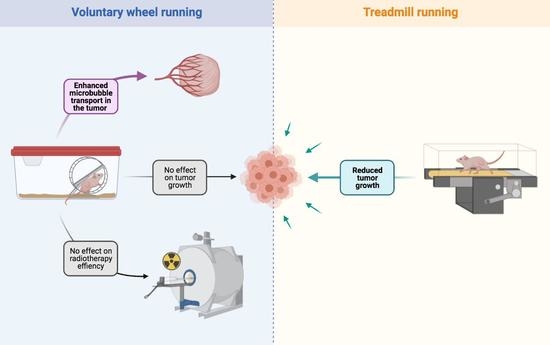
Voluntary Wheel Running Does Not Enhance Radiotherapy Efficiency in a Preclinical Model of Prostate Cancer: The Importance of Physical Activity Modalities?
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Animals and Tumor Models
2.3. Physical Activity Models
2.4. Radiotherapy Treatment
2.5. Assessment of Microbubble Transport in the Tumor
2.6. Immunohistochemistry Analyses
2.7. Western Blot Analyses
2.8. Statistical Analyses
3. Results
3.1. VWR Alters Microbubbles Apparent Transport in the Tumor Tissue
3.2. VWR Does Not Impact PC-3 Tumor Growth
3.3. VWR Does Not Impact RT Efficiency in PC-3 Xenografts
3.4. VWR Is Not Affected by RT and Does Not Result in Weight Loss
3.5. RT but Not VWR Is Effective at Remodeling Tumor Vasculature
3.6. VWR Does Not Influence the RT-Induced Inhibition of Cell Proliferation and Survival
3.7. VWR Does Impact Tumor Cell Death, Even When Combined with RT
3.8. TR, But Not VWR, Slows Down Tumor Growth in Both PC-3 and PPC-1 Xenografts
4. Discussion
4.1. VWR Does Not Slow Down Tumor Growth but Modulates Microbubble Transport in the Prostate Tumor Tissue
4.2. VWR Does Not Affect Tumor Growth and the RT Response
4.3. TR Slows Down PC-3 and PPC-1 Tumor Growth
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christensen, J.F.; Simonsen, C.; Hojman, P. Exercise Training in Cancer Control and Treatment. Compr. Physiol. 2018, 9, 165–205. [Google Scholar] [CrossRef]
- Patel, A.V.; Friedenreich, C.M.; Moore, S.C.; Hayes, S.C.; Silver, J.K.; Campbell, K.L.; Winters-Stone, K.; Gerber, L.H.; George, S.M.; Fulton, J.E.; et al. American College of Sports Medicine Roundtable Report on Physical Activity, Sedentary Behavior, and Cancer Prevention and Control. Med. Sci. Sports Exerc. 2019, 51, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- Friedenreich, C.M.; Stone, C.R.; Cheung, W.Y.; Hayes, S.C. Physical Activity and Mortality in Cancer Survivors: A Systematic Review and Meta-Analysis. JNCI. Cancer Spectr. 2020, 4. [Google Scholar] [CrossRef] [PubMed]
- Ashcraft, K.A.; Peace, R.M.; Betof, A.S.; Dewhirst, M.W.; Jones, L.W. Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res. 2016, 76, 4032–4050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedersen, L.; Christensen, J.F.; Hojman, P. Effects of Exercise on Tumor Physiology and Metabolism. Cancer J. 2015, 21, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Ashcraft, K.A.; Warner, A.B.; Jones, L.W.; Dewhirst, M.W. Exercise as Adjunct Therapy in Cancer. Semin. Radiat. Oncol. 2019, 29, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Betof, A.S.; Lascola, C.D.; Weitzel, D.H.; Landon, C.D.; Scarbrough, P.M.; Devi, G.R.; Palmer, G.M.; Jones, L.W.; Dewhirst, M.W. Modulation of Murine Breast Tumor Vascularity, Hypoxia, and Chemotherapeutic Response by Exercise. J. Natl. Cancer Inst. 2015, 107, djv040. [Google Scholar] [CrossRef] [Green Version]
- Schadler, K.L.; Thomas, N.J.; Galie, P.A.; Bhang, D.H.; Roby, K.C.; Addai, P.; Till, J.E.; Sturgeon, K.; Zaslavsky, A.; Chen, C.S.; et al. Tumor Vessel Normalization after Aerobic Exercise Enhances Chemotherapeutic Efficacy. Oncotarget 2016, 7, 65429–65440. [Google Scholar] [CrossRef] [Green Version]
