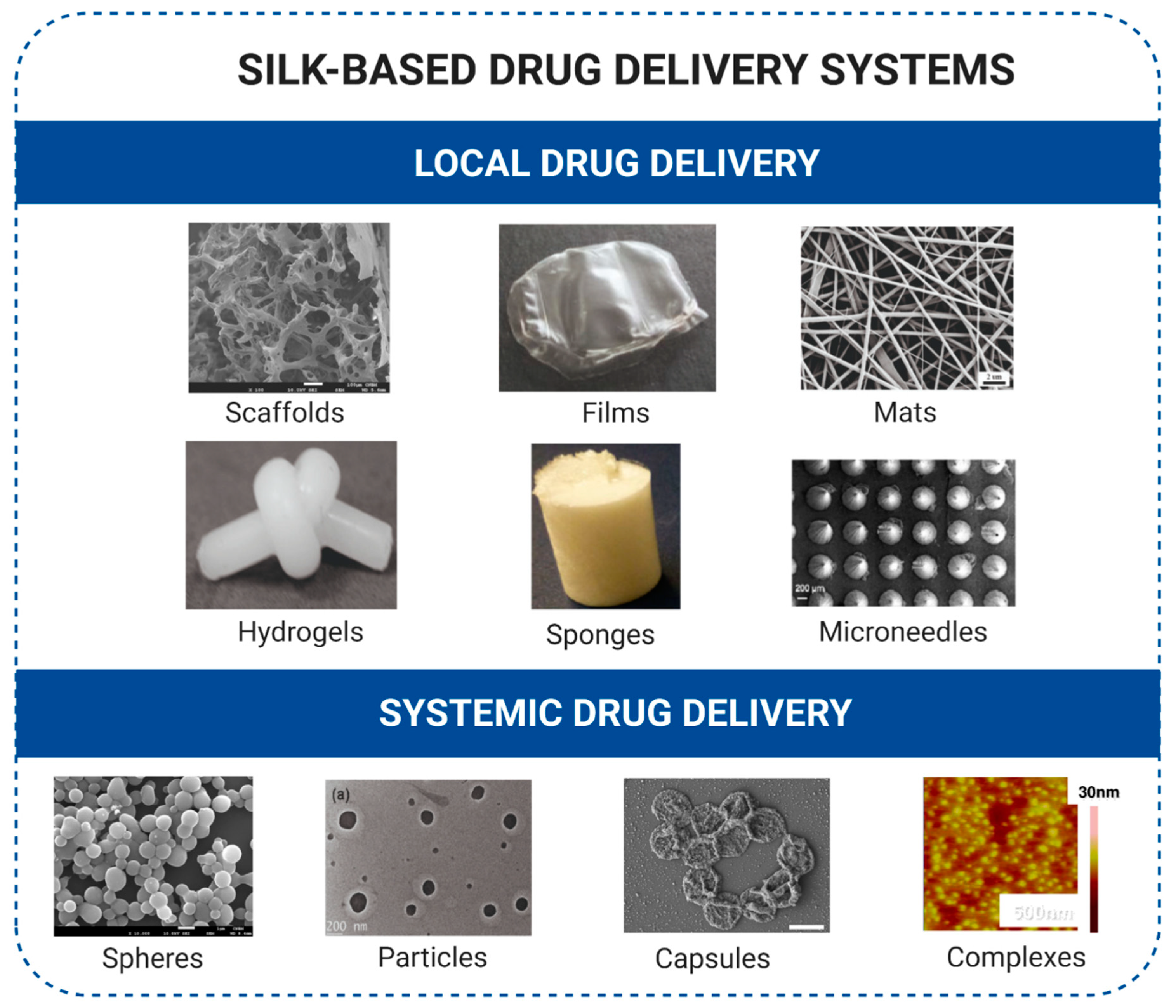
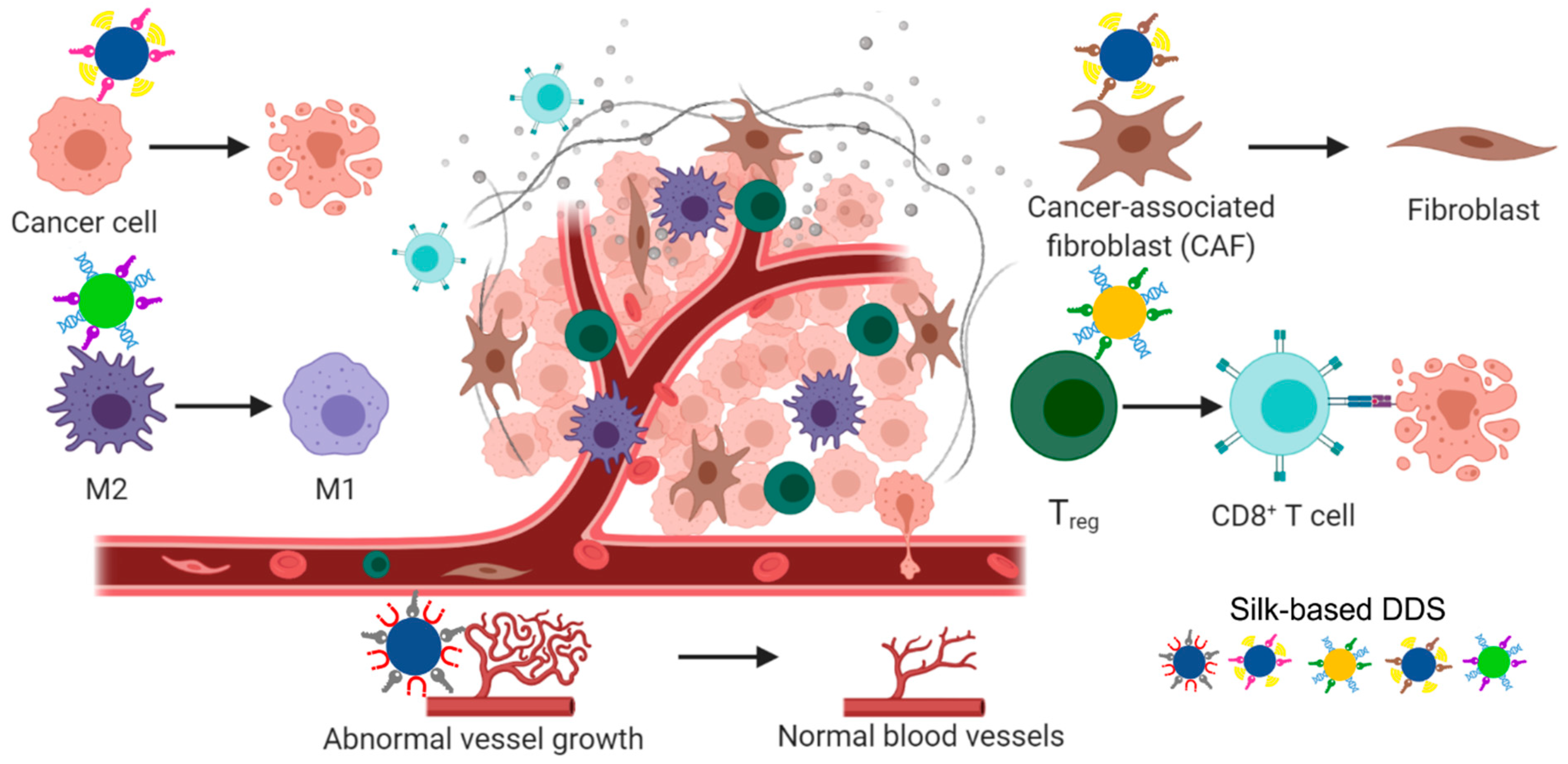
6. Silk-Based Biomaterials for Drug Delivery in Cancer Treatment
6.1. Local Drug Delivery
6.2. Systemic Drug Delivery
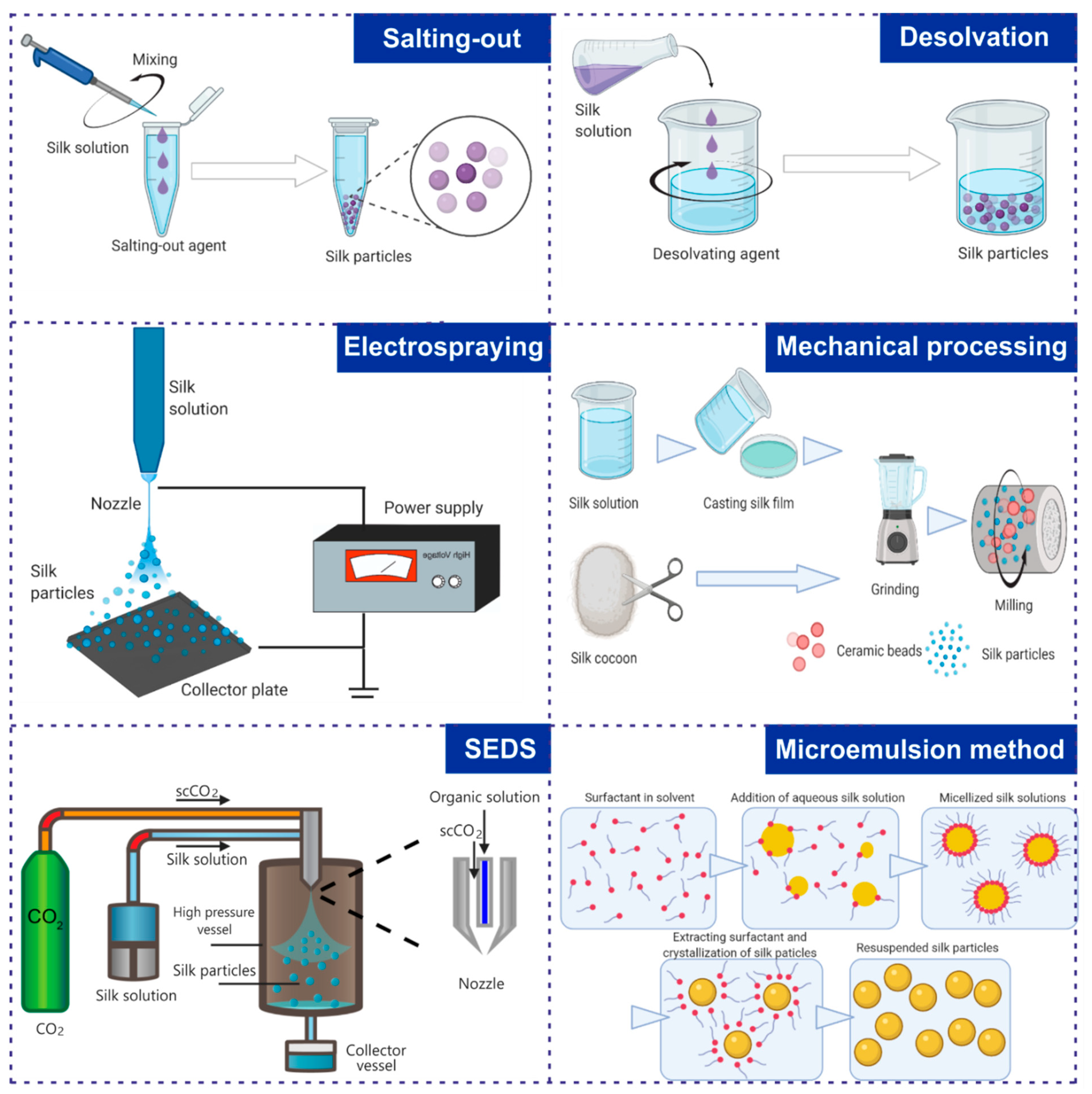
7. Preparation of Nanoparticles Made of Different Silk Proteins
8. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Majumder, J.; Taratula, O.; Minko, T. Nanocarrier-based systems for targeted and site specific therapeutic delivery. Adv. Drug Deliv. Rev. 2019, 144, 57–77. [Google Scholar] [CrossRef]
- Aquib, M.; Juthi, A.Z.; Farooq, M.A.; Ali, M.G.; Janabi, A.H.W.; Bavi, S.; Banerjee, P.; Bhosale, R.; Bavi, R.; Wang, B. Advances in local and systemic drug delivery systems for post-surgical cancer treatment. J. Mater. Chem. B 2020, 8, 8507–8518. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.K.; Hamad, M.A.; Hafez, M.Z.; Wooley, K.L.; Elsabahy, M. Nanomedicine in management of hepatocellular carcinoma: Challenges and opportunities. Int. J. Cancer 2017, 140, 1475–1484. [Google Scholar] [CrossRef]
- Aftab, S.; Shah, A.; Nadhman, A.; Kurbanoglu, S.; Aysil Ozkan, S.; Dionysiou, D.D.; Shukla, S.S.; Aminabhavi, T.M. Nanomedicine: An effective tool in cancer therapy. Int. J. Pharm. 2018, 540, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Doppalapudi, S.; Jain, A.; Domb, A.J.; Khan, W. Biodegradable polymers for targeted delivery of anti-cancer drugs. Expert Opin. Drug Deliv. 2016, 13, 891–909. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef]
- Tulay, P.; Galam, N.; Adali, T. The Wonders of Silk Fibroin Biomaterials in the Treatment of Breast Cancer. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, B.; Chambre, L.; Kaplan, D.L. Extended release formulations using silk proteins for controlled delivery of therapeutics. Expert Opin. Drug Deliv. 2019, 16, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Deptuch, T.; Dams-Kozlowska, H. Silk Materials Functionalized via Genetic Engineering for Biomedical Applications. Materials 2017, 10, 1417. [Google Scholar] [CrossRef]
- Jastrzebska, K.; Kucharczyk, K.; Florczak, A.; Dondajewska, E.; Mackiewicz, A.; Dams-Kozlowska, H. Silk as an innovative biomaterial for cancer therapy. Rep. Pract. Oncol. Radiother. 2015, 20, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, D.; Tokareva, O.; Rim, N.G.; Wong, J.Y.; Kaplan, D.L.; Buehler, M.J. Silk-Its Mysteries, How It Is Made, and How It Is Used. ACS Biomater. Sci. Eng. 2015, 1, 864–876. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Kaplan, D.L. Mechanism of silk processing in insects and spiders. Nature 2003, 424, 1057–1061. [Google Scholar] [CrossRef] [PubMed]
- Tokareva, O.; Jacobsen, M.; Buehler, M.; Wong, J.; Kaplan, D.L. Structure-function-property-design interplay in biopolymers: Spider silk. Acta Biomater. 2014, 10, 1612–1626. [Google Scholar] [CrossRef]
- Huang, W.; Ling, S.; Li, C.; Omenetto, F.G.; Kaplan, D.L. Silkworm silk-based materials and devices generated using bio-nanotechnology. Chem. Soc. Rev. 2018, 47, 6486–6504. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Ghosh, A.K.; Kundu, S.C. Purification and characterization of fibroin from the tropical Saturniid silkworm, Antheraea mylitta. Insect Biochem. Mol. Biol. 2001, 31, 1013–1018. [Google Scholar] [CrossRef]
- Li, F.; Bian, C.; Li, D.; Shi, Q. Spider Silks: An Overview of Their Component Proteins for Hydrophobicity and Biomedical Applications. Protein Pept. Lett. 2021, 28, 255–269. [Google Scholar] [CrossRef]
- Shehata, N.; Hassounah, I.; Sobolciak, P.; Krupa, I.; Lewis, R.V.; Kandas, I. Chapter 9—Spider Silk Fibers: Synthesis, Characterization, and Related Biomedical Applications. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 289–307. [Google Scholar]
- Cao, T.T.; Zhang, Y.Q. Processing and characterization of silk sericin from Bombyx mori and its application in biomaterials and biomedicines. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 61, 940–952. [Google Scholar] [CrossRef]
- Seo, Y.K.; Choi, G.M.; Kwon, S.Y.; Lee, H.S.; Park, Y.S.; Song, K.Y.; Kim, Y.J.; Park, J.K. The Biocompatibility of Silk Scaffold for Tissue Engineered Ligaments. Key Eng. Mater. 2007, 342–343, 73–76. [Google Scholar] [CrossRef]
- Kundu, B.; Kurland, N.E.; Bano, S.; Patra, C.; Engel, F.B.; Yadavalli, V.K.; Kundu, S.C. Silk proteins for biomedical applications: Bioengineering perspectives. Prog. Polym. Sci. 2014, 39, 251–267. [Google Scholar] [CrossRef]
- Crivelli, B.; Perteghella, S.; Bari, E.; Sorrenti, M.; Tripodo, G.; Chlapanidas, T.; Torre, M.L. Silk nanoparticles: From inert supports to bioactive natural carriers for drug delivery. Soft Matter 2018, 14, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.; Harris, J.; Zakharov, A.D.; Poirier, J.; Ikegaki, N.; Kajdacsy-Balla, A.; Pilichowska, M.; Lyubimov, A.V.; Shimada, H.; Kaplan, D.L.; et al. Implantable chemotherapy-loaded silk protein materials for neuroblastoma treatment. Int. J. Cancer 2017, 140, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.M.; Harris, J.; Cunningham, R.; Zeki, J.; Kaplan, D.L.; Chiu, B. Manipulation of variables in local controlled release vincristine treatment in neuroblastoma. J. Pediatr. Surg. 2017, 52, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Kaplan, D.L. Doxorubicin-loaded silk films: Drug-silk interactions and in vivo performance in human orthotopic breast cancer. Biomaterials 2012, 33, 8442–8450. [Google Scholar] [CrossRef]
- Seib, F.P.; Pritchard, E.M.; Kaplan, D.L. Self-assembling doxorubicin silk hydrogels for the focal treatment of primary breast cancer. Adv. Funct. Mater. 2013, 23, 58–65. [Google Scholar] [CrossRef]
- Shahbazi, B.; Taghipour, M.; Rahmani, H.; Sadrjavadi, K.; Fattahi, A. Preparation and characterization of silk fibroin/oligochitosan nanoparticles for siRNA delivery. Colloids Surf. B Biointerfaces 2015, 136, 867–877. [Google Scholar] [CrossRef]
- Sun, W.; Gregory, D.A.; Tomeh, M.A.; Zhao, X. Silk Fibroin as a Functional Biomaterial for Tissue Engineering. Int. J. Mol. Sci. 2021, 22, 1499. [Google Scholar] [CrossRef]
- Asakura, T.; Okushita, K.; Williamson, M.P. Analysis of the structure of Bombyx mori silk fibroin by NMR. Macromolecules 2015, 48, 2345–2357. [Google Scholar] [CrossRef]
- Ha, S.W.; Gracz, H.S.; Tonelli, A.E.; Hudson, S.M. Structural study of irregular amino acid sequences in the heavy chain of Bombyx mori silk fibroin. Biomacromolecules 2005, 6, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk fibroin: Structural implications of a remarkable amino acid sequence. Proteins 2001, 44, 119–122. [Google Scholar] [CrossRef]
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the H-L complex of silk fibroin produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Kikuchi, Y.; Takagi, T.; Kikuchi, A.; Oyama, F.; Shimura, K.; Mizuno, S. Primary structure of the silk fibroin light chain determined by cDNA sequencing and peptide analysis. J. Mol. Biol. 1989, 210, 127–139. [Google Scholar] [CrossRef]
- Zafar, M.S.; Belton, D.J.; Hanby, B.; Kaplan, D.L.; Perry, C.C. Functional material features of Bombyx mori silk light versus heavy chain proteins. Biomacromolecules 2015, 16, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine organization of Bombyx mori fibroin heavy chain gene. Nucleic Acids Res. 2000, 28, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, J.; Greish, K.; Frandsen, J.; Cappello, J.; Ghandehari, H. Silk-elastinlike recombinant polymers for gene therapy of head and neck cancer: From molecular definition to controlled gene expression. J. Control. Release 2009, 140, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.X.; Wang, M.; Lin, Y.; Xu, Q.; Kaplan, D.L. Hydrophobic drug-triggered self-assembly of nanoparticles from silk-elastin-like protein polymers for drug delivery. Biomacromolecules 2014, 15, 908–914. [Google Scholar] [CrossRef]
- Wu, J.H.; Wang, Z.; Xu, S.Y. Preparation and characterization of sericin powder extracted from silk industry wastewater. Food Chem. 2007, 103, 1255–1262. [Google Scholar] [CrossRef]
- Aramwit, P.; Palapinyo, S.; Srichana, T.; Chottanapund, S.; Muangman, P. Silk sericin ameliorates wound healing and its clinical efficacy in burn wounds. Arch. Dermatol. Res. 2013, 305, 585–594. [Google Scholar] [CrossRef]
- Kundu, S.C.; Dash, B.C.; Dash, R.; Kaplan, D.L. Natural protective glue protein, sercin bioengineered by silkworms: Potential for biomedical and biotechnological applications. Prog. Polym. Sci. 2008, 33, 998–1012. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, J.; Huang, L.; Qi, C.; Xu, L.; Liu, X.; Wang, G.; Wang, L.; Wang, Z. Safe and Effective Reversal of Cancer Multidrug Resistance Using Sericin-Coated Mesoporous Silica Nanoparticles for Lysosome-Targeting Delivery in Mice. Small 2016, 13, 1602567. [Google Scholar] [CrossRef]
- Kunz, R.I.; Brancalhao, R.M.; Ribeiro, L.F.; Natali, M.R. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef]
- Mandal, B.B.; Kundu, S.C. Self-assembled silk sericin/poloxamer nanoparticles as nanocarriers of hydrophobic and hydrophilic drugs for targeted delivery. Nanotechnology 2009, 20, 355101. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, T.; Xu, Z.; Liu, D.; Yang, M.; Zhu, L. Self-stabilized silk sericin-based nanoparticles: In vivo biocompatibility and reduced doxorubicin-induced toxicity. Acta Biomater. 2018, 74, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Deng, L.; Yu, J.; Chen, Z.; Woo, Y.; Liu, H.; Li, T.; Lin, T.; Chen, H.; Zhao, M.; et al. Sericin nanomicelles with enhanced cellular uptake and pH-triggered release of doxorubicin reverse cancer drug resistance. Drug Deliv. 2018, 25, 1103–1116. [Google Scholar] [CrossRef] [PubMed]
- Shuai, Y.; Yang, S.; Li, C.; Zhu, L.; Mao, C.; Yang, M. In situ protein-templated porous protein-hydroxylapatite nanocomposite microspheres for pH-dependent sustained anticancer drug release. J. Mater. Chem. B 2017, 5, 3945–3954. [Google Scholar] [CrossRef]
- Deng, L.; Guo, W.; Li, G.; Hu, Y.; Zhang, L.M. Hydrophobic IR780 loaded sericin nanomicelles for phototherapy with enhanced antitumor efficiency. Int. J. Pharm. 2019, 566, 549–556. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhang, Y.Q. Three-layered sericins around the silk fibroin fibre from Bombyx mori cocoon and their amino acid composition. Adv. Mater. Res. 2011, 175–176, 158–163. [Google Scholar] [CrossRef]
