Co-Occurrence of Differentiated Thyroid Cancer and Second Primary Malignancy: Correlation with Expression Profiles of Mismatch Repair Protein and Cell Cycle Regulators
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Histopathology
2.2. Tissue Microarray (TMA) Construction
2.3. Immunohistochemistry (IHC)
2.4. IHC Interpretation
2.5. Statistical Methods
3. Results
3.1. Clinical Features of DTC Patients
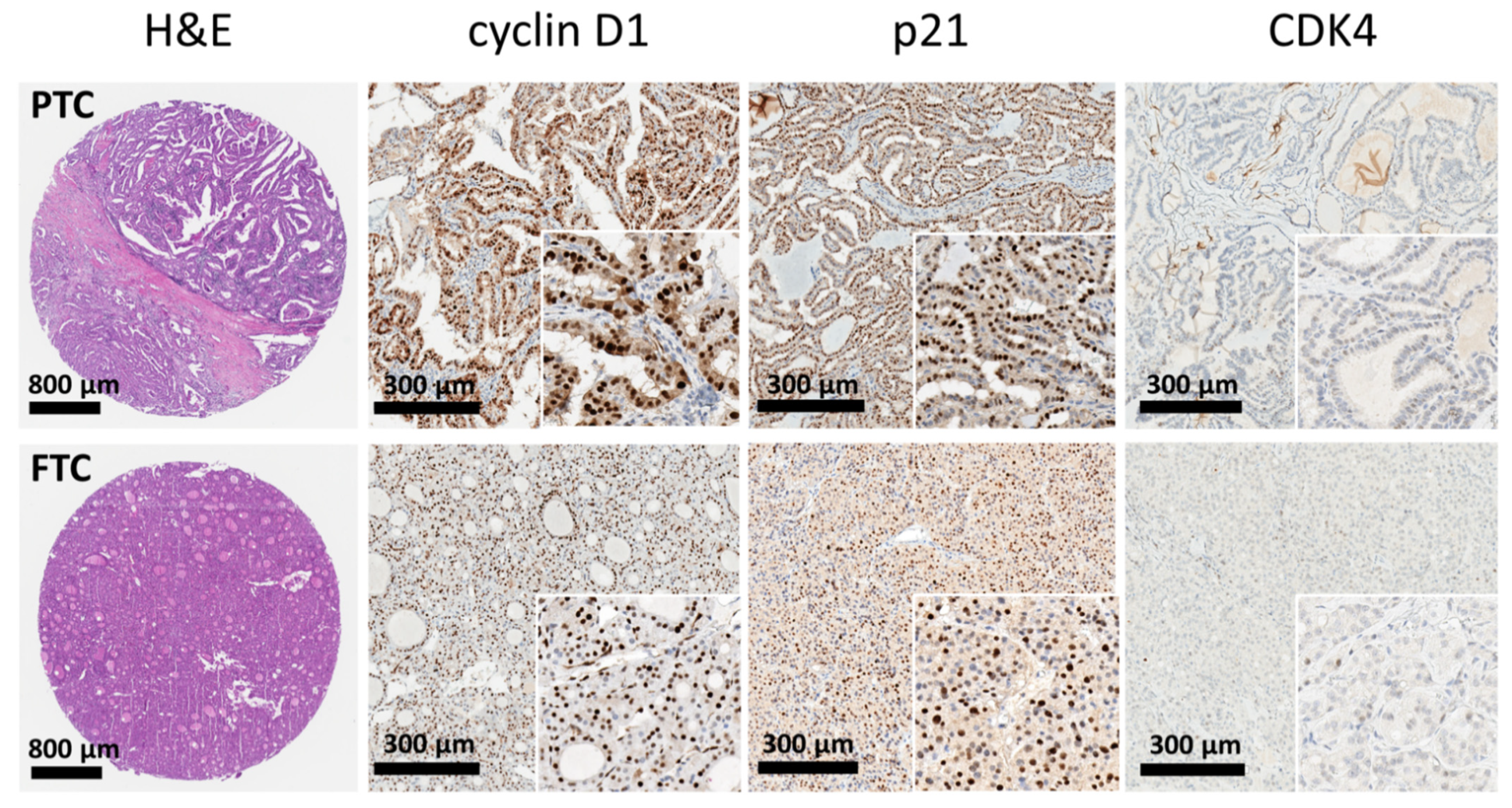
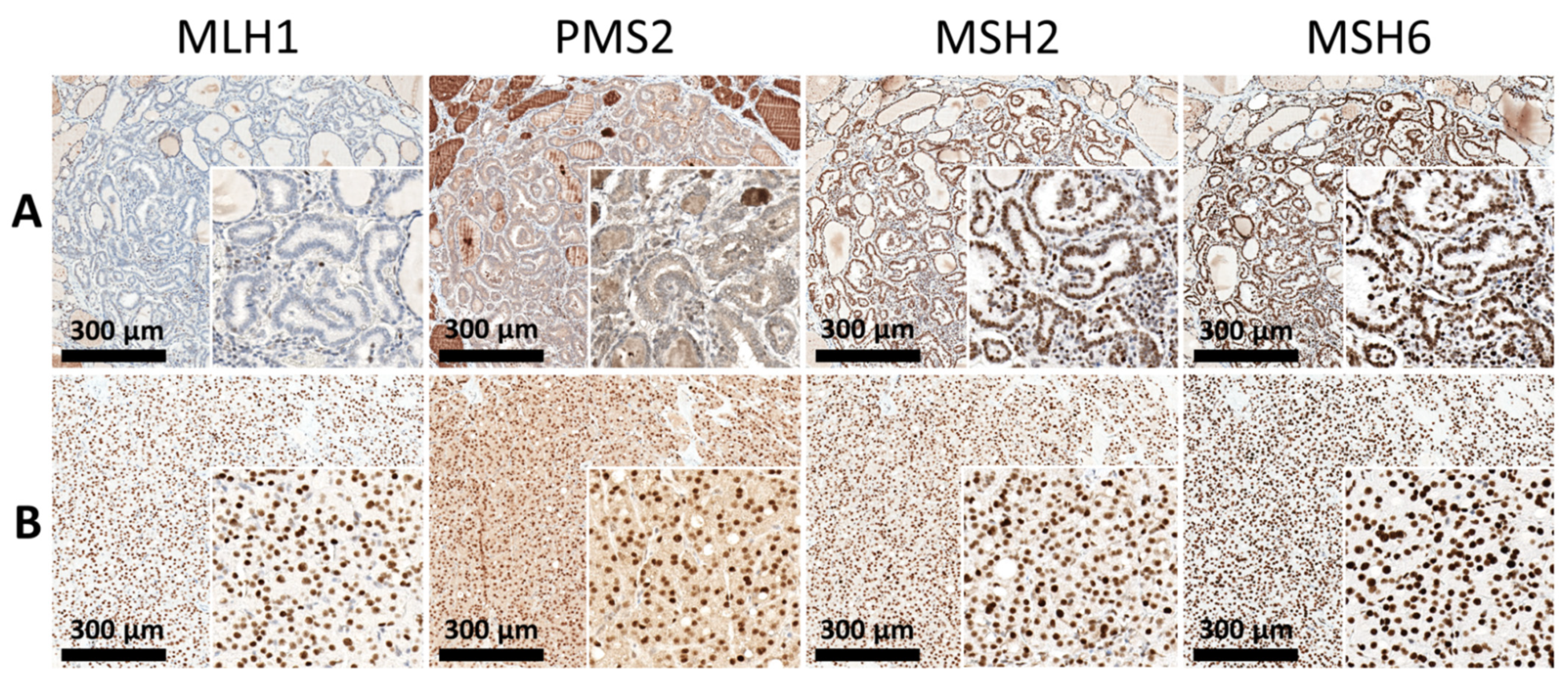
3.2. Expression of Mismatch Repair (MMR) Proteins and Cell-Cycle Regulatory Proteins
3.3. Immunoexpression Patterns in the Papillary Carcinoma (PTC) and Follicular Carcinoma (FTC) Subtypes and the Interaction between the SPC and DPC Groups
3.4. Immunoexpression Patterns of DTC and the Interrelationship with Significant Biomarkers
3.5. Multimarker Expression Model with Potential Implications of a Second Primary Malignancy
4. Discussion
4.1. Differences in Expression of Cell-Cycle Regulators between Individuals with and without Second Primary Malignancy
4.2. Possible Clinical Significance of Altered DNA Mismatch Repair Capacity in Patients with Double Primary Malignancies
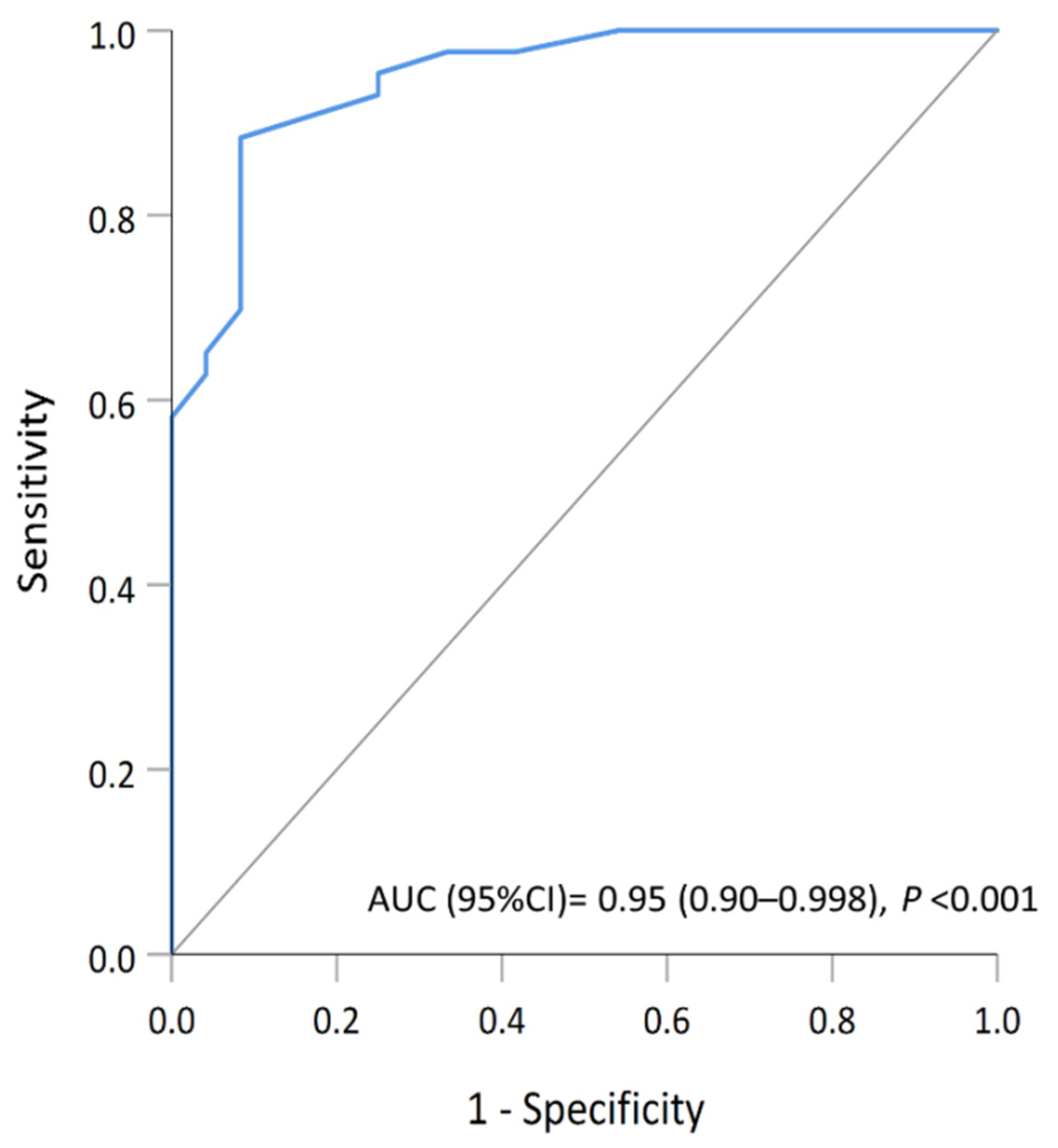
4.3. A Predictive Model Based on the Four Biomarkers (dMMR, pRb, CDK4, and CDK6)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macerola, E.; Poma, A.; Vignali, P.; Basolo, A.; Ugolini, C.; Torregrossa, L.; Santini, F.; Basolo, F. Molecular Genetics of Follicular-Derived Thyroid Cancer. Cancers 2021, 13, 1139. [Google Scholar] [CrossRef]
- Lloyd, R.V.; Osamura, R.Y.; Klöppel, G.; Rosai, J. WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Karaköse, M.; Çordan, I.; Can, M.; Kocabaş, M.; Kulaksızoğlu, M.; Karakurt, F.; Kulaksizoğlu, M. Incidence of second primary malignancies in patients with thyroid cancer in the Turkish population. Turk. J. Med. Sci. 2019, 49, 1529–1533. [Google Scholar] [CrossRef]
- Endo, M.; Liu, J.B.; Dougan, M.; Lee, J.S. Incidence of Second Malignancy in Patients with Papillary Thyroid Cancer from Surveillance, Epidemiology, and End Results 13 Dataset. J. Thyroid. Res. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corrêa, N.L.; De Sá, L.V.; De Mello, R.C.R. Estimation of Second Primary Cancer Risk after Treatment with Radioactive Iodine for Differentiated Thyroid Carcinoma. Thyroid 2017, 27, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Hakala, T.T.; Sand, J.A.; Jukkola, A.; Huhtala, H.S.; Metso, S.; Kellokumpu-Lehtinen, P.-L. Increased risk of certain second primary malignancies in patients treated for well-differentiated thyroid cancer. Int. J. Clin. Oncol. 2015, 21, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.Y.; Lim, J.; Oh, C.-M.; Ryu, J.; Jung, K.-W.; Chung, J.H.; Won, Y.-J.; Kim, S.W. Elevated risks of subsequent primary malignancies in patients with thyroid cancer: A nationwide, population-based study in Korea. Cancer 2014, 121, 259–268. [Google Scholar] [CrossRef]
