Voltage-Gated Sodium Channels as Potential Biomarkers and Therapeutic Targets for Epithelial Ovarian Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. IRB Approval
2.2. RNAseq Data
2.3. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.4. Kaplan–Meier Analysis
2.5. Cell Lines and Culture Conditions
2.6. Drugs
2.7. Cell Proliferation Assays
2.8. Statistical Analysis
3. Results
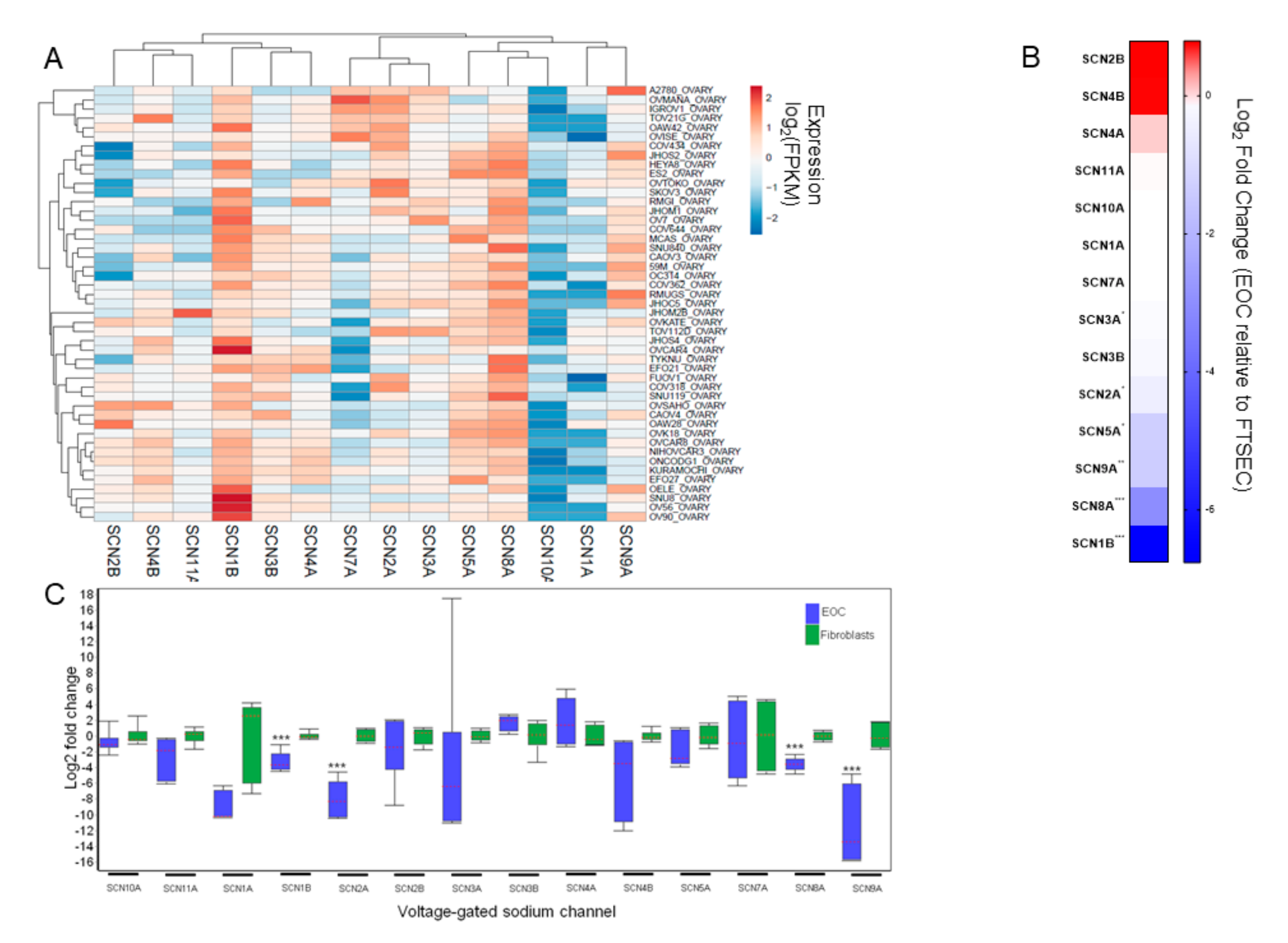
3.1. VGSC Expression in Ovarian Cancer Cell Lines
3.2. Prognostic Implications for VGSC Expression in Ovarian Cancer
3.3. Effects of AEDs on EOC Cells
3.4. Effects of Local Anesthetics on Cell Proliferation
3.5. Impact of Local Anesthetic Exposure on Subsequent Response to Chemotherapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Kurta, M.L.; Edwards, R.P.; Moysich, K.B.; McDonough, K.; Bertolet, M.; Weissfeld, J.L.; Catov, J.M.; Modugno, F.; Bunker, C.H.; Ness, R.B.; et al. Prognosis and Conditional Disease-Free Survival Among Patients with Ovarian Cancer. J. Clin. Oncol. 2014, 32, 4102–4112. [Google Scholar] [CrossRef] [Green Version]
- Dao, F.; Schlappe, B.A.; Tseng, J.; Lester, J.; Nick, A.M.; Lutgendorf, S.K.; McMeekin, S.; Coleman, R.L.; Moore, K.N.; Karlan, B.Y.; et al. Characteristics of 10-year survivors of high-grade serous ovarian carcinoma. Gynecol. Oncol. 2016, 141, 260–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brevet, M.; Ahidouch, A.; Sevestre, H.; Merviel, P.; El Hiani, Y.; Robbe, M.; Ouadid-Ahidouch, H. Expression of K+ channels in normal and cancerous human breast. Histol. Histopathol. 2008, 23, 965–972. [Google Scholar]
- Bon, E.; Driffort, V.; Gradek, F.; Martinez-Caceres, C.; Anchelin, M.; Pelegrin, P.; Cayuela, M.-L.; Lambot, S.M.; Oullier, T.; Guibon, R.; et al. SCN4B acts as a metastasis-suppressor gene preventing hyperactivation of cell migration in breast cancer. Nat. Commun. 2016, 7, 13648. [Google Scholar] [CrossRef] [Green Version]
- Lastraioli, E.; Iorio, J.; Arcangeli, A. Ion channel expression as promising cancer biomarker. Biochim. Biophys. Acta 2015, 1848, 2685–2702. [Google Scholar] [CrossRef] [Green Version]
- Fraser, S.P.; Diss, J.K.J.; Chioni, A.-M.; Mycielska, M.E.; Pan, H.; Yamaci, R.F.; Pani, F.; Siwy, Z.; Krasowska, M.; Grzywna, Z.; et al. Voltage-Gated Sodium Channel Expression and Potentiation of Human Breast Cancer Metastasis. Clin. Cancer Res. 2005, 11, 5381–5389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laniado, M.E.; Lalani, E.-N.; Fraser, S.P.; Grimes, J.A.; Bhangal, G.; Djamgoz, M.B.; Abel, P.D. Expression and functional analysis of voltage-activated Na+ channels in human prostate cancer cell lines and their contribution to invasion in vitro. Am. J. Pathol. 1997, 150, 1213–1221. [Google Scholar] [PubMed]