- Nayak, S.; Talukdar, S.; Kundu, S.C. Potential of 2D crosslinked sericin membranes with improved biostability for skin tissue engineering. Cell Tissue Res. 2012, 347, 783–794. [Google Scholar] [CrossRef]
- Hu, X.; Vasanthavada, K.; Kohler, K.; McNary, S.; Moore, A.M.; Vierra, C.A. Molecular mechanisms of spider silk. Cell. Mol. Life Sci. 2006, 63, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Yarger, J.; Cherry, B.; van der Vaart, A. Uncovering the structure–function relationship in spider silk. Nat. Rev. Mater. 2018, 3, 18008. [Google Scholar] [CrossRef]
- Hinman, M.B.; Lewis, R.V. Isolation of a clone encoding a second dragline silk fibroin. Nephila clavipes dragline silk is a two-protein fiber. J. Biol. Chem. 1992, 267, 19320–19324. [Google Scholar] [PubMed]
- Xu, M.; Lewis, R.V. Structure of a protein superfiber: Spider dragline silk. Proc. Natl. Acad. Sci. USA 1990, 87, 7120–7124. [Google Scholar] [CrossRef] [PubMed]
- Sponner, A.; Schlott, B.; Vollrath, F.; Unger, E.; Grosse, F.; Weisshart, K. Characterization of the protein components of Nephila clavipes dragline silk. Biochemistry 2005, 44, 4727–4736. [Google Scholar] [CrossRef] [PubMed]
- Heim, M.; Romer, L.; Scheibel, T. Hierarchical structures made of proteins. The complex architecture of spider webs and their constituent silk proteins. Chem. Soc. Rev. 2010, 39, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Eisoldt, L.; Smith, A.; Scheibel, T. Decoding the secrets of spider silk. Mater. Today 2011, 14, 80–86. [Google Scholar] [CrossRef]
- Ittah, S.; Barak, N.; Gat, U. A proposed model for dragline spider silk self-assembly: Insights from the effect of the repetitive domain size on fiber properties. Biopolymers 2010, 93, 458–468. [Google Scholar] [CrossRef]
- Askarieh, G.; Hedhammar, M.; Nordling, K.; Saenz, A.; Casals, C.; Rising, A.; Johansson, J.; Knight, S.D. Self-assembly of spider silk proteins is controlled by a pH-sensitive relay. Nature 2010, 465, 236–238. [Google Scholar] [CrossRef]
- Hagn, F.; Eisoldt, L.; Hardy, J.G.; Vendrely, C.; Coles, M.; Scheibel, T.; Kessler, H. A conserved spider silk domain acts as a molecular switch that controls fibre assembly. Nature 2010, 465, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.V. Spider silk: Ancient ideas for new biomaterials. Chem. Rev. 2006, 106, 3762–3774. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T. Structure and Dynamics of Spider Silk Studied with Solid-State Nuclear Magnetic Resonance and Molecular Dynamics Simulation. Molecules 2020, 25, 2634. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, C.Y.; Shipley, N.H.; Lewis, R.V. Hypotheses that correlate the sequence, structure, and mechanical properties of spider silk proteins. Int. J. Biol. Macromol. 1999, 24, 271–275. [Google Scholar] [CrossRef]
- Bratzel, G.; Buehler, M.J. Sequence-structure correlations in silk: Poly-Ala repeat of N. clavipes MaSp1 is naturally optimized at a critical length scale. J. Mech. Behav. Biomed. Mater. 2012, 7, 30–40. [Google Scholar] [CrossRef] [PubMed]
- van Beek, J.D.; Hess, S.; Vollrath, F.; Meier, B.H. The molecular structure of spider dragline silk: Folding and orientation of the protein backbone. Proc. Natl. Acad. Sci. USA 2002, 99, 10266–10271. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Oroudjev, E.; Mutz, S.; Cleveland, J.P.; Hansma, P.K.; Hayashi, C.Y.; Makarov, D.E.; Hansma, H.G. Molecular nanosprings in spider capture-silk threads. Nat. Mater. 2003, 2, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.M.; van der Vaart, A.; Guo, C.; Jones, J.; Onofrei, D.; Cherry, B.R.; Lewis, R.V.; Yarger, J.L.; Holland, G.P. Secondary Structure Adopted by the Gly-Gly-X Repetitive Regions of Dragline Spider Silk. Int. J. Mol. Sci. 2016, 17, 2023. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.E.; Creager, M.S.; Butler, E.B.; Lewis, R.V.; Yarger, J.L.; Holland, G.P. Solid-state NMR evidence for elastin-like beta-turn structure in spider dragline silk. Chem. Commun. 2010, 46, 6714–6716. [Google Scholar] [CrossRef]
- Brooks, A.E.; Steinkraus, H.B.; Nelson, S.R.; Lewis, R.V. An investigation of the divergence of major ampullate silk fibers from Nephila clavipes and Argiope aurantia. Biomacromolecules 2005, 6, 3095–3099. [Google Scholar] [CrossRef]
- Sponner, A.; Unger, E.; Grosse, F.; Weisshart, K. Differential polymerization of the two main protein components of dragline silk during fibre spinning. Nat. Mater. 2005, 4, 772–775. [Google Scholar] [CrossRef]
- Huemmerich, D.; Scheibel, T.; Vollrath, F.; Cohen, S.; Gat, U.; Ittah, S. Novel assembly properties of recombinant spider dragline silk proteins. Curr. Biol. 2004, 14, 2070–2074. [Google Scholar] [CrossRef]
- Rammensee, S.; Slotta, U.; Scheibel, T.; Bausch, A.R. Assembly mechanism of recombinant spider silk proteins. Proc. Natl. Acad. Sci. USA 2008, 105, 6590–6595. [Google Scholar] [CrossRef]
- Kiseleva, A.P.; Krivoshapkin, P.V.; Krivoshapkina, E.F. Recent Advances in Development of Functional Spider Silk-Based Hybrid Materials. Front. Chem. 2020, 8, 554. [Google Scholar] [CrossRef]
- Zheng, K.; Ling, S. De Novo Design of Recombinant Spider Silk Proteins for Material Applications. Biotechnol. J. 2019, 14, e1700753. [Google Scholar] [CrossRef]
- Meyer, D.E.; Chilkoti, A. Genetically encoded synthesis of protein-based polymers with precisely specified molecular weight and sequence by recursive directional ligation: Examples from the elastin-like polypeptide system. Biomacromolecules 2002, 3, 357–367. [Google Scholar] [CrossRef]
- Tokareva, O.; Michalczechen-Lacerda, V.A.; Rech, E.L.; Kaplan, D.L. Recombinant DNA production of spider silk proteins. Microb. Biotechnol. 2013, 6, 651–663. [Google Scholar] [CrossRef]
- Huemmerich, D.; Helsen, C.W.; Quedzuweit, S.; Oschmann, J.; Rudolph, R.; Scheibel, T. Primary structure elements of spider dragline silks and their contribution to protein solubility. Biochemistry 2004, 43, 13604–13612. [Google Scholar] [CrossRef]
- Aigner, T.B.; DeSimone, E.; Scheibel, T. Biomedical Applications of Recombinant Silk-Based Materials. Adv. Mater. 2018, 30, e1704636. [Google Scholar] [CrossRef]
- Herold, H.M.; Scheibel, T. Applicability of biotechnologically produced insect silks. Z. Naturforsch. C. J. Biosci. 2017, 72, 365–385. [Google Scholar] [CrossRef]
- Elsner, M.B.; Herold, H.M.; Muller-Herrmann, S.; Bargel, H.; Scheibel, T. Enhanced cellular uptake of engineered spider silk particles. Biomater. Sci. 2015, 3, 543–551. [Google Scholar] [CrossRef]
- Kozlowska, A.K.; Florczak, A.; Smialek, M.; Dondajewska, E.; Mackiewicz, A.; Kortylewski, M.; Dams-Kozlowska, H. Functionalized bioengineered spider silk spheres improve nuclease resistance and activity of oligonucleotide therapeutics providing a strategy for cancer treatment. Acta Biomater. 2017, 59, 221–233. [Google Scholar] [CrossRef]
- Numata, K.; Subramanian, B.; Currie, H.A.; Kaplan, D.L. Bioengineered silk protein-based gene delivery systems. Biomaterials 2009, 30, 5775–5784. [Google Scholar] [CrossRef]
- Numata, K.; Hamasaki, J.; Subramanian, B.; Kaplan, D.L. Gene delivery mediated by recombinant silk proteins containing cationic and cell binding motifs. J. Control. Release 2010, 146, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Kaplan, D.L. Silk-based gene carriers with cell membrane destabilizing peptides. Biomacromolecules 2010, 11, 3189–3195. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Mieszawska-Czajkowska, A.J.; Kvenvold, L.A.; Kaplan, D.L. Silk-based nanocomplexes with tumor-homing peptides for tumor-specific gene delivery. Macromol. Biosci. 2012, 12, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Reagan, M.R.; Goldstein, R.H.; Rosenblatt, M.; Kaplan, D.L. Spider silk-based gene carriers for tumor cell-specific delivery. Bioconjug. Chem. 2011, 22, 1605–1610. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.; Edwards, R.J. HIV-1-trans-activating (Tat) protein: Both a target and a tool in therapeutic approaches. Biochem. Pharmacol. 1999, 58, 1521–1528. [Google Scholar] [CrossRef]
- Laakkonen, P.; Vuorinen, K. Homing peptides as targeted delivery vehicles. Integr. Biol. (Camb.) 2010, 2, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Florczak, A.; Jastrzebska, K.; Mackiewicz, A.; Dams-Kozlowska, H. Blending two bioengineered spider silks to develop cancer targeting spheres. J. Mater. Chem. B 2017, 5, 3000–3011. [Google Scholar] [CrossRef]
- Florczak, A.; Mackiewicz, A.; Dams-Kozlowska, H. Functionalized spider silk spheres as drug carriers for targeted cancer therapy. Biomacromolecules 2014, 15, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Florczak, A.; Deptuch, T.; Lewandowska, A.; Penderecka, K.; Kramer, E.; Marszalek, A.; Mackiewicz, A.; Dams-Kozlowska, H. Functionalized silk spheres selectively and effectively deliver a cytotoxic drug to targeted cancer cells in vivo. J. Nanobiotechnol. 2020, 18, 177. [Google Scholar] [CrossRef]
- Saric, M.; Scheibel, T. Engineering of silk proteins for materials applications. Curr. Opin. Biotechnol. 2019, 60, 213–220. [Google Scholar] [CrossRef]
- Florczak, A.; Mackiewicz, A.; Dams-Kozlowska, H. Cellular uptake, intracellular distribution and degradation of Her2-targeting silk nanospheres. Int. J. Nanomed. 2019, 14, 6855–6865. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Li, C.; Kaplan, D.L. Enzymatic Degradation of Bombyx mori Silk Materials: A Review. Biomacromolecules 2020, 21, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Guo, C.; Fitzpatrick, V.; Ibrahim, A.; Zwierstra, M.J.; Hanna, P.; Lechtig, A.; Nazarian, A.; Lin, S.J.; Kaplan, D.L. Design of biodegradable, implantable devices towards clinical translation. Nat. Rev. Mater. 2020, 5, 61–81. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, B.; Li, M.; Zuo, B.; Kaplan, D.L.; Huang, Y.; Zhu, H. Degradation mechanism and control of silk fibroin. Biomacromolecules 2011, 12, 1080–1086. [Google Scholar] [CrossRef]
- Numata, K.; Cebe, P.; Kaplan, D.L. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials 2010, 31, 2926–2933. [Google Scholar] [CrossRef]