- Hsu, C.H.; Huang, C.L.; Iqbal, U.; Nguyen, P.A.; Jian, W.S. Co-occurrence of second primary malignancy in patients with thyroid cancer. QJM Int. J. Med. 2014, 107, 643–648. [Google Scholar] [CrossRef] [Green Version]
- Zafon, C.; Obiols, G.; Mesa, J. Second primary cancer in patients with papillary thyroid carcinoma. Anticancer Res. 2013, 33, 337–340. [Google Scholar]
- Lu, C.-H.; Lee, K.-D.; Chen, P.-T.; Chen, C.-C.; Kuan, F.-C.; Huang, C.-E.; Chen, M.-F.; Chen, M.-C. Second primary malignancies following thyroid cancer: A population-based study in Taiwan. Eur. J. Endocrinol. 2013, 169, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Lang, B.H.-H.; Wong, K.P. Risk Factors for Nonsynchronous Second Primary Malignancy and Related Death in Patients with Differentiated Thyroid Carcinoma. Ann. Surg. Oncol. 2011, 18, 3559–3565. [Google Scholar] [CrossRef] [Green Version]
- Sawka, A.M.; Thabane, L.; Parlea, L.; Ibrahim-Zada, I.; Tsang, R.; Brierley, J.D.; Straus, S.; Ezzat, S.; Goldstein, D.P. Second Primary Malignancy Risk After Radioactive Iodine Treatment for Thyroid Cancer: A Systematic Review and Meta-analysis. Thyroid 2009, 19, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.P.; Chen, J.; Hitchcock, Y.J.; Szabo, A.; Shrieve, D.C.; Tward, J. The Risk of Second Primary Malignancies up to Three Decades after the Treatment of Differentiated Thyroid Cancer. J. Clin. Endocrinol. Metab. 2008, 93, 504–515. [Google Scholar] [CrossRef] [Green Version]
- Verkooijen, R.B.T.; Smit, J.W.A.; Romijn, J.A.; Stokkel, M.P.M. The incidence of second primary tumors in thyroid cancer patients is increased, but not related to treatment of thyroid cancer. Eur. J. Endocrinol. 2006, 155, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Sandeep, T.C.; Strachan, M.W.J.; Reynolds, R.; Brewster, D.; Scélo, G.; Pukkala, E.; Hemminki, K.; Anderson, A.; Tracey, E.; Friis, S.; et al. Second Primary Cancers in Thyroid Cancer Patients: A Multinational Record Linkage Study. J. Clin. Endocrinol. Metab. 2006, 91, 1819–1825. [Google Scholar] [CrossRef]