- Prevarskaya, N.; Skryma, R.; Bidaux, G.; Flourakis, M.; Shuba, Y. Ion channels in death and differentiation of prostate cancer cells. Cell Death Differ. 2007, 14, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.; Yang, M.; Millican-Slater, R.; Brackenbury, W.J. Nav1.5 regulates breast tumor growth and metastatic dissemination in vivo. Oncotarget 2015, 6, 32914–32929. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.; Yang, M.; Dowle, A.A.; Thomas, J.R.; Brackenbury, W.J. The sodium channel-blocking antiepileptic drug phenytoin inhibits breast tumour growth and metastasis. Mol. Cancer 2015, 14, 13–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul, M.; Hoosein, N. Voltage-gated sodium ion channels in prostate cancer: Expression and activity. Anticancer Res. 2002, 22, 1727–1730. [Google Scholar]
- Olsen, C.M.; Meussen-Elholm, E.T.; Røste, L.S.; Taubøll, E. Antiepileptic drugs inhibit cell growth in the human breast cancer cell line MCF7. Mol. Cell. Endocrinol. 2004, 213, 173–179. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, G.; Wang, L.; Song, C.; Leng, Y.; Wang, X.; Kang, J. VPA inhibits breast cancer cell migration by specifically targeting HDAC2 and down-regulating Survivin. Mol. Cell. Biochem. 2011, 361, 39–45. [Google Scholar] [CrossRef]
- Boselli, E.; Duflo, F.; DeBon, R.; Allaouchiche, B.; Chassard, D.; Thomas, L.; Portoukalian, J. The Induction of Apoptosis by Local Anesthetics: A Comparison between Lidocaine and Ropivacaine. Anesth. Analg. 2003, 96, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Bundscherer, A.; Malsy, M.; Gebhardt, K.; Metterlein, T.; Plank, C.; Wiese, C.; Gruber, M.; Graf, B. Effects of ropivacaine, bupivacaine and sufentanil in colon and pancreatic cancer cells in vitro. Pharmacol. Res. 2015, 95-96, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Hsu, Y.-C.; Liu, C.-L.; Huang, S.-Y.; Hu, M.-C.; Cheng, S.-P. Local Anesthetics Induce Apoptosis in Human Thyroid Cancer Cells through the Mitogen-Activated Protein Kinase Pathway. PLoS ONE 2014, 9, e89563. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.-C.; Liu, C.-L.; Chen, M.-J.; Hsu, Y.-W.; Chen, S.-N.; Lin, C.-H.; Chen, C.-M.; Yang, F.-M.; Hu, M.-C. Local Anesthetics Induce Apoptosis in Human Breast Tumor Cells. Anesth. Analg. 2014, 118, 116–124. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.R.; Whipple, R.A.; Balzer, E.M.; Cho, E.H.; Matrone, M.A.; Peckham, M.; Martin, S.S. Local anesthetics inhibit kinesin motility and microtentacle protrusions in human epithelial and breast tumor cells. Breast Cancer Res. Treat. 2011, 129, 691–701. [Google Scholar] [CrossRef] [Green Version]
- Beverly, A.; Kaye, A.D.; Ljungqvist, O.; Urman, R.D. Essential Elements of Multimodal Analgesia in Enhanced Recovery After Surgery (ERAS) Guidelines. Anesthesiol. Clin. 2017, 35, e115–e143. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, U.O.; Oppelstrup, H.; Thorell, A.; Nygren, J.; Ljungqvist, O. Adherence to the ERAS protocol is Associated with 5-Year Survival After Colorectal Cancer Surgery: A Retrospective Cohort Study. World J. Surg. 2016, 40, 1741–1747. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, G.S.; Fitzgerald, P.; Streicher, L.F.; Marcus, R.J.; McCarthy, R.J. Systemic lidocaine to improve postoperative quality of recovery after ambulatory laparoscopic surgery. Anesth. Analg. 2012, 115, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Marret, E.; Rolin, M.; Beaussier, M.; Bonnet, F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. BJS 2008, 95, 1331–1338. [Google Scholar] [CrossRef]