- Totten, J.D.; Wongpinyochit, T.; Seib, F.P. Silk nanoparticles: Proof of lysosomotropic anticancer drug delivery at single-cell resolution. J. Drug Target. 2017, 25, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Chlapanidas, T.; Perteghella, S.; Farago, S.; Boschi, A.; Tripodo, G.; Vigani, B.; Crivelli, B.; Renzi, S.; Dotti, S.; Preda, S.; et al. Platelet lysate and adipose mesenchymal stromal cells on silk fibroin nonwoven mats for wound healing. J. Appl. Polym. Sci. 2016, 133, 42942. [Google Scholar] [CrossRef]
- Panilaitis, B.; Altman, G.H.; Chen, J.; Jin, H.J.; Karageorgiou, V.; Kaplan, D.L. Macrophage responses to silk. Biomaterials 2003, 24, 3079–3085. [Google Scholar] [CrossRef]
- Xie, H.; Wang, J.; He, Y.; Gu, Z.Y.; Xu, J.; Li, L.; Ye, Q. Biocompatibility and safety evaluation of a silk fibroin-doped calcium phosphate scaffold copolymer in vitro and in vivo. RSC Adv. 2017, 7, 46036–46044. [Google Scholar] [CrossRef]
- Deptuch, T.; Florczak, A.; Lewandowska, A.; Leporowska, E.; Penderecka, K.; Marszalek, A.; Mackiewicz, A.; Dams-Kozlowska, H. MS1-type bioengineered spider silk nanoparticles do not exhibit toxicity in an in vivo mouse model. Nanomedicine 2021, 16, 1553–1565. [Google Scholar] [CrossRef] [PubMed]
- Helfricht, N.; Klug, M.; Mark, A.; Kuznetsov, V.; Blum, C.; Scheibel, T.; Papastavrou, G. Surface properties of spider silk particles in solution. Biomater. Sci. 2013, 1, 1166–1171. [Google Scholar] [CrossRef]
- Kwon, K.; Seok, H. Silk protein-based membrane for guided bone regeneration. Appl. Sci. 2018, 8, 1214. [Google Scholar] [CrossRef]
- Yucel, T.; Lovett, M.L.; Kaplan, D.L. Silk-based biomaterials for sustained drug delivery. J. Control. Release 2014, 190, 381–397. [Google Scholar] [CrossRef]
- Dubey, P.; Murab, S.; Karmakar, S.; Chowdhury, P.K.; Ghosh, S. Modulation of Self-Assembly Process of Fibroin: An Insight for Regulating the Conformation of Silk Biomaterials. Biomacromolecules 2015, 16, 3936–3944. [Google Scholar] [CrossRef]
- Lammel, A.; Schwab, M.; Slotta, U.; Winter, G.; Scheibel, T. Processing conditions for the formation of spider silk microspheres. ChemSusChem 2008, 1, 413–416. [Google Scholar] [CrossRef]
- Wenk, E.; Wandrey, A.J.; Merkle, H.P.; Meinel, L. Silk fibroin spheres as a platform for controlled drug delivery. J. Control. Release 2008, 132, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Park, J.; Karageorgiou, V.; Kim, U.J.; Valluzzi, R.; Cebe, P.; Kaplan, D.L. Water-Stable Silk Films with Reduced β-Sheet Content. Adv. Funct. Mater. 2005, 15, 1241–1247. [Google Scholar] [CrossRef]
- Subia, B.; Kundu, S.C. Drug loading and release on tumor cells using silk fibroin-albumin nanoparticles as carriers. Nanotechnology 2013, 24, 035103. [Google Scholar] [CrossRef] [PubMed]
- Kucharczyk, K.; Rybka, J.D.; Hilgendorff, M.; Krupinski, M.; Slachcinski, M.; Mackiewicz, A.; Giersig, M.; Dams-Kozlowska, H. Composite spheres made of bioengineered spider silk and iron oxide nanoparticles for theranostics applications. PLoS ONE 2019, 14, e0219790. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef] [PubMed]
- Blüm, C.; Scheibel, T. Control of drug loading and release properties of spider silk sub-microparticles. BioNanoScience 2012, 2, 67–74. [Google Scholar] [CrossRef]
- Doblhofer, E.; Scheibel, T. Engineering of recombinant spider silk proteins allows defined uptake and release of substances. J. Pharm. Sci. 2015, 104, 988–994. [Google Scholar] [CrossRef]
- Jastrzebska, K.; Florczak, A.; Kucharczyk, K.; Lin, Y.; Wang, Q.; Mackiewicz, A.; Kaplan, D.L.; Dams-Kozlowska, H. Delivery of chemotherapeutics using spheres made of bioengineered spider silks derived from MaSp1 and MaSp2 proteins. Nanomedicine 2018, 13, 439–454. [Google Scholar] [CrossRef]
- Hines, D.J.; Kaplan, D.L. Characterization of small molecule controlled release from silk films. Macromol. Chem. Phys. 2012, 214, 280–294. [Google Scholar] [CrossRef]
- Wang, S.H.; Xu, T.; Yang, Y.H.; Shao, Z.Z. Colloidal Stability of Silk Fibroin Nanoparticles Coated with Cationic Polymer for Effective Drug Delivery. ACS Appl. Mater. Interfaces 2015, 7, 21254–21262. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Weiss, M.; Jastrzebska, K.; Luczak, M.; Ptak, A.; Kozak, M.; Mackiewicz, A.; Dams-Kozlowska, H. Bioengineering the spider silk sequence to modify its affinity for drugs. Int. J. Nanomed. 2018, 13, 4247–4261. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef]
- Murphy, A.R.; Kaplan, D.L. Biomedical applications of chemically-modified silk fibroin. J. Mater. Chem. 2009, 19, 6443–6450. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, A.; Algieri, F.; De Matteis, L.; Lozano-Perez, A.A.; Garrido-Mesa, J.; Vezza, T.; de la Fuente, J.M.; Cenis, J.L.; Galvez, J.; Rodriguez-Cabezas, M.E. Intestinal anti-inflammatory effects of RGD-functionalized silk fibroin nanoparticles in trinitrobenzenesulfonic acid-induced experimental colitis in rats. Int. J. Nanomed. 2016, 11, 5945–5958. [Google Scholar] [CrossRef]
- Herold, H.M.; Dobl, A.; Wohlrab, S.; Humenik, M.; Scheibel, T. Designed Spider Silk-Based Drug Carrier for Redox- or pH-Triggered Drug Release. Biomacromolecules 2020, 21, 4904–4912. [Google Scholar] [CrossRef]
- Megeed, Z.; Haider, M.; Li, D.; O’Malley, B.W., Jr.; Cappello, J.; Ghandehari, H. In vitro and in vivo evaluation of recombinant silk-elastinlike hydrogels for cancer gene therapy. J. Control. Release 2004, 94, 433–445. [Google Scholar] [CrossRef]
- Price, R.; Gustafson, J.; Greish, K.; Cappello, J.; McGill, L.; Ghandehari, H. Comparison of silk-elastinlike protein polymer hydrogel and poloxamer in matrix-mediated gene delivery. Int. J. Pharm. 2012, 427, 97–104. [Google Scholar] [CrossRef]
- Mottaghitalab, F.; Kiani, M.; Farokhi, M.; Kundu, S.C.; Reis, R.L.; Gholami, M.; Bardania, H.; Dinarvand, R.; Geramifar, P.; Beiki, D.; et al. Targeted Delivery System Based on Gemcitabine-Loaded Silk Fibroin Nanoparticles for Lung Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 31600–31611. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Florczak, A.; Deptuch, T.; Penderecka, K.; Jastrzebska, K.; Mackiewicz, A.; Dams-Kozlowska, H. Drug affinity and targeted delivery: Double functionalization of silk spheres for controlled doxorubicin delivery into Her2-positive cancer cells. J. Nanobiotechnol. 2020, 18, 56. [Google Scholar] [CrossRef]
- Kucharczyk, K.; Kaczmarek, K.; Jozefczak, A.; Slachcinski, M.; Mackiewicz, A.; Dams-Kozlowska, H. Hyperthermia treatment of cancer cells by the application of targeted silk/iron oxide composite spheres. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111654. [Google Scholar] [CrossRef]
- Mao, B.; Liu, C.; Zheng, W.; Li, X.; Ge, R.; Shen, H.; Guo, X.; Lian, Q.; Shen, X.; Li, C. Cyclic cRGDfk peptide and Chlorin e6 functionalized silk fibroin nanoparticles for targeted drug delivery and photodynamic therapy. Biomaterials 2018, 161, 306–320. [Google Scholar] [CrossRef]
- Rnjak-Kovacina, J.; DesRochers, T.M.; Burke, K.A.; Kaplan, D.L. The effect of sterilization on silk fibroin biomaterial properties. Macromol. Biosci. 2015, 15, 861–874. [Google Scholar] [CrossRef]
- Gil, E.S.; Park, S.H.; Hu, X.; Cebe, P.; Kaplan, D.L. Impact of sterilization on the enzymatic degradation and mechanical properties of silk biomaterials. Macromol. Biosci. 2014, 14, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Hedhammar, M.; Bramfeldt, H.; Baris, T.; Widhe, M.; Askarieh, G.; Nordling, K.; Aulock, S.; Johansson, J. Sterilized recombinant spider silk fibers of low pyrogenicity. Biomacromolecules 2010, 11, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Lucke, M.; Winter, G.; Engert, J. The effect of steam sterilization on recombinant spider silk particles. Int. J. Pharm. 2015, 481, 125–131. [Google Scholar] [CrossRef]
- Hofmann, S.; Stok, K.S.; Kohler, T.; Meinel, A.J.; Muller, R. Effect of sterilization on structural and material properties of 3-D silk fibroin scaffolds. Acta Biomater. 2014, 10, 308–317. [Google Scholar] [CrossRef]
- Amornthep, K.; Prateep, M.; Boonya, S.; Lertyot, T.; Rachanee, U.; Bovornlak, O. Effect of gamma radiation on biodegradation of Bombyx mori silk fibroin. Int. Biodeterior. Biodegrad. 2008, 62, 487–490. [Google Scholar] [CrossRef]
- Lai, W.L.; Goh, K.L. Consequences of Ultra-Violet Irradiation on the Mechanical Properties of Spider Silk. J. Funct. Biomater. 2015, 6, 901–916. [Google Scholar] [CrossRef]
- Masuhiro, T.; Guiliano, F.; Norihiko, M. Changes in the fine structure of silk fibroin fibers following gamma irradiation. J. Appl. Polym. Sci. 1994, 51, 823–829. [Google Scholar] [CrossRef]
- Florczak, A.; Grzechowiak, I.; Deptuch, T.; Kucharczyk, K.; Kaminska, A.; Dams-Kozlowska, H. Silk Particles as Carriers of Therapeutic Molecules for Cancer Treatment. Materials 2020, 13, 4946. [Google Scholar] [CrossRef]
- Gao, Y.; Hou, M.; Yang, R.; Zhang, L.; Xu, Z.; Kang, Y.; Xue, P. Highly Porous Silk Fibroin Scaffold Packed in PEGDA/Sucrose Microneedles for Controllable Transdermal Drug Delivery. Biomacromolecules 2019, 20, 1334–1345. [Google Scholar] [CrossRef]
- Yavuz, B.; Zeki, J.; Coburn, J.M.; Ikegaki, N.; Levitin, D.; Kaplan, D.L.; Chiu, B. In vitro and in vivo evaluation of etoposide—Silk wafers for neuroblastoma treatment. J. Control. Release 2018, 285, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Yucel, T.; Lovett, M.L.; Giangregorio, R.; Coonahan, E.; Kaplan, D.L. Silk fibroin rods for sustained delivery of breast cancer therapeutics. Biomaterials 2014, 35, 8613–8620. [Google Scholar] [CrossRef]
- Zeki, J.; Taylor, J.S.; Yavuz, B.; Coburn, J.; Ikegaki, N.; Kaplan, D.L.; Chiu, B. Disseminated injection of vincristine-loaded silk gel improves the suppression of neuroblastoma tumor growth. Surgery 2018, 164, 909–915. [Google Scholar] [CrossRef]
- Dondajewska, E.; Juzwa, W.; Mackiewicz, A.; Dams-Kozlowska, H. Heterotypic breast cancer model based on a silk fibroin scaffold to study the tumor microenvironment. Oncotarget 2018, 9, 4935–4950. [Google Scholar] [CrossRef]
- Pollini, M.; Paladini, F. Bioinspired Materials for Wound Healing Application: The Potential of Silk Fibroin. Materials 2020, 13, 3361. [Google Scholar] [CrossRef]
- Yin, Y.; Xiong, J. Finite Element Analysis of Electrospun Nanofibrous Mats under Biaxial Tension. Nanomaterials 2018, 8, 348. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Yang, Y.; Shao, Z. Physically crosslinked biocompatible silk-fibroin-based hydrogels with high mechanical performance. Adv. Funct. Mater. 2016, 26, 872–880. [Google Scholar] [CrossRef]