- Canchola, A.J.; Horn-Ross, P.L.; Purdie, D.M. Risk of Second Primary Malignancies in Women with Papillary Thyroid Cancer. Am. J. Epidemiol. 2006, 163, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Rubino, C.; De Vathaire, F.; Dottorini, M.E.; Hall, P.; Schvartz, C.; Couette, J.E.; Dondon, M.-G.; Abbas, M.T.; Langlois, C.; Schlumberger, M. Second primary malignancies in thyroid cancer patients. Br. J. Cancer 2003, 89, 1638–1644. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Bi, X.; Pan, D.; Chen, Y.; Carling, T.; Ma, S.; Udelsman, R.; Zhang, Y. The Risk of Second Cancers after Diagnosis of Primary Thyroid Cancer Is Elevated in Thyroid Microcarcinomas. Thyroid 2013, 23, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schonfeld, S.J.; Morton, L.M.; de González, A.B.; Curtis, R.E.; Kitahara, C.M. Risk of second primary papillary thyroid cancer among adult cancer survivors in the United States, 2000-2015. Cancer Epidemiol. 2019, 64, 101664. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, J.; Shen, Y.; Zhao, X.; Zhang, L.; Wang, B.; Li, P.; Wang, Y.; Yi, M.; Yang, J. Clinicopathological features, survival and risk in breast cancer survivors with thyroid cancer: An analysis of the SEER database. BMC Public Health 2019, 19, 1592. [Google Scholar] [CrossRef] [PubMed]
- Joseph, K.R.; Edirimanne, S.; Eslick, G.D. The association between breast cancer and thyroid cancer: A meta-analysis. Breast Cancer Res. Treat. 2015, 152, 173–181. [Google Scholar] [CrossRef]
- Huang, J.; Walker, R.; Groome, P.G.; Shelley, W.; Mackillop, W.J. Risk of thyroid carcinoma in a female population after radiotherapy for breast carcinoma. Cancer 2001, 92, 1411–1418. [Google Scholar] [CrossRef]
- An, J.H.; Hwangbo, Y.; Ahn, H.Y.; Keam, B.; Lee, K.E.; Han, W.; Park, D.J.; Park, I.A.; Noh, D.-Y.; Youn, Y.-K.; et al. A Possible Association between Thyroid Cancer and Breast Cancer. Thyroid 2015, 25, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B. The Epidemiology of Second Primary Cancers. Cancer Epidemiol. Biomark. Prev. 2006, 15, 2020–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, S.; Masago, K. Alteration of DNA mismatch repair capacity underlying the co-occurrence of non-small-cell lung cancer and nonmedullary thyroid cancer. Sci. Rep. 2021, 11, 3597. [Google Scholar] [CrossRef]
- Dong, M.; Wei, H.; Hou, J.M.; Gao, S.; Yang, D.Z.; Lin, Z.H.; Jia, Y.; Ren, X.P.; Gao, M.H. Possible prognostic sig-nificance of p53, cyclin D1 and Ki-67 in the second primary malignancy of patients with double primary ma-lignancies. Int. J. Clin. Exp. Pathol. 2014, 7, 3975–3983. [Google Scholar] [PubMed]