- Scott, D.B.; Jebson, P.J.R.; Braid, D.P.; Ortengren, B.; Frisch, P. Factors affecting plasma levels of lignocaine and prilocaine. Br. J. Anaesth. 1972, 44, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Sharrock, N.E.; Mineo, R.; Stanton, J.; Ennis, W.J., 3rd; Urmey, W.F.; Arthur, G.R. Single versus staged epidural injections of 0.75% bupivacaine: Pharmacokinetic and pharmacodynamic effects. Anesth. Analg. 1994, 79, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-K.; Miao, C.-H. The Effect of Anesthetic Technique on Survival in Human Cancers: A Meta-Analysis of Retrospective and Prospective Studies. PLoS ONE 2013, 8, e56540. [Google Scholar] [CrossRef] [Green Version]
- Cummings, K.C.; Xu, F.; Cummings, L.C.; Cooper, G.S. A comparison of epidural analgesia and traditional pain management effects on survival and cancer recurrence after colectomy: A population-based study. Anesthesiology 2012, 116, 797–806. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, G.S.; Ahmad, S.; Schink, J.C.; Singh, D.K.; Fitzgerald, P.C.; McCarthy, R.J. Intraoperative neuraxial anesthesia but not postoperative neuraxial analgesia is associated with increased relapse-free survival in ovarian cancer patients after primary cytoreductive surgery. Reg. Anesth. Pain Med. 2011, 36, 271–277. [Google Scholar] [CrossRef]
- Exadaktylos, A.K.; Buggy, D.J.; Moriarty, D.C.; Mascha, E.; Sessler, D.I. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology 2006, 105, 660–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forget, P.; Tombal, B.; Scholtès, J.-L.; Nzimbala, J.; Meulders, C.; Legrand, C.; Van Cangh, P.; Cosyns, J.-P.; De Kock, M. Do intraoperative analgesics influence oncological outcomes after radical prostatectomy for prostate cancer? Eur. J. Anaesthesiol. 2011, 28, 830–835. [Google Scholar] [CrossRef] [PubMed]
- Lacassie, H.J.; Cartagena, J.; Brañes, J.; Assel, M.; Echevarría, G.C. The Relationship between Neuraxial Anesthesia and Advanced Ovarian Cancer-Related Outcomes in the Chilean Population. Anesth. Analg. 2013, 117, 653–660. [Google Scholar] [CrossRef]
- Merquiol, F.; Montelimard, A.S.; Nourissat, A.; Molliex, S.; Zufferey, P.J. Cervical epidural anesthesia is associated with increased cancer-free survival in laryngeal and hypopharyngeal cancer surgery: A retrospective propensity-matched analysis. Reg. Anesth. Pain Med. 2013, 38, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Roiss, M.; Schiffmann, J.; Tennstedt, P.; Kessler, T.; Blanc, I.; Goetz, A.; Schlomm, T.; Graefen, M.; Reuter, D. Oncological long-term outcome of 4772 patients with prostate cancer undergoing radical prostatectomy: Does the anaesthetic technique matter? Eur. J. Surg. Oncol. 2014, 40, 1686–1692. [Google Scholar] [CrossRef]