- Yavuz, B.; Chambre, L.; Harrington, K.; Kluge, J.; Valenti, L.; Kaplan, D.L. Silk Fibroin Microneedle Patches for the Sustained Release of Levonorgestrel. ACS Appl. Bio Mater. 2020, 3, 5375–5382. [Google Scholar] [CrossRef]
- Kundu, J.; Chung, Y.I.; Kim, Y.H.; Tae, G.; Kundu, S.C. Silk fibroin nanoparticles for cellular uptake and control release. Int. J. Pharm. 2010, 388, 242–250. [Google Scholar] [CrossRef]
- Li, L.; Puhl, S.; Meinel, L.; Germershaus, O. Silk fibroin layer-by-layer microcapsules for localized gene delivery. Biomaterials 2014, 35, 7929–7939. [Google Scholar] [CrossRef] [PubMed]
- Chiu, B.; Coburn, J.; Pilichowska, M.; Holcroft, C.; Seib, F.P.; Charest, A.; Kaplan, D.L. Surgery combined with controlled-release doxorubicin silk films as a treatment strategy in an orthotopic neuroblastoma mouse model. Br. J. Cancer 2014, 111, 708–715. [Google Scholar] [CrossRef]
- Yavuz, B.; Zeki, J.; Taylor, J.; Harrington, K.; Coburn, J.M.; Ikegaki, N.; Kaplan, D.L.; Chiu, B. Silk Reservoirs for Local Delivery of Cisplatin for Neuroblastoma Treatment: In Vitro and In Vivo Evaluations. J. Pharm. Sci. 2019, 108, 2748–2755. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Wang, H.; Wei, K.; Yang, Y.; Zheng, R.Y.; Kim, I.S.; Zhang, K.Q. A Review of Structure Construction of Silk Fibroin Biomaterials from Single Structures to Multi-Level Structures. Int. J. Mol. Sci. 2017, 18, 237. [Google Scholar] [CrossRef] [PubMed]
- Terada, D.; Yokoyama, Y.; Hattori, S.; Kobayashi, H.; Tamada, Y. The outermost surface properties of silk fibroin films reflect ethanol-treatment conditions used in biomaterial preparation. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 119–126. [Google Scholar] [CrossRef]
- Sagnella, A.; Pistone, A.; Bonetti, S.; Donnadio, A.; Saracino, E.; Nocchetti, M.; Dionigi, C.; Ruani, G.; Muccini, M.; Posati, T. Effect of different fabrication methods on the chemo-physical properties of silk fibroin films and on their interaction with neural cells. RSC Adv. 2016, 6, 9304–9314. [Google Scholar] [CrossRef]
- Jiang, C.S.; Wang, X.; Gunawidjaja, R.; Lin, Y.H.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical properties of robust ultrathin silk fibroin films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Huemmerich, D.; Slotta, U.; Scheibel, T. Proccessing and modification of films made from recombinant spider silk proteins. Appl. Phys. A 2006, 82, 219–222. [Google Scholar] [CrossRef]
- Pandey, V.; Haider, T.; Jain, P.; Gupta, P.N.; Soni, V. Silk as leading-edge biological macromolecule for improved drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101294. [Google Scholar] [CrossRef]
- Chouhan, D.; Janani, G.; Chakraborty, B.; Nandi, S.K.; Mandal, B.B. Functionalized PVA-silk blended nanofibrous mats promote diabetic wound healing via regulation of extracellular matrix and tissue remodelling. J. Tissue Eng. Regen. Med. 2018, 12, e1559–e1570. [Google Scholar] [CrossRef]
- Fan, S.; Zhang, Y.; Shao, H.; Hu, X. Electrospun regenerated silk fibroin mats with enhanced mechanical properties. Int. J. Biol. Macromol. 2013, 56, 83–88. [Google Scholar] [CrossRef]
- Xie, M.; Fan, D.; Chen, Y.; Zhao, Z.; He, X.; Li, G.; Chen, A.; Wu, X.; Li, J.; Li, Z.; et al. An implantable and controlled drug-release silk fibroin nanofibrous matrix to advance the treatment of solid tumour cancers. Biomaterials 2016, 103, 33–43. [Google Scholar] [CrossRef]
- Kapoor, S.; Kundu, S.C. Silk protein-based hydrogels: Promising advanced materials for biomedical applications. Acta Biomater. 2016, 31, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Sommer, M.R.; Schaffner, M.; Carnelli, D.; Studart, A.R. 3D Printing of Hierarchical Silk Fibroin Structures. ACS Appl. Mater. Interfaces 2016, 8, 34677–34685. [Google Scholar] [CrossRef]
- Bellas, E.; Lo, T.J.; Fournier, E.P.; Brown, J.E.; Abbott, R.D.; Gil, E.S.; Marra, K.G.; Rubin, J.P.; Leisk, G.G.; Kaplan, D.L. Injectable silk foams for soft tissue regeneration. Adv. Healthc. Mater. 2015, 4, 452–459. [Google Scholar] [CrossRef]
- Chambre, L.; Parker, R.N.; Allardyce, B.J.; Valente, F.; Rajkhowa, R.; Dilley, R.J.; Wang, X.; Kaplan, D.L. Tunable biodegradable silk-based memory foams with controlled release of antibiotics. ACS Appl. Bio Mater. 2020, 3, 2466–2472. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; McCrudden, M.T.; Donnelly, R.F. Transdermal Drug Delivery: Innovative Pharmaceutical Developments Based on Disruption of the Barrier Properties of the stratum corneum. Pharmaceutics 2015, 7, 438–470. [Google Scholar] [CrossRef]
- Tsioris, K.; Raja, W.K.; Pritchard, E.M.; Panilaitis, B.; Kaplan, D.L.; Omenetto, F.G. Fabrication of silk microneedles for controlled-release drug delivery. Adv. Funct. Mater. 2012, 22, 330–335. [Google Scholar] [CrossRef]
- DeMuth, P.C.; Min, Y.; Irvine, D.J.; Hammond, P.T. Implantable silk composite microneedles for programmable vaccine release kinetics and enhanced immunogenicity in transcutaneous immunization. Adv. Healthc. Mater. 2014, 3, 47–58. [Google Scholar] [CrossRef]
- Stinson, J.A.; Raja, W.K.; Lee, S.; Kim, H.B.; Diwan, I.; Tutunjian, S.; Panilaitis, B.; Omenetto, F.G.; Tzipori, S.; Kaplan, D.L. Silk fibroin microneedles for transdermal vaccine delivery. ACS Biomater. Sci. Eng. 2017, 3, 360–369. [Google Scholar] [CrossRef]
- Seib, F.P.; Coburn, J.; Konrad, I.; Klebanov, N.; Jones, G.T.; Blackwood, B.; Charest, A.; Kaplan, D.L.; Chiu, B. Focal therapy of neuroblastoma using silk films to deliver kinase and chemotherapeutic agents in vivo. Acta Biomater. 2015, 20, 32–38. [Google Scholar] [CrossRef]
- Coburn, J.M.; Na, E.; Kaplan, D.L. Modulation of vincristine and doxorubicin binding and release from silk films. J. Control. Release 2015, 220, 229–238. [Google Scholar] [CrossRef]
- Li, H.J.; Zhu, J.; Chen, S.; Jia, L.; Ma, Y. Fabrication of aqueous-based dual drug loaded silk fibroin electrospun nanofibers embedded with curcumin-loaded RSF nanospheres for drugs controlled release. RSC Adv. 2017, 7, 56550–56558. [Google Scholar] [CrossRef]
- Harris, J.C.; Coburn, J.M.; Kajdacsy-Balla, A.; Kaplan, D.L.; Chiu, B. Sustained delivery of vincristine inside an orthotopic mouse sarcoma model decreases tumor growth. J. Pediatr. Surg. 2016, 51, 2058–2062. [Google Scholar] [CrossRef][Green Version]
- Wu, H.; Liu, S.; Xiao, L.; Dong, X.D.; Lu, Q.; Kaplan, D.L. Injectable and pH-responsive silk nanofiber hydrogels for sustained anticancer drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 17118–17126. [Google Scholar] [CrossRef]
- Shchepelina, O.; Drachuk, I.; Gupta, M.K.; Lin, J.; Tsukruk, V.V. Silk-on-silk layer-by-layer microcapsules. Adv. Mater. 2011, 23, 4655–4660. [Google Scholar] [CrossRef]
- Wang, X.; Yucel, T.; Lu, Q.; Hu, X.; Kaplan, D.L. Silk nanospheres and microspheres from silk/pva blend films for drug delivery. Biomaterials 2010, 31, 1025–1035. [Google Scholar] [CrossRef]
- Seib, F.P.; Jones, G.T.; Rnjak-Kovacina, J.; Lin, Y.; Kaplan, D.L. pH-dependent anticancer drug release from silk nanoparticles. Adv. Healthc. Mater. 2013, 2, 1606–1611. [Google Scholar] [CrossRef] [PubMed]
- Wongpinyochit, T.; Johnston, B.F.; Seib, F.P. Manufacture and Drug Delivery Applications of Silk Nanoparticles. J. Vis. Exp. JoVE 2016, 116, 54669. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Liu, F.; Chen, Y.; Yu, T.; Lou, D.; Guo, Y.; Li, P.; Wang, Z.; Ran, H. Drug release from core-shell PVA/silk fibroin nanoparticles fabricated by one-step electrospraying. Sci. Rep. 2017, 7, 11913. [Google Scholar] [CrossRef]
- Montoya, N.V.; Peterson, R.; Ornell, K.J.; Albrecht, D.R.; Coburn, J.M. Silk Particle Production Based on silk/PVA Phase Separation Using a Microfabricated Co-flow Device. Molecules 2020, 25, 890. [Google Scholar] [CrossRef]
- Sun, N.; Lei, R.; Xu, J.; Kundu, S.C.; Cai, Y.; Yao, J.; Ni, Q. Fabricated porous silk fibroin particles for pHresponsive drug delivery and targeting of tumor cells. J. Mater. Sci. 2019, 54, 3319–3330. [Google Scholar] [CrossRef]
- Wongpinyochit, T.; Uhlmann, P.; Urquhart, A.J.; Seib, F.P. PEGylated Silk Nanoparticles for Anticancer Drug Delivery. Biomacromolecules 2015, 16, 3712–3722. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Xing, T.; Kundu, B.; Li, M.; Kundu, S.C.; Lu, S. Ion-induced fabrication of silk fibroin nanoparticles from Chinese oak tasar Antheraea pernyi. Int. J. Biol. Macromol. 2015, 79, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, J.; Mao, L.; Li, G.L. Antheraea Pernyi Silk Fibroin Nanoparticles for Drug Delivery. J. Nano Res. 2014, 27, 75–81. [Google Scholar] [CrossRef]
- Subia, B.; Chandra, S.; Talukdar, S.; Kundu, S.C. Folate conjugated silk fibroin nanocarriers for targeted drug delivery. Integr. Biol. (Camb.) 2014, 6, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shao, Z.; Chen, X. Paclitaxel-loaded silk fibroin nanospheres. J. Biomed. Mater. Res. Part A 2012, 100, 203–210. [Google Scholar] [CrossRef]
- Wu, P.; Liu, Q.; Li, R.; Wang, J.; Zhen, X.; Yue, G.; Wang, H.; Cui, F.; Wu, F.; Yang, M.; et al. Facile preparation of paclitaxel loaded silk fibroin nanoparticles for enhanced antitumor efficacy by locoregional drug delivery. ACS Appl. Mater. Interfaces 2013, 5, 12638–12645. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, W.; Chen, S.; Yao, J.; Shao, Z.; Chen, X. Size-controllable dual drug-loaded silk fibroin nanospheres through a facile formation process. J. Mater. Chem. B 2018, 6, 1179–1186. [Google Scholar] [CrossRef]
- Perteghella, S.; Sottani, C.; Cocce, V.; Negri, S.; Cavicchini, L.; Alessandri, G.; Cottica, D.; Torre, M.L.; Grignani, E.; Pessina, A. Paclitaxel-Loaded Silk Fibroin Nanoparticles: Method Validation by UHPLC-MS/MS to Assess an Exogenous Approach to Load Cytotoxic Drugs. Pharmaceutics 2019, 11, 285. [Google Scholar] [CrossRef] [PubMed]
- Bian, X.; Wu, P.; Sha, H.; Qian, H.; Wang, Q.; Cheng, L.; Yang, Y.; Yang, M.; Liu, B. Anti-EGFR-iRGD recombinant protein conjugated silk fibroin nanoparticles for enhanced tumor targeting and antitumor efficiency. OncoTargets Ther. 2016, 9, 3153–3162. [Google Scholar] [CrossRef][Green Version]
