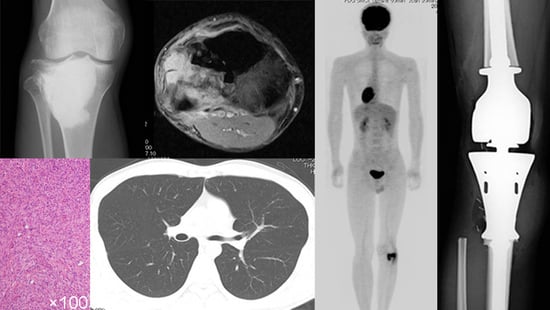
Effect of Adjuvant Chemotherapy on Localized Malignant Giant Cell Tumor of Bone: A Systematic Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Literature Search and Study Selection
2.3. Data Collection and Presentation
2.4. Data Summary, Synthesis and Meta-Analysis
2.5. Assessment of Methodological Quality
2.6. Search Results
2.7. Demographic Data and Ratio of the Patients Who Were Treated with Surgery Combined with Adjuvant Chemotherapy and Surgery Alone
2.8. Methodological Quality of the Included Studies
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stewart, F.W.; Coley, B.L.; Farrow, J.H. Malignant Giant Cell Tumor of Bone. Am. J. Pathol. 1938, 14, 515–536. [Google Scholar] [PubMed]
- Hutter, R.V.; Worcester, J.N.; Francis, K.C.; Foote, F.W., Jr.; Stewart, F.W. Benign and Malignant Giant Cell Tumors of Bone. A Clinicopathological Analysis of the Natural History of the Disease. Cancer 1962, 15, 653–690. [Google Scholar] [CrossRef]
- Dahlin, D.C.; Cupps, R.E.; Johnson, E.W. Giant-Cell Tumor: A Study of 195 Cases. Cancer 1970, 25, 1061–1070. [Google Scholar] [CrossRef]
- Flanagan, A.M.; Larousserie, F.; O’Donnell, P.G.; Yoshida, A. Giant Cell Tumour of Bone. In WHO Classification of Tumours, 5th ed.; Soft Tissue and Bone Tumours; The WHO Classification of Tumours Editorial Board; International Arctic Research Center: Lyon, France, 2020; pp. 440–446. [Google Scholar]
- Palmerini, E.; Picci, P.; Reichardt, P.; Downey, G. Malignancy in Giant Cell Tumor of Bone: A Review of the Literature. Technol. Cancer Res. Treat. 2019, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wülling, M.; Delling, G.; Kaiser, E. The origin of the neoplastic stromal cell in giant cell tumor of bone. Hum. Pathol. 2003, 34, 983–993. [Google Scholar] [CrossRef]
- Robinson, D.; Segal, M.; Nevo, Z. Giant cell tumor of bone. The role of fibroblast growth factor 3 positive mesenchymal stem cells in its pathogenesis. Pathobiology 2002 2003, 70, 333–342. [Google Scholar] [CrossRef]
- Fittall, M.W.; Lyskjaer, I.; Ellery, P.; Lombard, P.; Ijaz, J.; Strobl, A.-C.; Oukrif, D.; Tarabichi, M.; Sill, M.; Koelsche, C.; et al. Drivers Underpinning the Malignant Transformation of Giant Cell Tumour of Bone. J. Pathol. 2020, 252, 433–440. [Google Scholar] [CrossRef]
- Domovitov, S.V.; Healey, J.H. Primary Malignant Giant-Cell Tumor of Bone Has High Survival Rate. Ann. Surg. Oncol. 2010, 17, 694–701. [Google Scholar] [CrossRef]
- Rock, M.G.; Sim, F.H.; Unni, K.K.; Witrak, G.A.; Frassica, F.J.; Schray, M.F.; Beabout, J.W.; Dahlin, D.C. Secondary Malignant Giant-Cell Tumor of Bone. Clinicopathological Assessment of Nineteen Patients. J. Bone Jt. Surg. Am. 1986, 68, 1073–1079. [Google Scholar] [CrossRef]