- Zagouri, F.; Kotoula, V.; Kouvatseas, G.; Sotiropoulou, M.; Koletsa, T.; Gavressea, T.; Valavanis, C.; Trihia, H.; Bobos, M.; Lazaridis, G.; et al. Protein expression patterns of cell cycle regulators in operable breast cancer. PLoS ONE 2017, 12, e0180489. [Google Scholar] [CrossRef] [Green Version]
- Myong, N.-H. Cyclin D1 Overexpression, p16 Loss, and pRb Inactivation Play a Key Role in Pulmonary Carcinogenesis and have a Prognostic Implication for the Long-term Survival in Non-small Cell Lung Carcinoma Patients. Cancer Res. Treat. 2008, 40, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Ebihara, Y.; Miyamoto, M.; Shichinohe, T.; Kawarada, Y.; Cho, Y.; Fukunaga, A.; Murakami, S.; Uehara, H.; Kaneko, H.; Hashimoto, H.; et al. Over-expression of E2F-1 in esophageal squamous cell carcinoma correlates with tumor progression. Dis. Esophagus 2004, 17, 150–154. [Google Scholar] [CrossRef]
- Kjellman, P.; Wallin, G.; Höög, A.; Auer, G.; Larsson, C.; Zedenius, J. MIB-1 Index in Thyroid Tumors: A Predictor of the Clinical Course in Papillary Thyroid Carcinoma? Thyroid 2003, 13, 371–380. [Google Scholar] [CrossRef]
- Anwar, F.; Emond, M.J.; Schmidt, R.A.; Hwang, H.C.; Bronner, M.P. Retinoblastoma Expression in Thyroid Neoplasms. Mod. Pathol. 2000, 13, 562–569. [Google Scholar] [CrossRef]
- Cao, H.-H.; Zhang, S.-Y.; Shen, J.-H.; Wu, Z.-Y.; Wu, J.-Y.; Wang, S.-H.; Li, E.-M.; Xu, L.-Y. A three-protein signature and clinical outcome in esophageal squamous cell carcinoma. Oncotarget 2014, 6, 5435–5448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, H.-H.; Zheng, C.-P.; Wang, S.-H.; Wu, J.-Y.; Shen, J.-H.; Xu, X.-E.; Fu, J.-H.; Wu, Z.-Y.; Li, E.-M.; Xu, L.-Y. A Molecular Prognostic Model Predicts Esophageal Squamous Cell Carcinoma Prognosis. PLoS ONE 2014, 9, e106007. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Wu, Y.; Liu, F.; Fu, L.; Tong, Z. Characteristics and survival of patients with metachronous or synchronous double primary malignancies: Breast and thyroid cancer. Oncotarget 2016, 7, 52450–52459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunbanjerdsuk, S.; Vorasan, N.; Saethang, T.; Pongrujikorn, T.; Pangpunyakulchai, D.; Mongkonsiri, N.; Arsa, L.; Thokanit, N.; Pongsapich, W.; Anekpuritanang, T.; et al. Oncoproteomic and gene expression analyses identify prognostic biomarkers for second primary malignancy in patients with head and neck squamous cell carcinoma. Mod. Pathol. 2019, 32, 943–956. [Google Scholar] [CrossRef]