- Qu, J.; Liu, Y.; Yu, Y.; Li, J.; Luo, J.; Li, M. Silk fibroin nanoparticles prepared by electrospray as controlled release carriers of cisplatin. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 166–174. [Google Scholar] [CrossRef]
- Kim, S.Y.; Naskar, D.; Kundu, S.C.; Bishop, D.P.; Doble, P.A.; Boddy, A.V.; Chan, H.K.; Wall, I.B.; Chrzanowski, W. Formulation of Biologically-Inspired Silk-Based Drug Carriers for Pulmonary Delivery Targeted for Lung Cancer. Sci. Rep. 2015, 5, 11878. [Google Scholar] [CrossRef]
- Lozano-Pérez, A.A.; Gil, A.L.; Pérez, S.A.; Cutillas, N.; Meyer, H.; Pedreño, M.; Aznar-Cervantes, S.D.; Janiak, C.; Cenis, J.L.; Ruiz, J. Antitumor properties of platinum(iv) prodrug-loaded silk fibroin nanoparticles. Dalton Trans. 2015, 44, 13513–13521. [Google Scholar] [CrossRef]
- Li, H.; Tian, J.; Wu, A.; Wang, J.; Ge, C.; Sun, Z. Self-assembled silk fibroin nanoparticles loaded with binary drugs in the treatment of breast carcinoma. Int. J. Nanomed. 2016, 11, 4373–4380. [Google Scholar] [CrossRef]
- Rahmani, H.; Fattahi, A.; Sadrjavadi, K.; Khaledian, S.; Shokoohinia, Y. Preparation and Characterization of Silk Fibroin Nanoparticles as a Potential Drug Delivery System for 5-Fluorouracil. Adv. Pharm. Bull. 2019, 9, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Reneeta, N.P.; Thiyonila, B.; Aathmanathan, V.S.; Ramya, T.; Chandrasekar, P.; Subramanian, N.; Prajapati, V.K.; Krishnan, M. Encapsulation and Systemic Delivery of 5-Fluorouracil Conjugated with Silkworm Pupa Derived Protein Nanoparticles for Experimental Lymphoma Cancer. Bioconjug. Chem. 2018, 29, 2994–3009. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yang, W.; Chen, S.; Chen, M.; Liu, Y.; Shao, Z.; Chen, X. Floxuridine-loaded silk fibroin nanospheres. RSC Adv. 2014, 4, 18171–18177. [Google Scholar] [CrossRef]
- Chen, A.Z.; Chen, L.Q.; Wang, S.B.; Wang, Y.Q.; Zha, J.Z. Study of magnetic silk fibroin nanoparticles for massage-like transdermal drug delivery. Int. J. Nanomed. 2015, 10, 4639–4651. [Google Scholar] [CrossRef] [PubMed]
- Montalban, M.G.; Coburn, J.M.; Lozano-Perez, A.A.; Cenis, J.L.; Villora, G.; Kaplan, D.L. Production of Curcumin-Loaded Silk Fibroin Nanoparticles for Cancer Therapy. Nanomaterials 2018, 8, 126. [Google Scholar] [CrossRef]
- Xie, M.; Fan, D.; Li, Y.; He, X.; Chen, X.; Chen, Y.; Zhu, J.; Xu, G.; Wu, X.; Lan, P. Supercritical carbon dioxide-developed silk fibroin nanoplatform for smart colon cancer therapy. Int. J. Nanomed. 2017, 12, 7751–7761. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Aseh, A.; Rios, C.N.; Aggarwal, B.B.; Mathur, A.B. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 2009, 4, 115–122. [Google Scholar] [CrossRef]
- Suktham, K.; Koobkokkruad, T.; Wutikhun, T.; Surassmo, S. Efficiency of resveratrol-loaded sericin nanoparticles: Promising bionanocarriers for drug delivery. Int. J. Pharm. 2018, 537, 48–56. [Google Scholar] [CrossRef]
- Ding, B.; Wahid, M.A.; Wang, Z.; Xie, C.; Thakkar, A.; Prabhu, S.; Wang, J. Triptolide and celastrol loaded silk fibroin nanoparticles show synergistic effect against human pancreatic cancer cells. Nanoscale 2017, 9, 11739–11753. [Google Scholar] [CrossRef]
- Cheema, S.K.; Gobin, A.S.; Rhea, R.; Lopez-Berestein, G.; Newman, R.A.; Mathur, A.B. Silk fibroin mediated delivery of liposomal emodin to breast cancer cells. Int. J. Pharm. 2007, 341, 221–229. [Google Scholar] [CrossRef]
- Gobin, A.S.; Rhea, R.; Newman, R.A.; Mathur, A.B. Silk-fibroin-coated liposomes for long-term and targeted drug delivery. Int. J. Nanomed. 2006, 1, 81–87. [Google Scholar] [CrossRef]
- Pham, D.T.; Saelim, N.; Tiyaboonchai, W. Alpha mangostin loaded crosslinked silk fibroin-based nanoparticles for cancer chemotherapy. Colloids Surf. B Biointerfaces 2019, 181, 705–713. [Google Scholar] [CrossRef]
- Liu, Y.; You, R.; Liu, G.; Li, X.; Sheng, W.; Yang, J.; Li, M. Antheraea pernyi silk fibroin-coated PEI/DNA complexes for targeted gene delivery in HEK 293 and HCT 116 cells. Int. J. Mol. Sci. 2014, 15, 7049–7063. [Google Scholar] [CrossRef]
- Yalcin, E.; Kara, G.; Celik, E.; Pinarli, F.A.; Saylam, G.; Sucularli, C.; Ozturk, S.; Yilmaz, E.; Bayir, O.; Korkmaz, M.H.; et al. Preparation and characterization of novel albumin-sericin nanoparticles as siRNA delivery vehicle for laryngeal cancer treatment. Prep. Biochem. Biotechnol. 2019, 49, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Roy, K.; Patel, Y.S.; Kanwar, R.K.; Rajkhowa, R.; Wang, X.; Kanwar, J.R. Biodegradable Eri silk nanoparticles as a delivery vehicle for bovine lactoferrin against MDA-MB-231 and MCF-7 breast cancer cells. Int. J. Nanomed. 2016, 11, 25–44. [Google Scholar] [CrossRef]
- Lucke, M.; Mottas, I.; Herbst, T.; Hotz, C.; Romer, L.; Schierling, M.; Herold, H.M.; Slotta, U.; Spinetti, T.; Scheibel, T.; et al. Engineered hybrid spider silk particles as delivery system for peptide vaccines. Biomaterials 2018, 172, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jiang, X.; Chen, X.; Shao, Z.; Yang, W. Doxorubicin-loaded magnetic silk fibroin nanoparticles for targeted therapy of multidrug-resistant cancer. Adv. Mater. 2014, 26, 7393–7398. [Google Scholar] [CrossRef]
- Song, W.; Muthana, M.; Mukherjee, J.; Falconer, R.J.; Biggs, C.A.; Zhao, X. Magnetic-Silk Core-Shell Nanoparticles as Potential Carriers for Targeted Delivery of Curcumin into Human Breast Cancer Cells. ACS Biomater. Sci. Eng. 2017, 3, 1027–1038. [Google Scholar] [CrossRef]
- Song, W.X.; Gregory, D.A.; Al-Janabi, H.; Muthana, M.; Cai, Z.Q.; Zhao, X.B. Magnetic-silk/polyethyleneimine core-shell nanoparticles for targeted gene delivery into human breast cancer cells. Int. J. Pharm. 2019, 555, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Hou, M.; Gao, Y.; Lu, S.; Zhang, L.; Xu, Z.; Li, C.M.; Kang, Y.; Xue, P. Biomineralization-inspired Crystallization of Manganese Oxide on Silk Fibroin Nanoparticles for in vivo MR/fluorescence Imaging-assisted Tri-modal Therapy of Cancer. Theranostics 2019, 9, 6314–6333. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; ZhuGe, D.L.; Chen, P.P.; Tong, M.Q.; Lin, M.T.; Jiang, X.; Zheng, Y.W.; Chen, B.; Li, X.K.; Zhao, Y.Z. Silk fibroin nanoparticles dyeing indocyanine green for imaging-guided photo-thermal therapy of glioblastoma. Drug Deliv. 2018, 25, 364–375. [Google Scholar] [CrossRef]
- Vollrath, F.; Knight, D.P. Liquid crystalline spinning of spider silk. Nature 2001, 410, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Humenik, M.; Pawar, K.; Scheibel, T. Nanostructured, Self-Assembled Spider Silk Materials for Biomedical Applications. Adv. Exp. Med. Biol. 2019, 1174, 187–221. [Google Scholar] [CrossRef] [PubMed]
- Solomun, J.I.; Totten, J.D.; Wongpinyochit, T.; Florence, A.J.; Seib, F.P. Manual Versus Microfluidic-Assisted Nanoparticle Manufacture: Impact of Silk Fibroin Stock on Nanoparticle Characteristics. ACS Biomater. Sci. Eng. 2020, 6, 2796–2804. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Tavanai, H.; Moradi, A.R. Production of fibroin nanopowder through electrospinning. J. Nanoparticle Res. 2011, 13, 2089–2098. [Google Scholar] [CrossRef]
- Myung, S.J.; Kim, H.S.; Kim, Y.; Chen, P.; Jin, H.J. Fluorescent silk fibroin nanoparticles prepared using a reverse microemulsion. Macromol. Res. 2008, 16, 604–608. [Google Scholar] [CrossRef]
- Lozano-Perez, A.A.; Montalban, M.G.; Aznar-Cervantes, S.D.; Cragnolini, F.; Cenis, J.L.; Villora, G. Production of silk fibroin nanoparticles using ionic liquids and high-power ultrasounds. J. Appl. Polym. Sci. 2014, 132, 41702–41709. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, Z.; Ni, Y.; Xiong, Y.; Xu, W. Preparation and characterization of a novel composite based on hyperbranched polysilane and fullerene. J. Appl. Polym. Sci. 2007, 105, 821–826. [Google Scholar] [CrossRef]
- Zhao, Z.; Xie, M.; Li, Y.; Chen, A.; Li, G.; Zhang, J.; Hu, H.; Wang, X.; Li, S. Formation of curcumin nanoparticles via solution-enhanced dispersion by supercritical CO2. Int. J. Nanomed. 2015, 10, 3171–3181. [Google Scholar] [CrossRef]
- Rajkhowa, R.; Wang, L.; Wang, X. Ultra-fine silk powder preparation through rotary and ball milling. Powder Technol. 2008, 185, 87–95. [Google Scholar] [CrossRef]
- Kazemimostaghim, M.; Rajkhowa, R.; Tsuzuki, T.; Wang, X. Ultrafine silk powder from biocompatible surfactant-assisted milling. Powder Technol. 2013, 185, 87–95. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, Y.; Xie, M.B. Silk fibroin-based nanoparticles for drug delivery. Int. J. Mol. Sci. 2015, 16, 4880–4903. [Google Scholar] [CrossRef] [PubMed]
- Lammel, A.S.; Hu, X.; Park, S.H.; Kaplan, D.L.; Scheibel, T.R. Controlling silk fibroin particle features for drug delivery. Biomaterials 2010, 31, 4583–4591. [Google Scholar] [CrossRef]
- Mohammadi, P.; Jonkergouw, C.; Beaune, G.; Engelhardt, P.; Kamada, A.; Timonen, J.V.I.; Knowles, T.P.J.; Penttila, M.; Linder, M.B. Controllable coacervation of recombinantly produced spider silk protein using kosmotropic salts. J. Colloid Interface Sci. 2020, 560, 149–160. [Google Scholar] [CrossRef]
- Florczak, A.; Jastrzebska, K.; Bialas, W.; Mackiewicz, A.; Dams-Kozlowska, H. Optimization of spider silk sphere formation processing conditions to obtain carriers with controlled characteristics. J. Biomed. Mater. Res. Part A 2018, 106, 3211–3221. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Winter, G.; Myschik, J. Recombinant spider silk particles for controlled delivery of protein drugs. Biomaterials 2012, 33, 1554–1562. [Google Scholar] [CrossRef] [PubMed]
- Jenjob, R.; Phakkeeree, T.; Seidi, F.; Theerasilp, M.; Crespy, D. Emulsion Techniques for the Production of Pharmacological Nanoparticles. Macromol. Biosci. 2019, 19, e1900063. [Google Scholar] [CrossRef]
- Chen, B.Q.; Kankala, R.K.; He, G.Y.; Yang, D.Y.; Li, G.P.; Wang, P.; Wang, S.B.; Zhang, Y.S.; Chen, A.Z. Supercritical Fluid-Assisted Fabrication of Indocyanine Green-Encapsulated Silk Fibroin Nanoparticles for Dual-Triggered Cancer Therapy. ACS Biomater. Sci. Eng. 2018, 4, 3487–3497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Y.; Chen, A.Z.; Zheng, Z.J.; Hu, J.Y.; Li, J.S.; Li, G. Generation of silk fibroin nanoparticles via solution-enhanced dispersion by supercritical CO2. Ind. Eng. Chem. Res. 2013, 52, 3752–3761. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Z.; Li, G.; Kaplan, D.L.; Wang, X. Control of silk microsphere formation using polyethylene glycol (PEG). Acta Biomater. 2016, 39, 156–168. [Google Scholar] [CrossRef]