- Anract, P.; De Pinieux, G.; Cottias, P.; Pouillart, P.; Forest, M.; Tomeno, B. Malignant Giant-Cell Tumours of Bone. Clinico-Pathological Types and Prognosis: A Review of 29 Cases. Int. Orthop. 1998, 22, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Chan, C.M.; Gong, L.; Bui, M.M.; Han, G.; Letson, G.D.; Yang, Y.; Niu, X. Malignancy in Giant Cell Tumor of Bone in the Extremities. J. Bone Oncol. 2021, 26, 100334. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Campanacci, M.; Baldini, N.; Boriani, S.; Sudanese, A. Giant-Cell Tumor of Bone. J. Bone Jt. Surg. Am. 1987, 69, 106–114. [Google Scholar] [CrossRef]
- Boriani, S.; Sudanese, A.; Baldini, N.; Picci, P. Sarcomatous Degeneration of Giant Cell Tumours. Ital. J. Orthop. Traumatol. 1986, 12, 191–199. [Google Scholar]
- Oda, Y.; Sakamoto, A.; Saito, T.; Matsuda, S.; Tanaka, K.; Iwamoto, Y.; Tsuneyoshi, M. Secondary Malignant Giant-Cell Tumour of Bone: Molecular Abnormalities of P53 and H-Ras Gene Correlated with Malignant Transformation. Histopathology 2001, 39, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, F.; Bacchini, P.; Staals, E.L. Malignancy in Giant Cell Tumor of Bone. Cancer 2003, 97, 2520–2529. [Google Scholar] [CrossRef]
- Picci, P.; Sieberova, G.; Alberghini, M.; Balladelli, A.; Vanel, D.; Hogendoorn, P.C.W.; Mercuri, M. Late Sarcoma Development after Curettage and Bone Grafting of Benign Bone Tumors. Eur. J. Radiol. 2011, 77, 19–25. [Google Scholar] [CrossRef]
- Ogura, K.; Higashi, T.; Kawai, A. Statistics of Bone Sarcoma in Japan: Report from the Bone and Soft Tissue Tumor Registry in Japan. J. Orthop. Sci. 2017, 22, 133–143. [Google Scholar] [CrossRef]
- Palmerini, E.; Seeger, L.L.; Gambarotti, M.; Righi, A.; Reichardt, P.; Bukata, S.; Blay, J.-Y.; Dai, T.; Jandial, D.; Picci, P. Malignancy in Giant Cell Tumor of Bone: Analysis of an Open-Label Phase 2 Study of Denosumab. BMC Cancer 2021, 21, 89. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Righi, A.; Mavrogenis, A.F.; Akahane, M.; Honoki, K.; Tanaka, Y.; Donati, D.M.; Errani, C. Late Local Recurrence of Bone Giant Cell Tumors Associated with an Increased Risk for Malignant Transformation. Cancers 2021, 13, 3644. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, J.E.; Lee, Y.J.; Seo, H.-J.; Sheen, S.-S.; Hahn, S.; Jang, B.-H.; Son, H.-J. Testing a Tool for Assessing the Risk of Bias for Nonrandomized Studies Showed Moderate Reliability and Promising Validity. J. Clin. Epidemiol. 2013, 66, 408–414. [Google Scholar] [CrossRef]
- Ferrari, S.; Bielack, S.S.; Smeland, S.; Longhi, A.; Egerer, G.; Sundby Hall, K.; Donati, D.; Kevric, M.; Brosjö, O.; Comandone, A.; et al. EURO-B.O.S.S.: A European Study on Chemotherapy in Bone-Sarcoma Patients Aged over 40: Outcome in Primary High-Grade Osteosarcoma. Tumori 2018, 104, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rockberg, J.; Bach, B.A.; Amelio, J.; Hernandez, R.K.; Sobocki, P.; Engellau, J.; Bauer, H.C.F.; Liede, A. Incidence Trends in the Diagnosis of Giant Cell Tumor of Bone in Sweden Since 1958. J. Bone Jt. Surg. Am. 2015, 97, 1756–1766. [Google Scholar] [CrossRef] [PubMed]