- Sun, Y.; Li, T.; Gan, T.J. The Effects of Perioperative Regional Anesthesia and Analgesia on Cancer Recurrence and Survival After Oncology Surgery: A Systematic Review and Meta-Analysis. Reg. Anesth. Pain Med. 2015, 40, 589–598. [Google Scholar] [CrossRef]
- Wuethrich, P.Y.; Hsu Schmitz, S.F.; Kessler, T.M.; Thalmann, G.N.; Studer, U.E.; Stueber, F.; Burkhard, F.C. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: A retrospective study. Anesthesiology 2010, 113, 570–576. [Google Scholar] [CrossRef]
- Cancer Cell Line Encyclopedia Consortium; Genomics of Drug Sensitivity in Cancer Consortium. Pharmacogenomic agreement between two cancer cell line data sets. Nature 2015, 528, 84–87. [Google Scholar] [CrossRef]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Margolin, A.A.; Kim, S.; Wilson, C.J.; Lehár, J.; Kryukov, G.V.; Sonkin, D.; et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–607. [Google Scholar] [CrossRef]
- Elias, K.M.; Emori, M.M.; Westerling, T.; Long, H.; Budina-Kolomets, A.; Li, F.; Macduffie, E.; Davis, M.; Holman, A.; Lawney, B.; et al. Epigenetic remodeling regulates transcriptional changes between ovarian cancer and benign precursors. JCI Insight 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Györffy, B.; Lanczky, A.; Eklund, A.C.; Denkert, C.; Budczies, J.; Li, Q.; Szallasi, Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res. Treat. 2009, 123, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Birkbak, N.J.; Gyorffy, B.; Szallasi, Z.; Eklund, A.C. Jetset: Selecting the optimal microarray probe set to represent a gene. BMC Bioinform. 2011, 12, 474. [Google Scholar] [CrossRef] [Green Version]
- Mihály, Z.; Kormos, M.; Lánczky, A.; Dank, M.; Budczies, J.; Szász, A.M.; Győrffy, B. A meta-analysis of gene expression-based biomarkers predicting outcome after tamoxifen treatment in breast cancer. Breast Cancer Res. Treat. 2013, 140, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Emori, M.M.; Papp, E.; MacDuffie, E.; Konecny, G.E.; Velculescu, V.E.; Drapkin, R. Beyond genomics: Critical evaluation of cell line utility for ovarian cancer research. Gynecol. Oncol. 2015, 139, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Jang, I.S.; Neto, E.C.; Guinney, J.; Friend, S.H.; Margolin, A.A. Systematic assessment of analytical methods for drug sensitivity prediction from cancer cell line data. Biocomputing 2014 2013, 2014, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Biasiotta, A.; D’Arcangelo, D.; Passarelli, F.; Nicodemi, E.M.; Facchiano, A. Ion channels expression and function are strongly modified in solid tumors and vascular malformations. J. Transl. Med. 2016, 14, 285. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Plata, E.; Ortíz, C.S.; Marquina-Castillo, B.; Medina-Martínez, I.; Alfaro, A.; Berumen, J.; Rivera, M.; Gomora, J.C. Overexpression of NaV1.6 channels is associated with the invasion capacity of human cervical cancer. Int. J. Cancer 2011, 130, 2013–2023. [Google Scholar] [CrossRef] [PubMed]