- Gianak, O.; Kyzas, G.; Samanidou, V.; Deliyanni, E. A review for the synthesis of silk fibroin nanoparticles with different techniques and their ability to be used for drug delivery. Curr. Anal. Chem. 2019, 15, 339–348. [Google Scholar] [CrossRef]
- Holland, C.; Numata, K.; Rnjak-Kovacina, J.; Seib, F.P. The Biomedical Use of Silk: Past, Present, Future. Adv. Healthc. Mater. 2019, 8, e1800465. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.S.; Kim, D.K.; Park, C.H.; Lee, H.R. Clinical outcomes of silk patch in acute tympanic membrane perforation. Clin. Exp. Otorhinolaryngol. 2015, 8, 117–122. [Google Scholar] [CrossRef]
- Baoyong, L.; Jian, Z.; Denglong, C.; Min, L. Evaluation of a new type of wound dressing made from recombinant spider silk protein using rat models. Burns 2010, 36, 891–896. [Google Scholar] [CrossRef]
- Fredriksson, C.; Hedhammar, M.; Feinstein, R.; Nordling, K.; Kratz, G.; Johansson, J.; Huss, F.; Rising, A. Tissue Response to Subcutaneously Implanted Recombinant Spider Silk: An in Vivo Study. Materials 2009, 2, 1908–1922. [Google Scholar] [CrossRef]
- Schierling, M.B.; Doblhofer, E.; Scheibel, T. Cellular uptake of drug loaded spider silk particles. Biomater. Sci. 2016, 4, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Cheng, X.; Tang, Q.; Chen, L. The antigenicity of silk-based biomaterials: Sources, influential factors and applications. J. Mater. Chem. B 2021, 9, 8365–8377. [Google Scholar] [CrossRef]


| Biomaterial Format | Released Therapeutic | Target | In Vitro/ In Vivo Model | Findings | Ref |
|---|---|---|---|---|---|
| Film | Doxorubicin | Breast cancer | MDA-MB-231/ Orthotopic adrenal tumor xenograft in mice | Sustained release over 4 weeks Doxorubicin release rate could be controlled by manipulating silk crystallinity and beta-sheet content Doxorubicin-loaded silk films significantly greater inhibited primary tumor than intravenously administered drug Silk films loaded with doxorubicin reduced metastatic spread, no local or systemic toxicity | [25] |
| Doxorubicin | Neuroblastoma | KELLY, SK-N-AS, IMR-32, SH-SY5Y/ Tumor xenograft in mice | Controlled and sustained drug release up to 30 days Slower tumor growth after treatment with controlled-release silk film Effective treatment by combining surgical resection and local treatment with doxorubicin-loaded films | [148] | |
| Doxorubicin, Crizotinib | Neuroblastoma | KELLY/ Orthotopic tumor xenograft in mice | Sustained drug release up to 28 days Controllable release kinetics from the silk films by changing the amount and physical crosslinking of silk Intratumoral application of drug-loaded films was more effective in vivo comparing with systemic application of drugs | [167] | |
| Vincristine, Doxorubicin | ND | ND | Sustained drug release up to 14 days Control over drug release by altering silk film crystallinity and chemical composition | [168] | |
| Fiber mat | Curcumin, Doxorubicin | ND | ND | Dual drug delivery (curcumin-loaded nanoparticles and doxorubicin-loaded core/shell nanofibers) and sustained release Control over the amount of drug release from nanofibers by adjusting the crystal content of nanofibers with the water-annealing process at a different temperature, release up to 40 h | [169] |
| Curcumin | Colorectal carcinoma | HCT-116/ Tumor xenograft in mice | Curcumin-loaded nanofibrous matrices had enhanced anti-cancer effect as compared to free drug No toxic effect on normal NCM-460 cells Implantation of curcumin-loaded nanofibrous matrices resulted in tumor growth inhibition in vivo | [158] | |
| Gel | Vincristine, Doxorubicin | Neuroblastoma | KELLY/ Orthotopic tumor xenograft in mice | Dual drug delivery and sustained release of drugs up to 25 days Intratumoral delivery of vincristine and doxorubicin significantly slowed tumor growth and increased drug availability as compared to intravenous administration | [23] |
| Vincristine, Doxorubicin | Ewing’s sarcoma | A-673/ Tumor xenograft in mice | Combination of vincristine-loaded silk gels and doxorubicin-loaded silk foams Delivery of vincristine inside the sarcoma tumor with silk gel decreased tumor growth more effectively compared to silk foam | [170] | |
| Vincristine | Neuroblastoma | KELLY/ Orthotopic tumor xenograft in mice | Sustained release silk gels, Multiple injections of vincristine-loaded silk gels suppressed tumor growth Tumor growth more significantly suppressed by distributed injections compared to central injections of drug-loaded silk gel | [140] | |
| Hydrogel | Doxorubicin | Breast cancer | MDA-MB-231, MCF-7/ Tumor xenograft in mice | Controlled doxorubicin release Doxorubicin-loaded silk hydrogels reduced primary and metastatic tumors growth Reduced toxicity compared to systemic drug administration | [26] |
| Doxorubicin | Breast cancer | MDA-MB-231/ Tumor xenograft in mice | Silk hydrogels displayed thixotropic capacity allowing for easy injectability Sustained drug release over 8 weeks Dox-loaded silk hydrogels had a superior antitumor response in vitro and in vivo than free Dox | [171] | |
| Foam | Vincristine | Neuroblastoma | KELLY/ Orthotopic tumor xenograft in mice | Sustained drug release from the foam format over 21 days | [24] |
| Vincristine, Doxorubicin | Neuroblastoma | KELLY/ Orthotopic tumor xenograft in mice | Sustained drug release High drugs concentrations within the tumor resulting in slower tumor growth with less post-treatment side effects than equivalent systemic chemotherapy | [23] | |
| Reservoir | Anastrozole | ND | ND/ Sprague-Dawley rats | Biocompatibility of silk reservoir rods Sustained drug delivery for 91 days measured in a pharmacokinetic study in vivo Biodegradation profile suitable for long-term sustained delivery of breast cancer therapeutics | [139] |
| Cisplatin | Neuroblastoma | KELLY/ Orthotopic tumor xenograft in mice | Controlled release of the drug up to 30 days Intratumoral implantation of silk reservoirs decreased tumor growth significantly when compared to free cisplatin | [149] | |
| Wafer | Etoposide | Neuroblastoma | KELLY/ Orthotopic tumor xenograft in mice | Silk coated 6% wafers released the drug up to 45 days, while uncoated wafers for 30 days Intratumoral implantation was effective at decreasing tumor growth. Etoposide-loaded silk wafers induced tumor necrosis | [138] |
| Vincristine | Neuroblastoma | KELLY/ Orthotopic tumor xenograft in mice | Sustained drug release from the wafer reservoir for 7 weeks Intratumoral injection slowed tumor growth and increased drug availability as compared to intravenous administration | [24] | |
| Microneedles | Doxorubicin, Rhodamine, ICG | Cervical cancer | HeLa/ Live mouse skin | Microneedles fabricated using a PDMS mold packed with a fibroin scaffold Controlled release up to 144 h More rapid release of doxorubicin from the microneedles with a higher proportion of sucrose Tumor cell viability decreased faster under higher sucrose content in the applied microneedles The soluble sucrose content and fibroin scaffold within microneedles accelerated the transdermal release of the photothermal agent in vivo | [137] |
| Drug | Silk Source | Preparation Method | Particle Size | Characterization | Functionalization/Surface Modification | Target/ In Vitro/ In Vivo Model | Outcome/Findings | Ref |
|---|---|---|---|---|---|---|---|---|
| Doxorubicin | B. mori silk fibroin | Desolvation with acetone | 100 nm | SEM, DLS, Zeta potential Drug loading/release Cellular uptake (CLSM) Cytotoxicity | Breast cancer/ MCF-7 MCF-7-ADR/ND | pH-dependent drug release up to 6 days Enhanced endocytic uptake and lysosomal accumulation | [174] | |
| Nanoprecipitation with acetone | 106 nm | Size, Zeta potential SEM Encapsulation efficiency Cytotoxicity | Breast cancer/ MDA-MB-231/ ND | Simple, quick and reproducible method of particle preparation High drug encapsulation efficiency Sustained drug release | [175] | |||
| Electrospraying with PVA blends | 600–1800 nm | DLS, Zeta potential SEM, TEM Drug loading/release Cytotoxicity (MTT) Apoptosis assay In vivo study | Breast cancer/ MDA-MB-231/ tumor xenograft in mice | Very good monodispersity High drug encapsulation efficiency Controlled drug release for 72h External ultrasound triggered and accelerated drug release | [176] | |||
| Silk/PVA phase separation within microfluidics device | 2.8–6.8 µm | SEM Drug loading/release Cytotoxicity (MTT) Macrophage activation Cellular uptake (CLSM) | Neuroblastoma/ KELLY THP-1/ ND | High drug loading capacity and efficiency pH-dependent drug release Sustained drug release over 23 days Uptake by THP-1 monocytes Macrophage activation in response to silk particle exposure | [177] | |||
| Salting-out with potassium phosphate | 530 nm | Size, SEM, Zeta potential, FTIR, BET analysis (porous structure) Cytotoxicity (CCK-8) Cellular binding and internalization (FCM, CLSM) | FA-conjugated | Cervical cancer/ HeLa/ ND | FA-targeted and pH-responsive particles Controlled drug release up to 32 h Enhanced internalization in cancer cells overexpressing FA receptor Higher cytotoxicity against HeLa cells than particles without functionalization | [178] | ||
| Acetone nanoprecipitation | 116 nm | DLS, Zeta potential, SEM, FTIR, Drug loading/release Macrophage activation Cytotoxicity (MTT) Cellular uptake (CLSM) | PEGylated silk | Breast cancer/ MCF-7/ ND | Increased particle stability Increased clearance time than non-modified particles High drug entrapment efficiency and release capacity pH-dependent drug release over 14 days | [179] | ||
| A. pernyi silk fibroin | Ion-induced self- assembly | 100–500 nm | Size, Zeta potential, SEM, FTIR, XRD, Drug release Cytotoxicity (Alamar blue) | Liver cancer/ HepG-2/ ND | Self-assembly induced by cations (Na+, Ca2+, and Ce3+) RGD-containing silk fibroin material pH-sensitive and sustained drug release up to 11 days | [180] | ||
| Self-assembly | 30–1000 nm | SEM, FTIR, XRD Drug loading/release | ND | pH-sensitive and sustained release for over 23 days | [181] | |||