- Raymond, A.K.; Jaffe, N. Conditions That Mimic Osteosarcoma. Cancer Treat. Res. 2009, 152, 85–121. [Google Scholar] [CrossRef]
- Raymond, A.K.; Jaffe, N. Osteosarcoma Multidisciplinary Approach to the Management from the Pathologist’s Perspective. Cancer Treat. Res. 2009, 152, 63–84. [Google Scholar] [CrossRef]
- Bathurst, N.; Sanerkin, N.; Watt, I. Osteoclast-Rich Osteosarcoma. Br. J. Radiol. 1986, 59, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.G.; Huvos, A.G.; Marcove, R.C. Primary Malignant Giant Cell Tumor of Bone: A Study of Eight Cases and Review of the Literature. Cancer 1979, 44, 1393–1402. [Google Scholar] [CrossRef]
- Gong, L.; Liu, W.; Sun, X.; Sajdik, C.; Tian, X.; Niu, X.; Huang, X. Histological and Clinical Characteristics of Malignant Giant Cell Tumor of Bone. Virchows Arch. 2012, 460, 327–334. [Google Scholar] [CrossRef]
- Mondal, A.; Kundu, B.; Gupta, S.; Biswas, J. Secondary Malignant Giant Cell Tumour of Bone--a Study of Five Cases with Short Review of Literature. Indian J. Pathol. Microbiol. 2002, 45, 273–275. [Google Scholar] [PubMed]
- Longhi, A.; Errani, C.; De Paolis, M.; Mercuri, M.; Bacci, G. Primary Bone Osteosarcoma in the Pediatric Age: State of the Art. Cancer Treat. Rev. 2006, 32, 423–436. [Google Scholar] [CrossRef]
- Chen, R.; Wang, G.; Zheng, Y.; Hua, Y.; Cai, Z. Drug Resistance-Related MicroRNAs in Osteosarcoma: Translating Basic Evidence into Therapeutic Strategies. J. Cell. Mol. Med. 2019, 23, 2280–2292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.-L.; Wu, Y.-H.; Shi, Y.-F.; Lin, H.; Nisar, M.; Meftah, Z.; Xu, C.; Chen, J.-X.; Wang, X.-Y. Survival and Prognosis in Malignant Giant Cell Tumor of Bone: A Population-Based Analysis from 1984 to 2013. J. Bone Oncol. 2019, 19, 100260. [Google Scholar] [CrossRef]
- Chen, W.; Yan, Z.; Tirumala, V. Malignant Giant Cell Tumor of Bone or Soft Tissue Treated by Surgery with or without Radiotherapy. J. Orthop. Res. 2020, 38, 2139–2148. [Google Scholar] [CrossRef] [PubMed]






| Study | Type of Study | Mean Follow-Up Period after Diagnosis of Malignant | Mean Follow-Up Period after Diagnosis of GCTB in the Case of Secondary Malignant GCTB (Months) | Total Number of Patients with Localized Malignant GCTB |
|---|---|---|---|---|
| Boriani et al. [15] | SR | 30 | 125 | 9 |
| Anract et al. [11] | SR | 69 | NR | 24 |
| Oda et al. [16] | SR | 15 | 45 | 2 |
| Bertoni et al. [17] | SR | 58 | 144 | 14 |
| Picci et al. [18] | SR | 112 | 352 | 6 |
| Ogura et al. [19] | MR | 48 | NA | 5 |
| Liu et al. [12] | SR | 54 | 143 | 32 |
| Palmerini et al. [20] | MP | Median 48 | NR | 7 |