- Besson, P.; Driffort, V.; Bon, Émeline; Gradek, F.; Chevalier, S.; Roger, S. How do voltage-gated sodium channels enhance migration and invasiveness in cancer cells? Biochim. Biophys. Acta 2015, 1848, 2493–2501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, Y.; Wong, C.C.; Xu, J.; Chen, H.; Zhang, Y.; Kang, W.; Wang, H.; Zhang, L.; Li, W.; Chu, E.S.; et al. Sodium Channel Subunit SCNN1B Suppresses Gastric Cancer Growth and Metastasis via GRP78 Degradation. Cancer Res. 2017, 77, 1968–1982. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Ohno, S.; Uchida, S.; Amano, O.; Sakagami, H.; Nagasaka, H. Cytotoxicity and type of cell death induced by local anesthetics in human oral normal and tumor cells. Anticancer Res. 2012, 32, 2925–2933. [Google Scholar] [PubMed]
- Werdehausen, R.; Fazeli, S.; Braun, S.; Hermanns, H.; Essmann, F.; Hollmann, M.W.; Bauer, I.; Stevens, M.F. Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br. J. Anaesth. 2009, 103, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Xuan, W.; Zhao, H.; Hankin, J.; Chen, L.; Yao, S.; Ma, D. Local anesthetic bupivacaine induced ovarian and prostate cancer apoptotic cell death and underlying mechanisms in vitro. Sci. Rep. 2016, 6, 26277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, M.; Fulco, R.A.; Collecchi, P.; Zicca, A.; Cadoni, A.; Merlo, F.; Rosso, R.; Sobrero, A. Improved Therapeutic Index of Cisplatin by Procaine Hydrochloride. J. Natl. Cancer Inst. 1990, 82, 677–684. [Google Scholar] [CrossRef]
- Tabrizi, L.; Chiniforoshan, H. Cytotoxicity and cellular response mechanisms of water-soluble platinum(II) complexes of lidocaine and phenylcyanamide derivatives. BioMetals 2016, 30, 59–70. [Google Scholar] [CrossRef]
- Viale, M.; Pastrone, M.; Pellecchia, C.; Vannozzi, M.O.; Cafaggi, S.; Esposito, M. Combination of cisplatin-procaine complex DPR with anticancer drugs increases cytotoxicity against ovarian cancer cell lines. Anti-Cancer Drugs 1998, 9, 457–463. [Google Scholar] [CrossRef]
- Beaufort, C.M.; Helmijr, J.C.A.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; van Ijcken, W.; Heine, A.; Smid, M.; et al. Ovarian Cancer Cell Line Panel (OCCP): Clinical Importance of In Vitro Morphological Subtypes. PLoS ONE 2014, 9, e103988. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-N.; Chen, B.-S.; Wu, Y.-H.; Peng, H.; Chen, L.-T. The mechanism of the actions of oxaliplatin on ion currents and action potentials in differentiated NG108-15 neuronal cells. NeuroToxicology 2009, 30, 677–685. [Google Scholar] [CrossRef]
- Grolleau, F.; Gamelin, L.; Boisdron-Celle, M.; Lapied, B.; Pelhate, M.; Gamelin, E. A possible explanation for a neurotoxic effect of the anticancer agent oxaliplatin on neuronal voltage-gated sodium channels. J. Neurophysiol. 2001, 85, 2293–2297. [Google Scholar] [CrossRef] [PubMed]