- Saini, M.L.; Weynand, B.; Rahier, J.; Mourad, M.; Hamoir, M.; Marbaix, E. Cyclin D1 in well differentiated thyroid tumour of uncertain malignant potential. Diagn. Pathol. 2015, 10, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, D.; Zhang, H.; Hu, X.; Xin, S.; Duan, Z. Abnormality of pl6/p38MAPK/p53/Wipl pathway in papillary thyroid cancer. Gland. Surg. 2012, 1, 33–38. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, J.K.; Jin, S.M.; Lee, K.C.; Sohn, J.H.; Chae, S.W.; Kim, D.H. Expression of cell-cycle regulators (cyclin D1, cyclin E, p27kip1, p57kip2) in papillary thyroid carcinoma. Otolaryngol. Neck Surg. 2010, 142, 332–337. [Google Scholar] [CrossRef]
- Zafon, C.; Obiols, G.; Castellvi, J.; Cajal, S.R.Y.; Baena-Fustegueras, J.A.; Mesa, J. Expression of p21cip1, p27kip1, and p16INk4a Cyclin-Dependent Kinase Inhibitors in Papillary Thyroid Carcinoma: Correlation with Clinicopathological Factors. Endocr. Pathol. 2008, 19, 184–189. [Google Scholar] [CrossRef]
- Pešutić-Pisac, V.; Punda, A.; Glunčić, I.; Bedeković, V.; Pranić-Kragić, A.; Kunac, N. Cyclin D1 and p27 Expression as Prognostic Factor in Papillary Carcinoma of the Thyroid: Association with Clinicopathological Parameters. Croat. Med. J. 2008, 49, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Melck, A.; Masoudi, H.; Griffith, O.; Rajput, A.; Wilkins, G.; Bugis, S.; Jones, S.; Wiseman, S.M. Cell Cycle Regulators Show Diagnostic and Prognostic Utility for Differentiated Thyroid Cancer. Ann. Surg. Oncol. 2007, 14, 3403–3411. [Google Scholar] [CrossRef]
- Khoo, M.L.C.; Beasley, N.J.P.; Ezzat, S.; Freeman, J.L.; Asa, S.L. Overexpression of Cyclin D1 and Underexpression of p27 Predict Lymph Node Metastases in Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2002, 87, 1814–1818. [Google Scholar] [CrossRef]
- Wang, S.; Lloyd, R.V.; Hutzler, M.J.; Safran, M.S.; Patwardhan, N.A.; Khan, A. The Role of Cell Cycle Regulatory Protein, Cyclin D1, in the Progression of Thyroid Cancer. Mod. Pathol. 2000, 13, 882–887. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wuu, J.; Savas, L.; Patwardhan, N.; Khan, A. The role of cell cycle regulatory proteins, cyclin D1, cyclin E, and p27 in thyroid carcinogenesis. Hum. Pathol. 1998, 29, 1304–1309. [Google Scholar] [CrossRef]
- Temmim, L.; Ebraheem, A.K.; Baker, H.; Sinowatz, F. Cyclin D1 Protein Expression in Human Thyroid gland and Thyroid Cancer. Anat. Histol. Embryol. 2005, 35, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Muro-Cacho, C.A.; Holt, T.; Klotch, D.; Mora, L.; Livingston, S.; Futran, N. Cyclin D1 Expression as a Prognostic Parameter in Papillary Carcinoma of the Thyroid. Otolaryngol. Neck Surg. 1999, 120, 200–207. [Google Scholar] [CrossRef]