- Wennerberg, E.; Lhuillier, C.; Rybstein, M.D.; Dannenberg, K.; Rudqvist, N.-P.; Koelwyn, G.J.; Jones, L.W.; Demaria, S. Exercise Reduces Immune Suppression and Breast Cancer Progression in a Preclinical Model. Oncotarget 2020, 11, 452–461. [Google Scholar] [CrossRef] [Green Version]
- Dufresne, S.; Guéritat, J.; Chiavassa, S.; Noblet, C.; Assi, M.; Rioux-Leclercq, N.; Rannou-Bekono, F.; Lefeuvre-Orfila, L.; Paris, F.; Rébillard, A. Exercise Training Improves Radiotherapy Efficiency in a Murine Model of Prostate Cancer. FASEB J. 2020, 34, 4984–4996. [Google Scholar] [CrossRef]
- Escoffre, J.-M.; Novell, A.; Serrière, S.; Lecomte, T.; Bouakaz, A. Irinotecan Delivery by Microbubble-Assisted Ultrasound: In Vitro Validation and a Pilot Preclinical Study. Mol. Pharm. 2013, 10, 2667–2675. [Google Scholar] [CrossRef] [PubMed]
- Denis de Senneville, B.; Novell, A.; Arthuis, C.; Mendes, V.; Dujardin, P.-A.; Patat, F.; Bouakaz, A.; Escoffre, J.-M.; Perrotin, F. Development of a Fluid Dynamic Model for Quantitative Contrast-Enhanced Ultrasound Imaging. IEEE Trans. Med. Imaging 2018, 37, 372–383. [Google Scholar] [CrossRef] [Green Version]
- Horn, B.K.P.; Schunck, B.G. Determining Optical Flow. Artif. Intell. 1981, 17, 185–203. [Google Scholar] [CrossRef] [Green Version]
- Corpetti, T.; Memin, E.; Perez, P. Dense Estimation of Fluid Flows. IEEE Trans. Pattern Anal. Mach. Intell. 2002, 24, 365–380. [Google Scholar] [CrossRef] [Green Version]
- Denis de Senneville, B.; Frulio, N.; Laumonier, H.; Salut, C.; Lafitte, L.; Trillaud, H. Liver Contrast-Enhanced Sonography: Computer-Assisted Differentiation between Focal Nodular Hyperplasia and Inflammatory Hepatocellular Adenoma by Reference to Microbubble Transport Patterns. Eur. Radiol. 2020, 30, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Potiron, V.A.; Abderrahmani, R.; Clément-Colmou, K.; Marionneau-Lambot, S.; Oullier, T.; Paris, F.; Supiot, S. Improved Functionality of the Vasculature during Conventionally Fractionated Radiation Therapy of Prostate Cancer. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gałecki, A.; Burzykowski, T. Linear Mixed-Effects Models Using R: A Step-by-Step Approach; Springer Texts in Statistics; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-3899-1. [Google Scholar]
- Bristow, R.G.; Hill, R.P. Hypoxia and Metabolism. Hypoxia, DNA Repair and Genetic Instability. Nat. Rev. Cancer 2008, 8, 180–192. [Google Scholar] [CrossRef]
- Kinkade, C.W.; Castillo-Martin, M.; Puzio-Kuter, A.; Yan, J.; Foster, T.H.; Gao, H.; Sun, Y.; Ouyang, X.; Gerald, W.L.; Cordon-Cardo, C.; et al. Targeting AKT/MTOR and ERK MAPK Signaling Inhibits Hormone-Refractory Prostate Cancer in a Preclinical Mouse Model. J. Clin. Investig. 2008, 118, 3051–3064. [Google Scholar] [CrossRef] [Green Version]
- Sarker, D.; Reid, A.H.; Yap, T.A.; de Bono, J.S. Targeting the PI3K/AKT Pathway for the Treatment of Prostate Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 4799–4805. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Cui, X.-X.; Huang, M.-T.; Liu, Y.; Shih, W.J.; Lin, Y.; Lu, Y.P.; Wagner, G.C.; Conney, A.H. Inhibitory Effect of Voluntary Running Wheel Exercise on the Growth of Human Pancreas Panc-1 and Prostate PC-3 Xenograft Tumors in Immunodeficient Mice. Oncol. Rep. 2008, 19, 1583–1588. [Google Scholar]
