Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
2.1.1. HEGP Cohort
2.1.2. PRODIGE 17-ACCORD 20-MEGA Trial
2.1.3. Healthy Controls
2.2. Monocyte Isolation and Culture
2.3. Isolation of CD4+ T Cells
2.4. Monocyte/Lymphocyte Co-Culture
2.5. Immunostaining
2.6. Analysis of Cytokine Production
2.7. Statistical Analyses
3. Results
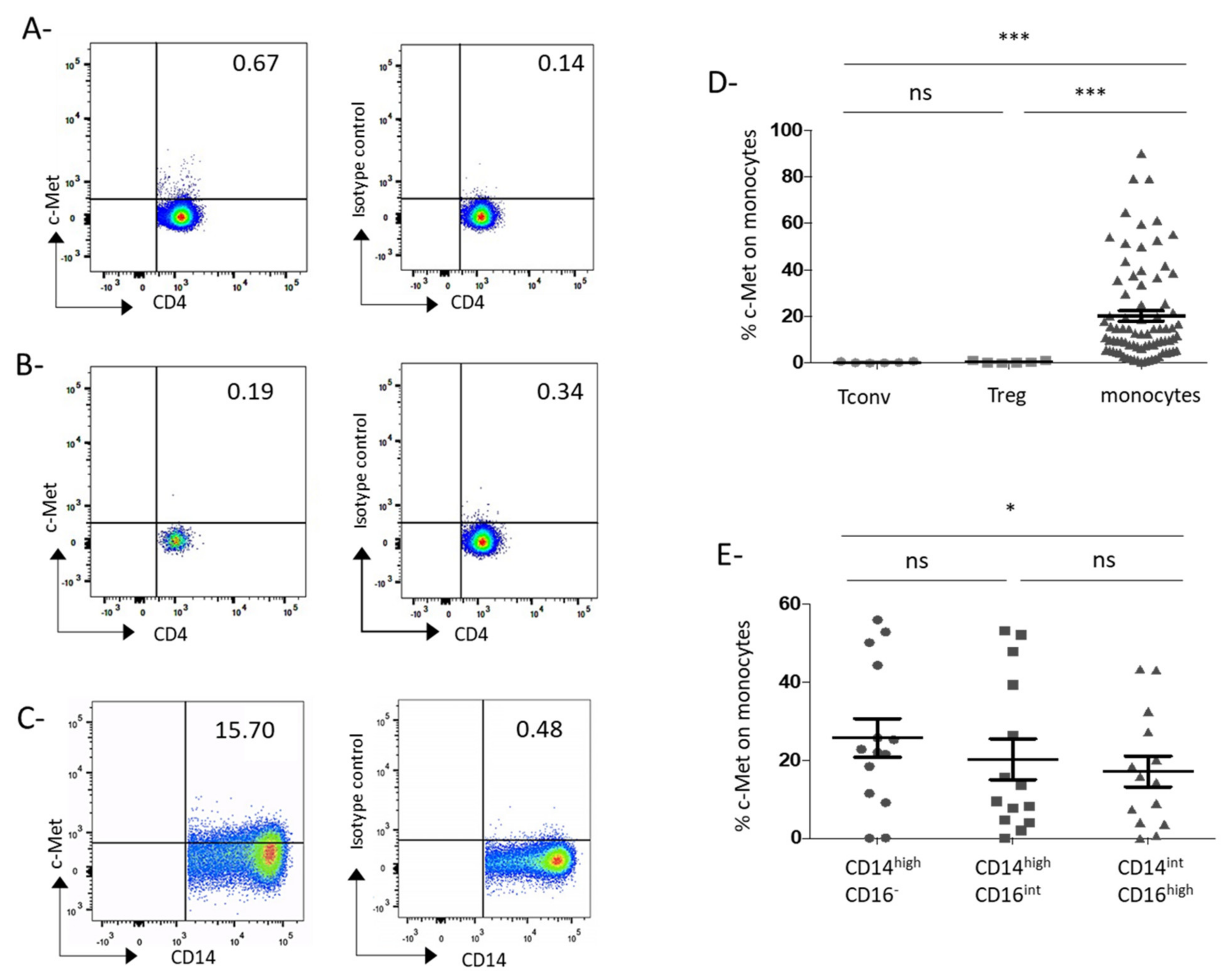
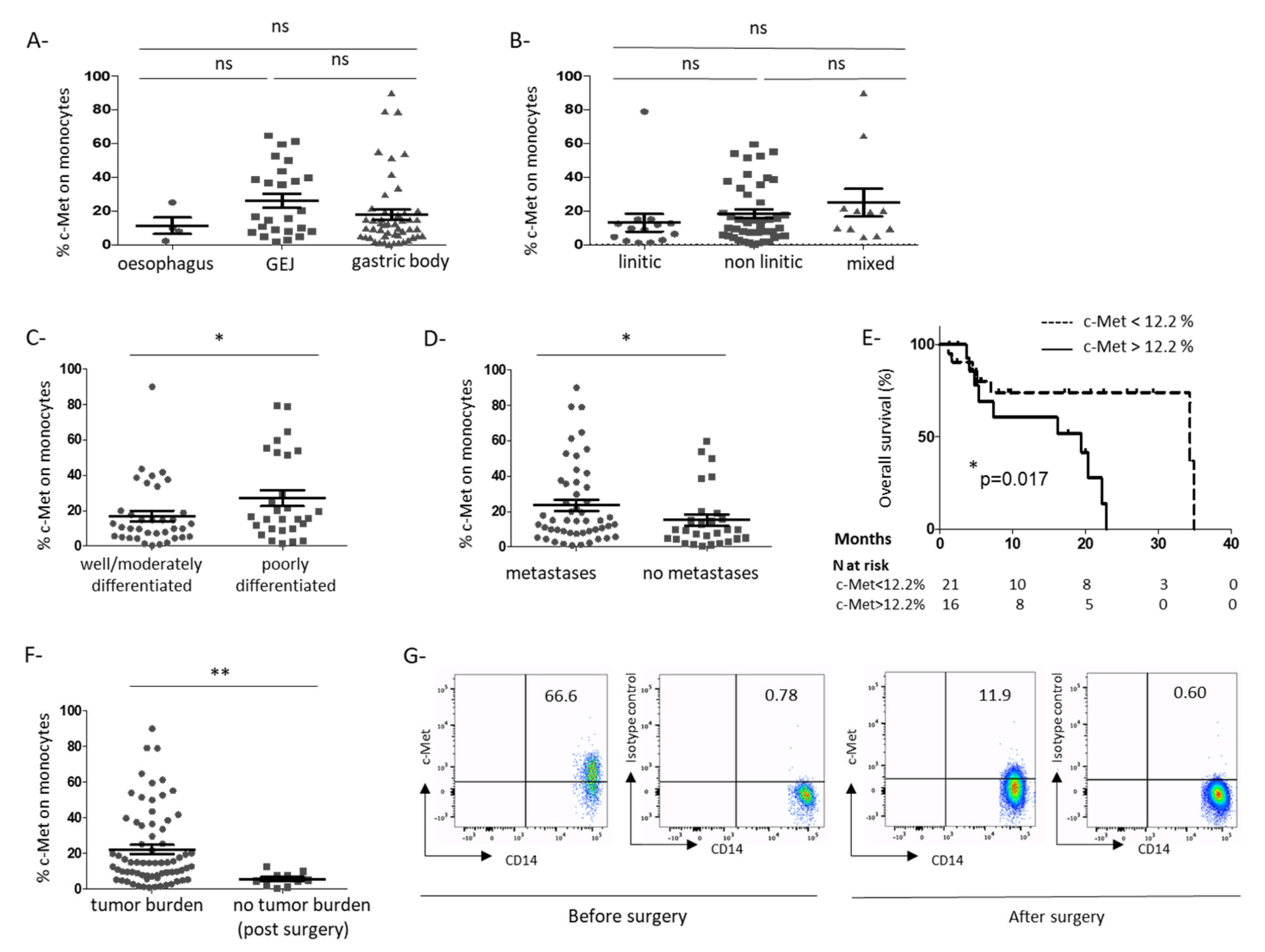
3.1. C-Met Receptor Is Expressed on Peripheral Blood Monocytes in GC Patients
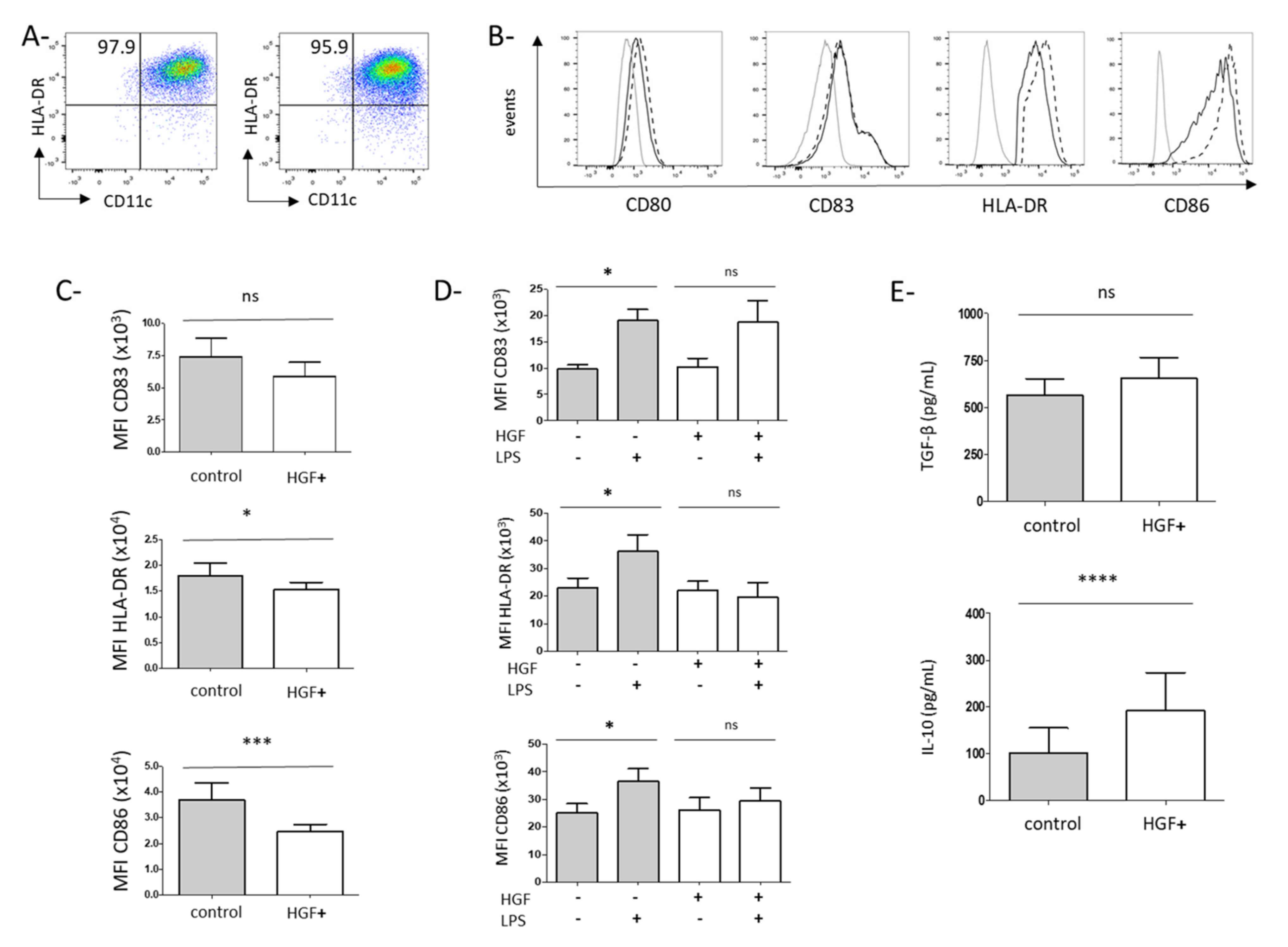
3.2. HGF Induces the Differentiation of Monocytes into Dendritic Cells with Tolerogenic Properties
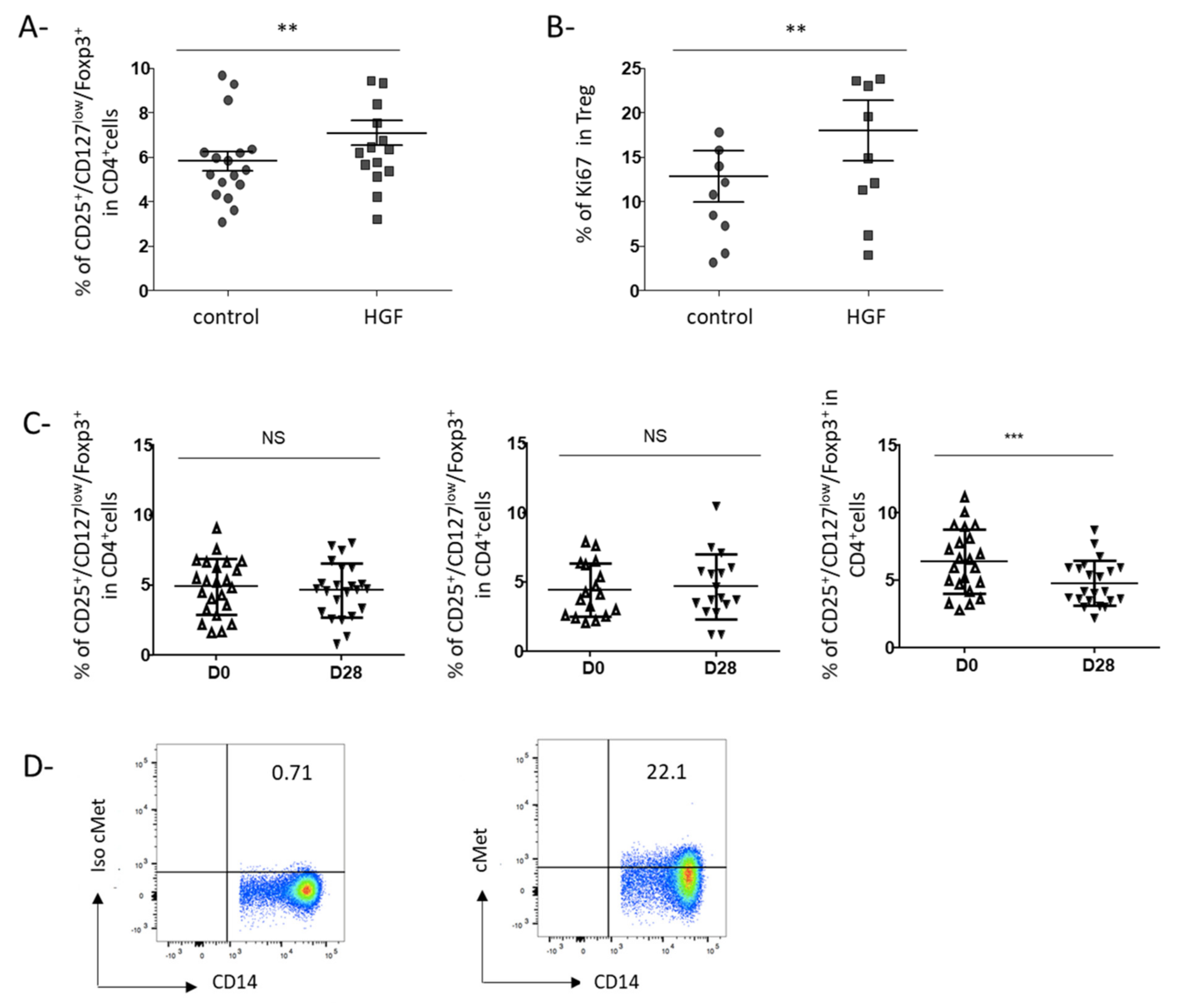
3.3. HGF-Generated DCs Induce the Development of Regulatory T Cells in Gastric Cancer Patients
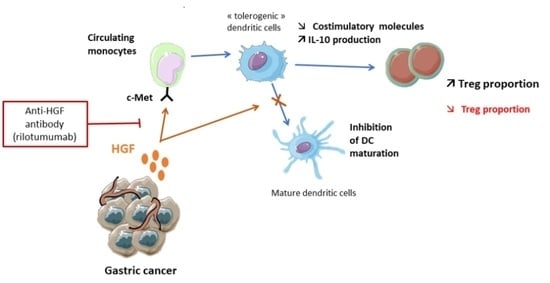
3.4. Rilotumumab, an Anti-HGF Antibody, Reduces Treg Proportion in the Peripheral Blood of Advanced GC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanchot, C.; Terme, M.; Pere, H.; Tran, T.; Benhamouda, N.; Strioga, M.; Banissi, C.; Galluzzi, L.; Kroemer, G.; Tartour, E. Tumor-infiltrating regulatory T cells: Phenotype, role, mechanism of expansion in situ and clinical significance. Cancer Microenviron. 