| A. mylitta silk fibroin | Desolvation with acetone | 150–170 nm | TEM, DLS, Zeta potential Drug loading/release Cellular binding and internalization (FCM, CLSM) Cytotoxicity (MTT) Macrophage activation | FA-conjugated | Breast cancer/ MDA-MB-231/ ND | Capable of sustained drug release up to 21 days Selective cancer cells targeting Enhanced cellular binding and uptake via endocytosis than non-functionalized particles | [182] | |
| B. mori silk sericin-chitosan | Two-step crosslinking with chitosan and EDC | 200–300 nm | Drug loading/release Zeta potential Cytotoxicity (CCK-8) Hemolysis assay Plasma coagulation assay In vivo studies | Breast cancer/ MCF-7 and Liver cancer/ HepG-2/ tumor xenograft in mice | Excellent colloidal stability Stable in the absence of cryoprotectants Biocompatible in animal study Low systemic toxicity of the released drug | [44] | ||
| A. pernyi silk sericin | Silk-templated hydroxyapatite (HAp) mineralization | 1.2 µm | SEM, TEM, DLS, FTIR, XRD Drug loading/release Cryo-SEM Cytotoxicity (Alamar blue) Cellular uptake (CLSM) | Breast cancer/ Bcap-37 and Cervical cancer/ HeLa/ ND | Uniform and porous microparticles pH-responsive characteristic due to the presence of pH-responsive HAp Controlled and sustained release of drug | [46] | ||
| Bioengineered silk (SELP) | Self-assembly with hydrophobic Dox | 50–142 nm | DLS, Drug loading/release Cytotoxicity (MTT) Cellular binding (FCM) and uptake (CLSM) | Cervical cancer/ HeLa/ ND | Fabricated and loaded with an aqueous process under mild conditions Simple method to control particle size High uptake of the nanoparticles by the cancer cells Internalization of the nanoparticles through endocytosis | [37] | ||
| Bioengineered N. clavipes spider silk (MS1) | Salting-out with potassium phosphate | 300–400 nm | Size, Zeta potential, SEM, FTIR, Drug loading/release Cellular binding (FCM) and uptake (CLSM) Cytotoxicity (MTT) | H2.1 and H2.2 peptides-conjugated (anti-Her2) | Breast cancer/ SKBR-3 and Ovarian cancer/ SKOV-3/ ND | pH-dependent drug release up to 15 days Enhanced targeted binding to Her2-overexpressing cells Enhanced internalization into targeted cancer cells Higher toxicity towards cancer cells than control cells No cytotoxic | [88,89] | |
| In vivo studies (toxicity, biodistribution, efficiency) | H2.1 peptide-conjugated | Breast cancer/ murine D2F2 and D2F2E2/ tumor in mice | Enhanced tumor-specific targeting in vivo than non-functionalized particles No systemic toxicity as compared to free Dox Suppression of cancer cell growth in vivo | [90] | ||||
| Bioengineered N. clavipes spider silk (MS1, MS2) | Salting-out with potassium phosphate | <400 nm | Size, SEM, Drug loading/release Cellular binding (FCM) and uptake (CLSM) Cytotoxicity (MTT) | H2.1 peptide/ DOX binding peptide-conjugated | Breast cancer/ SKBR-3/ ND | pH-dependent drug release up to 7 days Double functionalization of silk spheres for controlled Dox delivery into Her2-positive cancer cells Enhanced targeted binding and internalization into Her2-overexpressing cells Higher drug-loading capacity, binding per cell and cytotoxic effect compared with control spheres, Higher toxicity towards cancer cells than control cells | [125] | |
| Paclitaxel | B. mori silk fibroin | Desolvation with ethanol and freezing | 270–520 nm | Size, Zeta potential, FTIR, HRSEM, TEM Drug loading/release | ND | Easy and mild method of particle preparation Particles with controllable shape and size Drug release for over 9 days | [183] | |
| Desolvation with ethanol | 158–206 nm | Size, Zeta potential, TEM, FTIR, XRD, Drug loading/release Cellular binding (microscopy) Cytotoxicity (MTT) Apoptosis assay In vivo studies (toxicity, efficiency) | Gastric cancer/ BGC-823 and SGC-7901/ Tumor xenograft in mice | Sustained drug release for 100 h Drug-induced cytotoxicity when incorporated into nanoparticles Excellent antitumor efficacy in mice No systemic toxicity | [184] | |||
| Desolvation with ethanol | 100–600 nm | Size, Zeta potential TEM Drug loading/release Cellular uptake (CLSM) Cytotoxicity | Cervical cancer/ HeLa and Liver cancer/ HepG-2/ ND | Dual drug loading (Ptx, Dox) Controlled and sustained drug release for over 7 days High cellular uptake via endocytosis Suppression of cancer cell growth in vitro | [185] | |||
| Desolvation with acetone | 115 nm | DLS, SEM, FTIR Drug loading (UHPLC-MS/MS) Cytotoxicity (MTT) | Pancreatic cancer/ CFPAC-1/ ND | The drug-encapsulation in nanoparticles did not influence its cytotoxicity profile High dose-dependent cytotoxic activity of drug-loaded nanoparticles | [186] | |||
| Desolvation with ethanol | 186 nm | Size, Zeta potential, FTIR, TEM, Cytotoxicity (MTT) Cellular binding (fluorescence microscopy) In vivo study (biodistribution, efficiency) | Anti-iRGD-EGFR-conjugated | Cervical cancer/ HeLa/ Tumor xenograft in mice | High drug content and loading efficiency Enhanced tumor-specific targeting in vitro and in vivo than non-functionalized particles Good antitumor effect | [187] | ||
| A. mylitta silk sericin | Self-assembly with pluronic surfactants | 100–110 nm | DLS, TEM Fluorescence microscopy Cytotoxicity (MTT) Apoptosis assay (FCM, CLSM, western blot) | Breast cancer/ MCF-7/ ND | High loading of hydrophobic drug Stable in aqueous solution High cellular uptake Efficient cytotoxicity towards cancer cells when loaded with drug | [43] | ||
| Cisplatin | B. mori silk fibroin | Electrospraying | 59 nm | SEM, DLS, FTIR Drug loading/release Cytotoxicity (MTT) Apoptosis assay (FCM) | Lung cancer/ A-549/ ND | Drug release for more than 15 days Internalization into cancer cells Sustained and efficient killing of cancer cells Low toxicity in fibroblasts | [188] | |
| Spray-drying/spray-freeze-drying and crosslinking with genipin | 10.8–22.75 µm | DLS, SEM, AFM, XRD Aerosolization (NGI) Drug release Cytotoxicity (CCK-8, PicoGreen) Cell migration and invasion | Lung cancer/ A-549/ ND | Drug loading with or without cross-linking showing different release profiles Drug delivery directly to the lungs via powder inhalers Enhanced cytotoxicity when drug was delivered using the cross-linked particles | [189] | |||
| Precipitation with ionic liquids and high-power ultrasounds | 173 nm | DLS, TEM, XRD Drug loading/release Cytotoxicity (MTT) Apoptosis assay (flow cytometry) | Ovarian cancer/ A-780 and A-780-cisR and Breast cancer/ SK-BR-3, MCF-7 and MDA-MB-231/ ND | Efficient loading with Pt(IV) prodrug PtBz High cellular uptake Overcame drug resistance to cisplatin | [190] | |||
| 5-Fluorouracil | B. mori silk fibroin | Desolvation with acetone | 278.2–364.9 nm | Drug loading/release Cytotoxicity (CCK-8) Degradation Cellular uptake (CLSM) In vivo studies (toxicity, biodistribution, efficiency) | cRGDfk and Ce6-conjugated | Gastric cancer/ MGC-803/ tumor xenograft in mice | Targeted drug delivery and PDT Active tumor targeting Together with laser irradiation, the drug-loaded particles reduced the tumor burden Biocompatibility and safety in vivo | [127] |
| Desolvation and crosslinking with genipin | 217 nm | TEM, DLS, FTIR Drug loading/release Apoptosis assay (FCM) In vivo studies (toxicity, efficacy) | Murine breast cancer/ 4T1/ tumor-bearing mice | Binary drug loading (5-FU and curcumin), High loading efficacy Improvement in the cytotoxic activity and bioavailability compared with free drugs Toxic effect toward cancer cells in vitro and in vivo The anticancer effect observed may be induced by the apoptosis of cells via the generation of cellular ROS | [191] | |||
| Desolvation with acetone | 220 nm | DLS, Zeta potential, SEM, TEM, FTIR, XRD, Drug loading/release Cytotoxicity (MTT) | Breast cancer/ MCF-7 Colon cancer/ HT-29 | High loading efficiency Controlled and sustained drug release Enhanced cytotoxic effect on cancer cells | [192] | |||
| B. mori pupa protein (Pp) | Desolvation with ethanol | 162 nm | FTIR, Size, Zeta potential Drug loading/release Cytotoxicity (hemolysis assay, MTT) In vivo studies (toxicity, biodistribution, efficacy,) | Lymphoma/ DAL/ tumor-bearing mice | Particles that are easy to prepare, modify, with good biocompatibility and bio-adhesivity High entrapment efficiency and capacity Sustained drug release Anticancer efficiency in vivo without causing toxicity in the healthy tissue | [193] | ||
| FUDR | B. mori silk fibroin | Desolvation with ethanol and freezing | 210–510 nm | Size, Zeta potential, SEM, TEM, Drug loading/release Cytotoxicity (MTT) Cellular uptake (CLSM) | Cervical cancer/ HeLa/ ND | Controllable shape and size, without apparent aggregation Drug release time over 2 days Cancer cells growth inhibition Similar curative effect to kill or inhibit Hela cells to the free drug | [194] | |
| Methotrexate | B. mori silk fibroin | Suspension-enhanced dispersion by supercritical CO2 (SEDS) | 112 nm | FTIR, SEM Drug loading/release Cellular uptake (CLSM) | Skin from guinea pig | High drug loading efficiency Magnetic nanoparticles for transdermal drug delivery Improved penetration of drugs across the skin | [195] | |
| B. mori silk fibroin-albumin | Desolvation with acetone and crosslinking with glutaraldehyde | 152–176 nm | TEM, DLS, Zeta potential, FTIR, Drug loading/release Cellular uptake (CLSM) Cytotoxicity (MTT, hemolysis assay) | Breast cancer/ MDA-MB-231/ ND | Silk-albumin conjugates High drug loading efficiency Sustained drug release over 12 days | [109] | ||
| Gemcitabine | B. mori silk fibroin | Desolvation with DMSO | 302 nm | DLS, SEM, Zeta potential Cytotoxicity (MTT) Cellular uptake (CLSM) In vivo studies (biodistribution, toxicity, efficiency) | SP5-52 peptide-conjugated | Lung cancer/ LL/2/ tumor-bearing mice | Targeted delivery to lung cancer cells Higher cellular uptake and cytotoxicity in cancer cells in vitro than non-modified particles Increased accumulation in lung tissue than non-modified particles The improved therapeutic outcome in vivo and minimized systemic toxicity than free drug | [124] |
| Type of Anticancer Therapeutic | Therapeutic Agent | Silk Source | Preparation Method | Particle Size | Functionalization/Surface Msodification | Target/ In Vitro/ In Vivo Model | Outcome/Findings | Ref |
|---|---|---|---|---|---|---|---|---|
| Plant-derived therapeutic agents | Curcumin | B. mori silk fibroin | Precipitation with ionic liquids and high-power ultrasounds | 166–171 nm | Liver cancer/ Hep3B and Neuroblastoma/ KELLY/ ND | Sustained drug release up to 3 days Drug bioavailability Cytotoxic activity towards cancer cells No toxic effect in healthy cells | [196] | |