- Esser, K.A.; Harpole, C.E.; Prins, G.S.; Diamond, A.M. Physical Activity Reduces Prostate Carcinogenesis in a Transgenic Model. Prostate 2009, 69, 1372–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Cui, X.-X.; Gao, Z.; Zhao, Y.; Shi, Y.; Huang, M.-T.; Liu, Y.; Wagner, G.C.; Lin, Y.; Shih, W.J.; et al. Inhibitory Effect of Dietary Atorvastatin and Celecoxib Together with Voluntary Running Wheel Exercise on the Progression of Androgen-Dependent LNCaP Prostate Tumors to Androgen Independence. Exp. Ther. Med. 2011, 2, 221–228. [Google Scholar] [CrossRef]
- Zheng, X.; Cui, X.; Huang, M.; Liu, Y.; Wagner, G.C.; Lin, Y.; Shih, W.J.; Lee, M.; Yang, C.S.; Conney, A.H. Inhibition of Progression of Androgen-Dependent Prostate LNCaP Tumors to Androgen Independence in SCID Mice by Oral Caffeine and Voluntary Exercise. Nutr. Cancer 2012, 64, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Gueritat, J.; Lefeuvre-Orfila, L.; Vincent, S.; Cretual, A.; Ravanat, J.-L.; Gratas-Delamarche, A.; Rannou-Bekono, F.; Rebillard, A. Exercise Training Combined with Antioxidant Supplementation Prevents the Antiproliferative Activity of Their Single Treatment in Prostate Cancer through Inhibition of Redox Adaptation. Free Radic. Biol. Med. 2014, 77, 95–105. [Google Scholar] [CrossRef]
- Saedmocheshi, S.; Saghebjoo, M.; Vahabzadeh, Z.; Sheikholeslami-Vatani, D. Aerobic Training and Green Tea Extract Protect against N-Methyl-N-Nitrosourea-Induced Prostate Cancer. Med. Sci. Sports Exerc. 2019, 51, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- Vahabzadeh, Z.; Molodi, M.; Nikkho, B.; Saghebjoo, M.; Saedmocheshi, S.; Zamani, F.; Roshani, Y.; Babanzadeh, S. Aerobic Training and Hydroalcoholic Extracts of Green Tea Improve Pro-Oxidant-Antioxidant Balance and Histopathological Score in the N-Methyl-N-Nitrosourea-Induced Prostate Cancer Model of Rat. EXCLI J. 2020, 19, 762–772. [Google Scholar] [CrossRef]
- McCullough, D.J.; Nguyen, L.M.-D.; Siemann, D.W.; Behnke, B.J. Effects of Exercise Training on Tumor Hypoxia and Vascular Function in the Rodent Preclinical Orthotopic Prostate Cancer Model. J. Appl. Physiol. (1985) 2013, 115, 1846–1854. [Google Scholar] [CrossRef] [Green Version]
- Jones, L.W.; Antonelli, J.; Masko, E.M.; Broadwater, G.; Lascola, C.D.; Fels, D.; Dewhirst, M.W.; Dyck, J.R.B.; Nagendran, J.; Flores, C.T.; et al. Exercise Modulation of the Host-Tumor Interaction in an Orthotopic Model of Murine Prostate Cancer. J. Appl. Physiol. 2012, 113, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Baumfalk, D.R.; Opoku-Acheampong, A.B.; Caldwell, J.T.; Ade, C.J.; Copp, S.W.; Musch, T.I.; Behnke, B.J. Effects of Prostate Cancer and Exercise Training on Left Ventricular Function and Cardiac and Skeletal Muscle Mass. J. Appl. Physiol. (1985) 2019, 126, 668–680. [Google Scholar] [CrossRef] [Green Version]
- Patel, D.I.; Abuchowski, K.; Bedolla, R.; Rivas, P.; Musi, N.; Reddick, R.; Kumar, A.P. Nexrutine and Exercise Similarly Prevent High Grade Prostate Tumors in Transgenic Mouse Model. PLoS ONE 2019, 14, e0226187. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.I.; Wallace, D.; Abuchowski, K.; Rivas, P.; Gallegos, A.; Musi, N.; Kumar, A.P. Nexrutine® Preserves Muscle Mass Similar to Exercise in Prostate Cancer Mouse Model. Physiol. Rep. 2019, 7, e14217. [Google Scholar] [CrossRef] [PubMed]