2013, 6, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Terme, M.; Pernot, S.; Marcheteau, E.; Sandoval, F.; Benhamouda, N.; Colussi, O.; Dubreuil, O.; Carpentier, A.F.; Tartour, E.; Taieb, J. VEGFA-VEGFR Pathway Blockade Inhibits Tumor-Induced Regulatory T-cell Proliferation in Colorectal Cancer. Cancer Res. 2013, 73, 539–549. [Google Scholar] [CrossRef] [Green Version]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trusolino, L.; Bertotti, A.; Comoglio, P.M. MET signalling: Principles and functions in development, organ regeneration and cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Anestis, A.; Zoi, I.; Karamouzis, M.V. Current advances of targeting HGF/c-Met pathway in gastric cancer. Ann. Transl. Med. 2018, 6, 247. [Google Scholar] [CrossRef]
- Yu, S.; Yu, Y.; Zhao, N.; Cui, J.; Li, W.; Liu, T. c-Met as a Prognostic Marker in Gastric Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e79137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, Z.; Zhu, Y.; Wang, Q.; Gao, J.; Li, Y.; Li, Y.; Ge, S.; Shen, L. Prognostic Significance of MET Amplification and Expression in Gastric Cancer: A Systematic Review with Meta-Analysis. PLoS ONE 2014, 9, e84502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toiyama, Y.; Yasuda, H.; Saigusa, S.; Matushita, K.; Fujikawa, H.; Tanaka, K.; Mohri, Y.; Inoue, Y.; Goel, A.; Kusunoki, M. Co-expression of hepatocyte growth factor and c-Met predicts peritoneal dissemination established by autocrine hepatocyte growth factor/c-Met signaling in gastric cancer. Int. J. Cancer 2012, 130, 2912–2921. [Google Scholar] [CrossRef]
- Park, D.J.; Yoon, C.; Thomas, N.; Ku, G.Y.; Janjigian, Y.Y.; Kelsen, D.P.; Ilson, D.H.; Goodman, K.A.; Tang, L.H.; Strong, V.E.; et al. Prognostic Significance of Targetable Angiogenic and Growth Factors in Patients Undergoing Resection for Gastric and Gastroesophageal Junction Cancers. Ann. Surg. Oncol. 2014, 21, 1130–1137. [Google Scholar] [CrossRef]
- Papaccio, F.; della Corte, C.M.; Viscardi, G.; di Liello, R.; Esposito, G.; Sparano, F.; Ciardiello, F.; Morgillo, F. HGF/MET and the Immune System: Relevance for Cancer Immunotherapy. Int. J. Mol. Sci. 2018, 19, 3595. [Google Scholar] [CrossRef] [Green Version]