| Suspension-enhanced dispersion by supercritical CO2 (SEDS) | <100 nm | Colon cancer/ HCT-116/ ND | Time-dependent intracellular uptake ability Improved inhibition effects on colon cancer cells No toxic effect in healthy cells | [197] | ||||
| Desolvation and cross-linking with genipin | 217 nm | Murine breast cancer/ 4T1/ Tumor in mice | Binary drug loading (5-FU and curcumin) High loading efficacy Improvement in the cytotoxic activity and bioavailability compared with free drugs Toxic effect toward cancer cells in vitro and in vivo | [191] | ||||
| B. mori silk fibroin-chitosan blend | Microdot capillary method | <100 nm | Breast cancer/ MCF-7 and MDA-MB-453/ ND | Sustained drug release over 9 days Efficacy against Her2-overexpressing cancer cells | [198] | |||
| Resveratrol | B. mori silk sericin | Desolvation with DMSO and pluronic F-68 | 200–400 nm | Colon cancer/ Caco-2/ ND | High drug encapsulation levels and stable drug release profile over 72 h High intra-cellular internalization efficiency The anticancer effect, but no toxicity towards healthy cells | [199] | ||
| Triptolide/ celastrol | B. mori silk fibroin | Desolvation with acetone and ethanol | 166 nm/ 170 nm | Pancreatic cancer/ MIA PaCA-2 and PANC-1/ ND | Improved bioavailability and pharmacokinetic properties compared to free drugs The pH-dependent sustained drug release over 192 h Increased therapeutic efficiency compared to free drugs | [200] | ||
| Emodin | B. mori silk fibroin | Lyophilisation of silk fibroin with emodin-loaded liposomes | 316 nm | Breast cancer/ MCF-7, BT-474 and MDA-MB-453/ ND | Silk coating of liposomes decreased drug release rate compared to uncoated liposomes Longer intracellular retention of silk coated liposomes than liposomes w/o coating lead to the longer availability of emodin for down-modulation of various Her2/neu pathways | [201,202] | ||
| α-mangostin | B. mori silk fibroin | Desolvation and crosslinking with EDC or PEI | 300 nm | Colon cancer/ Caco-2 and Breast cancer/ MCF-7/ ND | Increase in water solubility of the drug Maintained α-mangostin’s apoptotic effect Increased cytotoxic effect on cancer cells than the free drug Reduction of hematoxicity compared to free drug | [203] | ||
| Nucleic acid-based therapeutic agents | siRNA (anti-LUC) | B. mori silk fibroin-oligochitosan blend | Desolvation with acetone | 250–450 nm | Lung cancer/ H1299/ ND | Enhanced particle loading capacity than oligochitosan polyplexes Enhanced serum stability of siRNA than naked nucleic acid Increased gene silencing effect compared with oligochitosan polyplexes | [27] | |
| pDNA encoding GFP | A. pernyi silk fibroin (ASF) | Self-assembly with PEI/DNA complexes | 230–360 nm | Colon cancer/ HCT-116/ ND | PEI/DNA complexes coated with RGD-rich ASF Increased target specificity in comparison with PEI/DNA complexes alone Higher uptake of silk coated complexes in cancer cells due to the affinity of the RGD peptides from ASF for integrins, Lower post-transfection cell toxicity than uncoated complexes | [204] | ||
| siRNA (anti-CK2, anti-ASH2L, anti-Cyclin D1) | B. mori silk sericin-albumin | Desolvation with ethanol | 127–142 nm | poly-L-lysine (PLL)-conjugated and hyaluronic acid (HA)-conjugated | Laryngeal cancer/ Hep-2/ ND | Particles modified with PLL for siRNA binding and decorated HA to target cancer cells High siRNA entrapment Downregulation of target CK2, ASH2L and Cyclin D1 genes Higher silencing effect comparing with naked siRNA | [205] | |
| siRNA (anti-STAT3) | Bioengineered N. clavipes spider silk (MS2KN) | Salting out with potassium phosphate | 202 nm | poly-L-lysine (KN) | Macrophages/ J774/ ND | Approach for cancer immunotherapy Protection of CpG-siRNA therapeutics from degradation by serum nucleases CpG-STAT3-siRNA targeted delivery to TLR9-positive macrophages Prolonged siRNA presence in macrophages than naked siRNA Prolonged silencing effect on STAT3 expression than naked siRNA | [80] | |
| pDNA encoding LUC | Bioengineered N. clavipes spider silk (15mer) | Self-assembly with pDNA | 186 nm | poly-L-lysine and RGD-conjugated | Cervical cancer/ HeLa/ ND | High pDNA delivery efficiency Increased integrin-mediated transfection with RGD sequences than non-conjugated constructs | [82] | |
| 99 nm | poly-L-lysine and ppTG1-conjugated | Melanoma/ MDA-MB-435/ ND | High transfection rates Controlled enzymatic degradation rate of the silk-based pDNA complexes enables the regulation of the release profile of genes from the complexes | [83] | ||||
| 90 nm | poly-L-lysine and Lyp1 or F3 peptide-conjugated | Melanoma/ MDA-MB-435 and Breast cancer/ MDA-MB-231/ Tumors in mice | Enhanced pDNA delivery than non-functionalized complexes Low cytotoxicity Functionalization with F3 tumor homing peptide was the most effective in pDNA delivery to cancer cells | [84] | ||||
| Protein-based therapeutic agents | Lactoferrin | S. cynthia ricini Eri silk | Milling | 200–300 nm | Breast cancer/ MCF-7 and MDA-MB-231/ ND | Sustained release of therapeutic agents Higher stability in presence of proteolytic enzymes than bovine lactoferrin alone EGFR or TfR2 receptors-mediated endocytosis of nanoparticles Cytotoxic properties towards cancer cells | [206] | |
| Peptides from ovoalbumin (C16-OVA) | Recombinant A. diadematus spider silk | Salting-out with potassium phosphate using micromixing device | 369–386 nm | Bone marrow derived cells (BMDC)/ in vivo mouse model | Potential approach for cancer vaccine immunotherapy Preferential uptake by immunological cells Localization in lysosomes Particles cleaved by lysosomal cathepsins to release transported peptide Antigen-specific proliferation of T-cells and cytotoxicity of released peptides in vivo | [207] | ||
| Inorganic agents | IONPs/ Dox | Bioengineered N. clavipes spider silk (MS1, MS2, EMS2) | Salting-out with potassium phosphate | 500 nm | ND | The addition of silk did not influence magnetic properties of IONPs Efficient incorporation and sustained release of incorporated drug (Dox) Not cytotoxic in vitro | [110] | |
| ND | H2.1 peptide-conjugated (anti-Her2) | Breast cancer/ SK-BR-3/ ND | Specific affinity of functionalized magnetic silk particles towards Her2-overexpressing cancer cells, Efficient binding of doxorubicin Ability to generate heat upon application of magnetic field (MF) Induction of hyperthermia in targeted cancer cells | [126] | ||||
| IONPs/ Dox | B. mori silk fibroin | Salting-out with potassium phosphate | 171–206 nm | Breast cancer/ MCF-7 and MCF-7-ADR/ tumor xenograft in mice | High drug loading efficiency pH-dependent drug release up to 4 days Efficient magnetic targeting and intracellular delivery into both MCF-7 and MCF-7/ADR Ability to overcome multidrug resistance (MDR) The magnetic targeting to tumor in vivo | [208] | ||
| IONPs/ Mtx | Suspension-enhanced dispersion by supercritical CO2 (SEDS) | 112 nm | Skin from guinea pig (ex vivo studies) | High drug loading efficiency Magnetic nanoparticles for transdermal drug delivery Improved penetration of drugs across the skin upon application of MF | [195] | |||
| IONPs/ Cur | Salting-out with potassium phosphate | 90–350 nm | Breast cancer/ MDA-MB-231/ ND | The magnetic targeting to cancer cells in vitro Higher uptake of drug-loaded nanoparticles than free drug Lower viability of cancer cells than control cells | [209] | |||
| IONPs/ ODN (anti-c-myc) | B. mori silk fibroin mixed with PEI | Salting-out with sodium phosphate | <200 nm | Breast cancer/ MDA-MB-231/ ND | Magnetic-silk/PEI core-shell nanoparticles Lower surface charge and reduced cytotoxicity than magnetic-PEI-coated particles High cellular uptake, efficient magnetofection level | [210] | ||
| MnO2/ Dox/ICG | B. mori silk fibroin | Self-assembly induced by organic solvent | 140 nm | Breast cancer/ 4T1/ tumor-bearing mice | Strong and stable photothermal effect upon NIR irradiation Effective tumor-specific accumulation via EPR effect Combination chemotherapy, PDT and PTT under the guidance of NIR/MR imaging Reduced systemic toxicity | [211] | ||
| Photo-sensitive or photo-dynamic agents | ICG | B. mori silk fibroin | Desolvation with acetone | 210 nm | Glioblastoma/ C6/ Tumor xenograft in mice | A therapeutic nano-platform for imaging and PTT of glioblastoma High encapsulation efficiency of photosensitive agent and slow drug release profile in vitro Increased stability of PTT effect under NIR irradiation than free drug Internalization of particles by cancer cells in vitro Accumulation of particles in site of tumor and tumor growth suppression in vivo | [212] | |
| Ce6/ 5-FU | B. mori silk fibroin | Desolvation with acetone | 278.2–364.9 nm | cRGDfk and Ce6-conjugated | Gastric cancer/ MGC-803/ Tumor xenograft in mice | Combination of targeted drug delivery and (PDT) Active tumor targeting of integrin receptor-overexpressing cells Together with laser irradiation, the drug-loaded particles reduced the tumor burden Biocompatibility and safety in vivo | [127] | |
| IR780 | B. mori silk sericin-cholesterol | Self-assembly induced by DMSO | 105 nm | FA-conjugated | Gastric cancer/ BGC-823/ ND | Efficient incorporation of photosensitive substance IR780 Improved photo-stability and water solubility of IR780 Efficient absorption by FA-positive cancer cells Excellent PDT and PTT cytotoxicity towards cancer cells under NIR irradiation | [47] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Florczak, A.; Deptuch, T.; Kucharczyk, K.; Dams-Kozlowska, H. Systemic and Local Silk-Based Drug Delivery Systems for Cancer Therapy. Cancers 2021, 13, 5389. https://doi.org/10.3390/cancers13215389
Florczak A, Deptuch T, Kucharczyk K, Dams-Kozlowska H. Systemic and Local Silk-Based Drug Delivery Systems for Cancer Therapy. Cancers. 2021; 13(21):5389. https://doi.org/10.3390/cancers13215389
Chicago/Turabian StyleFlorczak, Anna, Tomasz Deptuch, Kamil Kucharczyk, and Hanna Dams-Kozlowska. 2021. "Systemic and Local Silk-Based Drug Delivery Systems for Cancer Therapy" Cancers 13, no. 21: 5389. https://doi.org/10.3390/cancers13215389
APA StyleFlorczak, A., Deptuch, T., Kucharczyk, K., & Dams-Kozlowska, H. (2021). Systemic and Local Silk-Based Drug Delivery Systems for Cancer Therapy. Cancers, 13(21), 5389. https://doi.org/10.3390/cancers13215389