- Opoku-Acheampong, A.B.; Baumfalk, D.R.; Horn, A.G.; Kunkel, O.N.; Ganta, C.K.; McCullough, D.J.; Siemann, D.W.; Muller-Delp, J.; Behnke, B.J. Prostate Cancer Cell Growth Characteristics in Serum and Prostate-Conditioned Media from Moderate-Intensity Exercise-Trained Healthy and Tumor-Bearing Rats. Am. J. Cancer Res. 2019, 9, 650–667. [Google Scholar] [PubMed]
- Taylor, R.A.; Farrelly, S.G.; Clark, A.K.; Watt, M.J. Early Intervention Exercise Training Does Not Delay Prostate Cancer Progression in Pten-/- Mice. Prostate 2020, 80, 906–914. [Google Scholar] [CrossRef] [PubMed]
- Koelwyn, G.J.; Quail, D.F.; Zhang, X.; White, R.M.; Jones, L.W. Exercise-Dependent Regulation of the Tumour Microenvironment. Nat. Rev. Cancer 2017, 17, 620–632. [Google Scholar] [CrossRef]
- Schumacher, O.; Galvão, D.A.; Taaffe, D.R.; Chee, R.; Spry, N.; Newton, R.U. Exercise Modulation of Tumour Perfusion and Hypoxia to Improve Radiotherapy Response in Prostate Cancer. Prostate Cancer Prostatic Dis. 2020, 1–14. [Google Scholar] [CrossRef]
- McCullough, D.J.; Stabley, J.N.; Siemann, D.W.; Behnke, B.J. Modulation of Blood Flow, Hypoxia, and Vascular Function in Orthotopic Prostate Tumors During Exercise. JNCI J. Natl. Cancer Inst. 2014, 106, dju036. [Google Scholar] [CrossRef] [Green Version]
- Garcia, E.; Becker, V.G.C.; McCullough, D.J.; Stabley, J.N.; Gittemeier, E.M.; Opoku-Acheampong, A.B.; Sieman, D.W.; Behnke, B.J. Blood Flow Responses to Mild-Intensity Exercise in Ectopic vs. Orthotopic Prostate Tumors; Dependence upon Host Tissue Hemodynamics and Vascular Reactivity. J. Appl. Physiol. 2016, 121, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Bertout, J.A.; Patel, S.A.; Simon, M.C. The Impact of O2 Availability on Human Cancer. Nat. Rev. Cancer 2008, 8, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Stewart, G.D.; Ross, J.A.; McLaren, D.B.; Parker, C.C.; Habib, F.K.; Riddick, A.C.P. The Relevance of a Hypoxic Tumour Microenvironment in Prostate Cancer. BJU Int. 2010, 105, 8–13. [Google Scholar] [CrossRef]
- Elming, P.B.; Sørensen, B.S.; Oei, A.L.; Franken, N.A.P.; Crezee, J.; Overgaard, J.; Horsman, M.R. Hyperthermia: The Optimal Treatment to Overcome Radiation Resistant Hypoxia. Cancers 2019, 11, 60. [Google Scholar] [CrossRef] [Green Version]
- Kenfield, S.A.; Stampfer, M.J.; Giovannucci, E.; Chan, J.M. Physical Activity and Survival After Prostate Cancer Diagnosis in the Health Professionals Follow-Up Study. J. Clin. Oncol. 2011, 29, 726–732. [Google Scholar] [CrossRef]