- Ferenc, T.; Lewinski, A.; Lange, D.; Niewiadomska, H.; Sygut, J.; Sporny, S.; Jarzab, B.; Satacińska-Los, E.; Kulig, A.; Włoch, J. Analysis of cyclin D1 and retinoblastoma protein immunoreactivity in follicular thyroid tumors. Pol. J. Pathol. 2005, 56, 27–35. [Google Scholar] [PubMed]
- Holm, R.; Nesland, J.M. Retinoblastoma and p53 tumour suppressor gene protein expression in carcinomas of the thyroid gland. J. Pathol. 1994, 172, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Evren, B.; Yılmaz, S.; Karadağ, N.; Sertkaya, A.; Topaloğlu, Ö.; Kılınç, F. DNA repair proteins may differentiate papillary thyroid cancer from chronic lymphocytic thyroiditis and nodular colloidal goiter. Sci. Rep. 2021, 11, 9932. [Google Scholar] [CrossRef]
- Javid, M.; Sasanakietkul, T.; Nicolson, N.G.; Gibson, C.E.; Callender, G.G.; Korah, R.; Carling, T. DNA Mismatch Repair Deficiency Promotes Genomic Instability in a Subset of Papillary Thyroid Cancers. World J. Surg. 2017, 42, 358–366. [Google Scholar] [CrossRef]
- Onda, M.; Nakamura, I.; Suzuki, S.; Takenoshita, S.; Brogren, C.H.; Stampanoni, S.; Li, D.; Rampino, N. Mi-crosatellite instability in thyroid cancer: Hot spots, clinicopathological implications, and prognostic significance. Clin. Cancer Res. 2001, 7, 3444–3449. [Google Scholar]
- Mitmaker, E.; Alvarado, C.; Bégin, L.R.; Trifiro, M. Microsatellite Instability in Benign and Malignant Thyroid Neoplasms. J. Surg. Res. 2008, 150, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Wangefjord, S.; Brändstedt, J.; Lindquist, K.E.; Nodin, B.; Jirström, K.; Eberhard, J. Associations of beta-catenin alterations and MSI screening status with expression of key cell cycle regulating proteins and survival from colorectal cancer. Diagn. Pathol. 2013, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tornillo, L.; Lugli, A.; Zlobec, I.; Willi, N.; Glatz, K.; Lehmann, F.; Spichtin, H.-P.; Maurer, R.; Stoios, D.; Sauter, G.; et al. Prognostic Value of Cell Cycle and Apoptosis Regulatory Proteins in Mismatch Repair–Proficient Colorectal Cancer. Am. J. Clin. Pathol. 2007, 127, 114–123. [Google Scholar] [CrossRef] [PubMed]

| Clinical Variable | Single Thyroid Cancer (STC) (n = 24) n (%) | Double Primary Cancer (DPC) (n = 43) n (%) |
|---|---|---|
| Gender | ||
| Male | 3 (12.5) | 9 (20.9) |
| Female | 21 (87.5) | 34 (79.1) |
| Age | ||
| <50 years | 14 (58.3) | 15 (34.9) |
| ≥50 years | 10 (41.7) | 28 (65.1) |
| Occurrence interval | ||
| <6 months | NA | 9 (20.9) |
| ≥6 months | NA | 34 (79.1) |
| Clinical outcome | ||
| Alive | 9 (37.5) | 29 (67.4) |
| Dead | 5 (20.8) | 8 (18.6) |
| Not accessible | 10 (41.7) | 6 (14.0) |