| Tsukamoto et al. [21] | MR | 32 | 155 | 13 |
| In the Group of Primary Malignant GCTBs | In the Group of Secondary Malignant GCTBs | |||||||
|---|---|---|---|---|---|---|---|---|
| Study | Number of Patients in Surgery Plus Adjuvant Chemotherapy Group | Number of Patients DOD in Surgery Plus Adjuvant Chemo- Therapy Group (Interval from Diagnosis of Malignant GCTB to DOD) | Number of Patients in Surgery-Only Group | Number of Patients DOD in Surgery-Only Group (Interval from Diagnosis of Malignant GCTB to DOD) | Number of Patients in Surgery Plus Adjuvant Chemotherapy Group | Number of Patients DOD in Surgery Plus Adjuvant Chemotherapy Group (Interval from Diagnosis of Malignant GCTB to DOD) | Number of Patients in Surgery-Only Group | Number of Patients DOD in Surgery-Only Group (Interval from Diagnosis of Malignant GCTB to DOD) |
| Boriani et al. [15] | 0 | 0 | 0 | 0 | 1 | 0 | 8 | 6 (mean 11 months) |
| Anract et al. [11] | 5 | 2 (mean 40 months) | 8 | 4 (mean 17.5 months) | 3 | 1 (20 months) | 8 | 3 (mean 36 months) |
| Oda et al. [16] | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 (6 months) |
| Bertoni et al. [17] | 2 | 0 | 3 | 1 (8 months) | 4 | 2 (mean 9 months) | 5 | 3 (mean 59 months) |
| Picci et al. [18] | 0 | 0 | 0 | 0 | 4 | 0 | 2 | 0 |
| Ogura et al. [19] | 1 | 1 (22 months) | 4 | 0 | 0 | 0 | 0 | 0 |
| Liu et al. [12] | 7 | 3 | 5 | 2 | 10 | 6 | 10 | 8 |
| Palmerini et al. [20] | 0 | 0 | 0 | 0 | 6 | 2 (mean 10 months) | 1 | 1 (2 months) |
| Tsukamoto et al. [21] | 0 | 0 | 4 | 1 (24 months) | 7 | 0 | 2 | 1 (19 months) |
| Study | Male vs. Female (Surgery + Chemo/Surgery) | Mean Age (Surgery + Chemo/Surgery) | Location of Tumor (Surgery + Chemo/Surgery) | Campanacci Stage of Malignant GCTB (Surgery + Chemo/Surgery) | Surgery of GCTB (in Case of Secondary Malignant GCTB) (Surgery + Chemo/Surgery) | Surgical Margin of Malignant GCTB (Surgery + Chemo/Surgery) | Histology (Surgery + Chemo/Surgery) | Mean Latent Period in Case of Secondary Malignant GCTB (Months) (Surgery + Chemo/Surgery) | Chemotherapy Regimen |
|---|---|---|---|---|---|---|---|---|---|
| Boriani et al. [15] | 1:0/7:1 | 34/37 | Ischium: 1/Distal radius: 1, Distal femur: 3, Proximal tibia: 3, Proximal femur: 1 | NR/NR | RT alone: 1/Curettage:6, Resection: 2 | NR/NR | MFH:1/MFH:3, OS:2, FS:3 | 30/103 | NR |
| Anract et al. [11] | 4:4/9:7 | 35/39 | Distal femur: 2, Proximal tibia: 2, Distal radius: 1, First cuneiform: 1, Distal tibia: 1, Proximal humerus: 1/Proximal femur: 1, Distal femur: 6, Proximal tibia: 4, Distal tibia: 2, Proximal humerus: 1, Pelvis: 1, L5:1, | NR/NR | Curettage: 3/Curettage: 5, RT alone: 3 | NR/NR | GCT grade III:4, OS:2, FS:2/GCT grade III:11, FS: 2, OS: 3 | NR/NR | NR |
| Oda et al. [16] | 1:0/0:1 | 25/42 | Acetabulum: 1/Distal femur: 1 | Stage 2: 1/NR | Resection: 1/Curettage: 1 | NR/NR | NR/NR | 24/36 | CDDP + DOX |