- Richman, E.L.; Kenfield, S.A.; Stampfer, M.J.; Paciorek, A.; Carroll, P.R.; Chan, J.M. Physical Activity after Diagnosis and Risk of Prostate Cancer Progression: Data from the Cancer of the Prostate Strategic Urologic Research Endeavor. Cancer Res. 2011, 71, 3889–3895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonn, S.E.; Sjölander, A.; Lagerros, Y.T.; Wiklund, F.; Stattin, P.; Holmberg, E.; Grönberg, H.; Bälter, K. Physical Activity and Survival among Men Diagnosed with Prostate Cancer. Cancer Epidemiol. Biomark. Prev. 2015, 24, 57–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedenreich, C.M.; Wang, Q.; Neilson, H.K.; Kopciuk, K.A.; McGregor, S.E.; Courneya, K.S. Physical Activity and Survival After Prostate Cancer. Eur. Urol. 2016, 70, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.J.; Kim, H.J.; Lee, W.J.; Seong, J.K. A Comparison of the Metabolic Effects of Treadmill and Wheel Running Exercise in Mouse Model. Lab. Anim. Res. 2020, 36, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.L.; Winters-Stone, K.M.; Wiskemann, J.; May, A.M.; Schwartz, A.L.; Courneya, K.S.; Zucker, D.S.; Matthews, C.E.; Ligibel, J.A.; Gerber, L.H.; et al. Exercise Guidelines for Cancer Survivors: Consensus Statement from International Multidisciplinary Roundtable. Med. Sci. Sports Exerc. 2019, 51, 2375–2390. [Google Scholar] [CrossRef] [Green Version]






| Protein | MW 1 (kDa) | Reference | Dilution | Source |
|---|---|---|---|---|
| cCASP3 | 17, 19 | Cell signaling 9661 | 1/500 | Rabbit |
| BAX | 20 | Cell signaling 2772 | 1/1000 | Rabbit |
| P21 | 21 | Cell signaling 2947 | 1/1000 | Rabbit |
| P27 | 27 | Cell signaling 2552 | 1/1000 | Rabbit |
| BCL-2 | 28 | Abcam ab7973 | 1/1000 | Rabbit |
| p-ERK1/2 | 42, 44 | Cell signaling 4376 | 1/1000 | Rabbit |
| ERK1/2 | 42, 44 | Santa Cruz sc-514302 | 1/1000 | Mouse |
| p-AKT | 60 | Cell signaling 9271 | 1/1000 | Rabbit |
| AKT | 60 | Cell signaling 9272 | 1/1000 | Rabbit |
| HSC70 | 70 | Santa Cruz sc-7298 | 1/5000 | Mouse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dufresne, S.; Richard, C.; Dieumegard, A.; Orfila, L.; Delpon, G.; Chiavassa, S.; Martin, B.; Rouvière, L.; Escoffre, J.-M.; Oujagir, E.; et al. Voluntary Wheel Running Does Not Enhance Radiotherapy Efficiency in a Preclinical Model of Prostate Cancer: The Importance of Physical Activity Modalities? Cancers 2021, 13, 5402. https://doi.org/10.3390/cancers13215402
Dufresne S, Richard C, Dieumegard A, Orfila L, Delpon G, Chiavassa S, Martin B, Rouvière L, Escoffre J-M, Oujagir E, et al. Voluntary Wheel Running Does Not Enhance Radiotherapy Efficiency in a Preclinical Model of Prostate Cancer: The Importance of Physical Activity Modalities? Cancers. 2021; 13(21):5402. https://doi.org/10.3390/cancers13215402
Chicago/Turabian StyleDufresne, Suzanne, Cindy Richard, Arthur Dieumegard, Luz Orfila, Gregory Delpon, Sophie Chiavassa, Brice Martin, Laurent Rouvière, Jean-Michel Escoffre, Edward Oujagir, and et al. 2021. "Voluntary Wheel Running Does Not Enhance Radiotherapy Efficiency in a Preclinical Model of Prostate Cancer: The Importance of Physical Activity Modalities?" Cancers 13, no. 21: 5402. https://doi.org/10.3390/cancers13215402
APA StyleDufresne, S., Richard, C., Dieumegard, A., Orfila, L., Delpon, G., Chiavassa, S., Martin, B., Rouvière, L., Escoffre, J.-M., Oujagir, E., Denis de Senneville, B., Bouakaz, A., Rioux-Leclercq, N., Potiron, V., & Rébillard, A. (2021). Voluntary Wheel Running Does Not Enhance Radiotherapy Efficiency in a Preclinical Model of Prostate Cancer: The Importance of Physical Activity Modalities? Cancers, 13(21), 5402. https://doi.org/10.3390/cancers13215402