| Variable | STC | DPC | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| (n = 24) | (n = 43) | Crude OR (95% CI) | p Value | Adjusted OR (95% CI) | p Value | |
| dMMR | 8 (33.3) | 32 (74.4) | 5.82 (1.95–17.32) | 0.002 | 10.34 (2.17–49.21) | 0.003 |
| cyclin D1 | 13 (54.2) | 36 (83.7) | 4.35 (1.39–13.61) | 0.011 | ||
| p21 | 5 (20.8) | 20 (46.5) | 3.30 (1.04–10.47) | 0.042 | ||
| pRb | 6 (25.0) | 26 (60.5) | 4.59 (1.52–13.89) | 0.007 | 62.71 (4.83–814.22) | 0.002 |
| p16INk4a | 3 (12.5) | 13 (30.2) | 3.03 (0.77–11.98) | 0.113 | ||
| CDK2 | 3 (12.5) | 19 (44.2) | 5.54 (1.43–21.40) | 0.013 | ||
| CDK4 | 13 (54.2) | 9 (20.9) | 0.22 (0.08–0.67) | 0.007 | 0.19 (0.04–0.99) | 0.049 |
| CDK6 | 8 (33.3) | 4 (9.3) | 0.21 (0.05–0.78) | 0.020 | 0.03 (0.002–0.44) | 0.011 |
| E2F1 | 8 (33.3) | 15 (34.9) | 1.07 (0.37–3.08) | 0.898 | ||
| Ki-67 | 2 (8.3) | 14 (32.6) | 5.31 (1.09–25.83) | 0.039 | ||
| Variable | Papillary Thyroid Cancer (PTC) | Follicular Thyroid Cancer (FTC) | |||||
|---|---|---|---|---|---|---|---|
| STC (n = 13) | DPC (n = 35) | OR (95% CI) | STC (n = 11) | DPC (n = 8) | OR (95% CI) | p Interaction | |
| dMMR | 4 (30.8) | 25 (71.4) | 5.62 (1.40–22.53) | 4 (36.4) | 7 (87.5) | 12.25 (1.08–138.99) | 0.586 |
| cyclin D1 | 8 (61.5) | 29 (82.9) | 3.02 (0.73–12.52) | 5 (45.5) | 7 (87.5) | 8.40 (0.76–93.34) | 0.474 |
| p21 | 2 (15.4) | 16 (45.7) | 4.63 (0.89–24.04) | 3 (27.3) | 4 (50.0) | 2.67 (0.39–18.17) | 0.669 |
| pRb | 0 (0.0) | 21 (60.0) | NA | 6 (54.5) | 5 (62.5) | 1.39 (0.22–8.92) | 0.998 |
| p16INk4a | 2 (15.4) | 13 (37.1) | 3.25 (0.62–17.01) | 1 (9.1) | 0 (0.0) | NA | 1.000 |
| CDK2 | 0 (0.0) | 17 (48.6) | NA | 3 (27.3) | 2 (25.0) | 0.89 (0.11–7.11) | 0.998 |
| CDK4 | 4 (30.8) | 5 (14.3) | 0.38 (0.08–1.70) | 9 (81.8) | 4 (50.0) | 0.22 (0.03–1.75) | 0.689 |
| CDK6 | 0 (0.0) | 2 (5.7) | NA | 8 (72.7) | 2 (25.0) | 0.13 (0.02–0.999) | 0.999 |
| E2F1 | 6 (46.2) | 12 (34.3) | 0.61 (0.17–2.22) | 2 (18.2) | 3 (37.5) | 2.70 (0.33–21.98) | 0.236 |
| Ki-67 | 2 (15.4) | 12 (34.3) | 2.87 (0.55–15.10) | 0 (0.0) | 2 (25.0) | NA | 0.999 |
| Variable | dMMR | MMR-Proficient | |||||
|---|---|---|---|---|---|---|---|
| STC (n = 8) | DPC (n = 32) | OR (95% CI) | STC (n = 16) | DPC (n = 11) | OR (95% CI) | p Interaction | |
| CDK4 | 3 (37.5) | 7 (21.9) | 0.47 (0.09–2.45) | 10 (62.5) | 2 (18.2) | 0.13 (0.02–0.84) | 0.321 |
| CDK6 | 2 (25.0) | 3 (9.4) | 0.31 (0.04–2.28) | 6 (37.5) | 1 (9.1) | 0.17 (0.02–1.65) | 0.688 |
| pRb | 2 (25.0) | 17 (53.1) | 3.40 (0.59–19.46) | 4 (25.0) | 9 (81.8) | 13.50 (2.01–90.69) | 0.295 |
| Variable | pRb = Positive | pRb = Negative | |||||