- Argyriou, A.A.; Cavaletti, G.; Antonacopoulou, A.; Genazzani, A.A.; Briani, C.; Bruna, J.; Terrazzino, S.; Velasco, R.; Alberti, P.; Campagnolo, M.; et al. Voltage-gated sodium channel polymorphisms play a pivotal role in the development of oxaliplatin-induced peripheral neurotoxicity: Results from a prospective multicenter study. Cancer 2013, 119, 3570–3577. [Google Scholar] [CrossRef] [PubMed]
- Palugulla, S.; Thakkar, D.N.; Kayal, S.; Narayan, S.; Dkhar, S. Association of Voltage-Gated Sodium Channel Genetic Polymorphisms with Oxaliplatin-Induced Chronic Peripheral Neuropathy in South Indian Cancer Patients. Asian Pac. J. Cancer Prev. 2017, 18, 3157–3165. [Google Scholar] [CrossRef] [PubMed]
- Sereno, M.; Gutiérrez-Gutiérrez, G.; Rubio, J.M.; Apellániz-Ruiz, M.; Sánchez-Barroso, L.; Casado, E.; Falagan, S.; López-Gómez, M.; Merino, M.; Gómez-Raposo, C.; et al. Genetic polymorphisms of SCN9A are associated with oxaliplatin-induced neuropathy. BMC Cancer 2017, 17, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casini, S.; Tan, H.L.; Demirayak, I.; Remme, C.A.; Amin, A.S.; Scicluna, B.P.; Chatyan, H.; Ruijter, J.M.; Bezzina, C.R.; van Ginneken, A.C.; et al. Tubulin polymerization modifies cardiac sodium channel expression and gating. Cardiovasc. Res. 2010, 85, 691–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dybkova, N.; Wagner, S.; Backs, J.; Hund, T.J.; Mohler, P.J.; Sowa, T.; Nikolaev, V.O.; Maier, L.S. Tubulin polymerization disrupts cardiac beta-adrenergic regulation of late INa. Cardiovasc. Res. 2014, 103, 168–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, K.M.; Kang, S.; Liu, X.; Horowitz, N.S.; Berkowitz, R.S.; Frendl, G. Anesthetic Selection and Disease-Free Survival Following Optimal Primary Cytoreductive Surgery for Stage III Epithelial Ovarian Cancer. Ann. Surg. Oncol. 2014, 22, 1341–1348. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brummelhuis, I.S.; Fiascone, S.J.; Hasselblatt, K.T.; Frendl, G.; Elias, K.M. Voltage-Gated Sodium Channels as Potential Biomarkers and Therapeutic Targets for Epithelial Ovarian Cancer. Cancers 2021, 13, 5437. https://doi.org/10.3390/cancers13215437
Brummelhuis IS, Fiascone SJ, Hasselblatt KT, Frendl G, Elias KM. Voltage-Gated Sodium Channels as Potential Biomarkers and Therapeutic Targets for Epithelial Ovarian Cancer. Cancers. 2021; 13(21):5437. https://doi.org/10.3390/cancers13215437
Chicago/Turabian StyleBrummelhuis, Iris S., Stephen J. Fiascone, Kathleen T. Hasselblatt, Gyorgy Frendl, and Kevin M. Elias. 2021. "Voltage-Gated Sodium Channels as Potential Biomarkers and Therapeutic Targets for Epithelial Ovarian Cancer" Cancers 13, no. 21: 5437. https://doi.org/10.3390/cancers13215437
APA StyleBrummelhuis, I. S., Fiascone, S. J., Hasselblatt, K. T., Frendl, G., & Elias, K. M. (2021). Voltage-Gated Sodium Channels as Potential Biomarkers and Therapeutic Targets for Epithelial Ovarian Cancer. Cancers, 13(21), 5437. https://doi.org/10.3390/cancers13215437