| Bertoni et al. [17] | 4:2/7:1 | 42/48 | Distal femur: 3, Iliac wing: 1, Ischiopubic arch: 1, Proximal radius: 1/Proximal tibia: 3, Distal tibia: 1, Proximal femur: 1, Distal fe-mur: 2, Distal ulna: 1 | NR/NR | Curettage: 3, RT alone: 1/Curet-tage: 3, Resection: 1, RT alone: 1 | NR/NR | OS:5, MFH:1/OS:6, MFH:1, FS:1 | 90/195 | NR |
| Picci et al. [18] | 3:1/2:0 | 53/57 | Distal femur: 1, Proximal radius: 1, Distal tibia: 1, Proximal tibia: 1/Proximal tibia: 2 | NR/NR | Curettage: 4/Curettage: 2 | NR/NR | OS:2, MFH:2/OS:2 | 228/264 | NR |
| Ogura et al. [19] | 1:0/2:2 | 24/49 | Distal femur: 1/Distal femur: 2, Rib: 1, Proximal tibia: 1 | NR/NR | NA | Inadequate: 1/Adequate: 2, Inadequate: 2 | NR/NR | NA | CDDP + DOX, IFO, MTX |
| Liu et al. [12] | 12:5/5:10 | 32/35 | All extremity/All extremity | Stage 2: 4, Stage 3: 13/Stage 2: 4, Stage 3: 11 | Curettage: 9, Resection: 1/Curettage: 8, Resection: 2 | Adequate: 12, Inadequate: 5/Adequate: 11, Inadequate: | UPS or FS: 3, OS: 14/UPS or FS: 8 OS: 7 | 79/112 | NR |
| Palmerini et al. [20] | NR/NR | 47/55 | Metatarsus: 1, Tibia: 2, Distal femur: 3/Sa- crum:1 | NR/NR | NR/NR | NR/NR | UPS: 4, OS: 2/UPS: 1 | 82/79 | NR |
| Tsukamoto et al. [21] | 3:4/6:0 | 31/47 | Sacrum: 2, Extremity: 5/Extremity: 6 | NR/NR | RT alone: 1, RT + Curettage:1, Curettage: 5/Curettage: 1, Resection: 1 | Adequate: 6, Carbon ion: 1/Adequate: 5, Inadequate: 1 | OS: 3, UPS: 4/OS: 1, UPS: 5 | 172/64 | CDDP, DOX, IFO, VP-16, MTX |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morii, R.; Tsukamoto, S.; Righi, A.; Honoki, K.; Tanaka, Y.; Kido, A.; Fujii, H.; Mavrogenis, A.F.; Tanaka, Y.; Errani, C. Effect of Adjuvant Chemotherapy on Localized Malignant Giant Cell Tumor of Bone: A Systematic Review. Cancers 2021, 13, 5410. https://doi.org/10.3390/cancers13215410
Morii R, Tsukamoto S, Righi A, Honoki K, Tanaka Y, Kido A, Fujii H, Mavrogenis AF, Tanaka Y, Errani C. Effect of Adjuvant Chemotherapy on Localized Malignant Giant Cell Tumor of Bone: A Systematic Review. Cancers. 2021; 13(21):5410. https://doi.org/10.3390/cancers13215410
Chicago/Turabian StyleMorii, Rokuro, Shinji Tsukamoto, Alberto Righi, Kanya Honoki, Yuu Tanaka, Akira Kido, Hiromasa Fujii, Andreas F. Mavrogenis, Yasuhito Tanaka, and Costantino Errani. 2021. "Effect of Adjuvant Chemotherapy on Localized Malignant Giant Cell Tumor of Bone: A Systematic Review" Cancers 13, no. 21: 5410. https://doi.org/10.3390/cancers13215410
APA StyleMorii, R., Tsukamoto, S., Righi, A., Honoki, K., Tanaka, Y., Kido, A., Fujii, H., Mavrogenis, A. F., Tanaka, Y., & Errani, C. (2021). Effect of Adjuvant Chemotherapy on Localized Malignant Giant Cell Tumor of Bone: A Systematic Review. Cancers, 13(21), 5410. https://doi.org/10.3390/cancers13215410