| STC (n = 6) | DPC (n = 26) | OR (95% CI) | STC (n = 18) | DPC (n = 17) | OR (95% CI) | p Interaction | |
| dMMR | 2 (33.3) | 17 (65.4) | 3.78 (0.58–24.75) | 6 (33.3) | 15 (88.2) | 15.00 (2.55–88.17) | 0.295 |
| CDK4 | 6 (100.0) | 6 (23.1) | NA | 7 (38.9) | 3 (17.6) | 0.34 (0.07–1.61) | 0.998 |
| CDK6 | 5 (83.3) | 4 (15.4) | 0.04 (0.00–0.40) | 3 (16.7) | 0 (0.0) | NA | 0.999 |
| Variable | CDK4 = Positive | CDK4 = Negative | |||||
| STC (n = 13) | DPC (n = 9) | OR (95% CI) | STC (n = 11) | DPC (n = 34) | OR (95% CI) | p Interaction | |
| dMMR | 3 (23.1) | 7 (77.8) | 11.67 (1.53–89.12) | 5 (45.5) | 25 (73.5) | 3.33 (0.81–13.66) | 0.321 |
| pRb | 6 (46.2) | 6 (66.7) | 2.33 (0.40–13.61) | 0 (0.0) | 20 (58.8) | NA | 0.998 |
| CDK6 | 8 (61.5) | 1 (11.1) | 0.08 (0.01–0.83) | 0 (0.0) | 3 (8.8) | NA | 0.999 |
| Variable | CDK6 = Positive | CDK6 = Negative | |||||
| STC (n = 8) | DPC (n = 4) | OR (95% CI) | STC (n = 16) | DPC (n = 39) | OR (95% CI) | p Interaction | |
| dMMR | 2 (25.0) | 3 (75.0) | 9.00 (0.56–143.89) | 6 (37.5) | 29 (74.4) | 4.83 (1.40–16.73) | 0.688 |
| pRb | 5 (62.5) | 4 (100.0) | NA | 1 (6.3) | 22 (56.4) | 19.41 (2.33–161.86) | 0.999 |
| CDK4 | 8 (100.0) | 1 (25.0) | NA | 5 (31.3) | 8 (20.5) | 0.57 (0.15–2.11) | 0.999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.-Y.; Huang, C.-S.; Huang, C.-C.; Ku, W.-C.; Shih, H.-Y.; Huang, C.-J. Co-Occurrence of Differentiated Thyroid Cancer and Second Primary Malignancy: Correlation with Expression Profiles of Mismatch Repair Protein and Cell Cycle Regulators. Cancers 2021, 13, 5486. https://doi.org/10.3390/cancers13215486
Liu C-Y, Huang C-S, Huang C-C, Ku W-C, Shih H-Y, Huang C-J. Co-Occurrence of Differentiated Thyroid Cancer and Second Primary Malignancy: Correlation with Expression Profiles of Mismatch Repair Protein and Cell Cycle Regulators. Cancers. 2021; 13(21):5486. https://doi.org/10.3390/cancers13215486
Chicago/Turabian StyleLiu, Chih-Yi, Ching-Shui Huang, Chi-Cheng Huang, Wei-Chi Ku, Hsing-Yu Shih, and Chi-Jung Huang. 2021. "Co-Occurrence of Differentiated Thyroid Cancer and Second Primary Malignancy: Correlation with Expression Profiles of Mismatch Repair Protein and Cell Cycle Regulators" Cancers 13, no. 21: 5486. https://doi.org/10.3390/cancers13215486
APA StyleLiu, C. -Y., Huang, C. -S., Huang, C. -C., Ku, W. -C., Shih, H. -Y., & Huang, C. -J. (2021). Co-Occurrence of Differentiated Thyroid Cancer and Second Primary Malignancy: Correlation with Expression Profiles of Mismatch Repair Protein and Cell Cycle Regulators. Cancers, 13(21), 5486. https://doi.org/10.3390/cancers13215486