- Ilangumaran, S.; Villalobos-Hernandez, A.; Bobbala, D.; Ramanathan, S. The hepatocyte growth factor (HGF)-MET receptor tyrosine kinase signaling pathway: Diverse roles in modulating immune cell functions. Cytokine 2016, 82, 125–139. [Google Scholar] [CrossRef]
- Benkhoucha, M.; Santiago-Raber, M.-L.; Schneiter, G.; Chofflon, M.; Funakoshi, H.; Nakamura, T.; Lalive, P.H. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2010, 107, 6424–6429. [Google Scholar] [CrossRef] [Green Version]
- Benkhoucha, M.; Molnarfi, N.; Dunand-Sauthier, I.; Merkler, D.; Schneiter, G.; Bruscoli, S.; Riccardi, C.; Tabata, Y.; Funakoshi, H.; Nakamura, T.; et al. Hepatocyte growth factor limits autoimmune neuroinflammation via glucocorticoid-induced leucine zipper expression in dendritic cells. J. Immunol. 2014, 193, 2743–2752. [Google Scholar] [CrossRef] [Green Version]
- Owusu, B.; Galemmo, R.; Janetka, J.; Klampfer, L. Hepatocyte Growth Factor, a Key Tumor-Promoting Factor in the Tumor Microenvironment. Cancers 2017, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Beppu, K.; Uchiyama, A.; Morisaki, T.; Matsumoto, K.; Nakamura, T.; Tanaka, M.; Katano, M. Hepatocyte growth factor production by peripheral blood mononuclear cells of recurrent cancer patients. Anticancer Res. 2001, 21, 2195–2200. [Google Scholar]
- Malka, D.; François, E.; Penault-Llorca, F.; Castan, F.; Bouché, O.; Bennouna, J.; Ghiringhelli, F.; de la Fouchardière, C.; Borg, C.; Samalin, E.; et al. FOLFOX alone or combined with rilotumumab or panitumumab as first-line treatment for patients with advanced gastroesophageal adenocarcinoma (PRODIGE 17-ACCORD 20-MEGA): A randomised, open-label, three-arm phase II trial. Eur. J. Cancer 2019, 115, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, H.; Kono, K.; Itakura, J.; Tang, R.F.; Takahashi, A.; An, F.Q.; Kamei, S.; Iizuka, H.; Fujii, H.; Matsumoto, Y. c-Met expression in gastric cancer with liver metastasis. Oncology 2002, 63, 286–296. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L. Blood Monocytes and Their Subsets: Established Features and Open Questions. Front. Immunol. 2015, 6, 423. [Google Scholar] [CrossRef] [PubMed]
- Pernot, S.; Voron, T.; Perkins, G.; Lagorce-Pages, C.; Berger, A.; Taieb, J. Signet-ring cell carcinoma of the stomach: Impact on prognosis and specific therapeutic challenge. World J. Gastroenterol. 2015, 21, 11428–11438. [Google Scholar] [CrossRef]
- Pernot, S.; Terme, M.; Radosevic-Robin, N.; Castan, F.; Badoual, C.; Marcheteau, E.; Penault-Llorca, F.; Bouche, O.; Bennouna, J.; Francois, E.; et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer 2020, 23, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Oku, M.; Okumi, M.; Shimizu, A.; Sahara, H.; Setoyama, K.; Nishimura, H.; Sada, M.; Scalea, J.; Ido, A.; Sachs, D.H.; et al. Hepatocyte growth factor sustains T regulatory cells and prolongs the survival of kidney allografts in major histocompatibility complex-inbred CLAWN-miniature swine. Transplantation 2012, 93, 148–155. [Google Scholar] [CrossRef]
- Molnarfi, N.; Benkhoucha, M.; Juillard, C.; Bjarnadóttir, K.; Lalive, P.H. The neurotrophic hepatocyte growth factor induces protolerogenic human dendritic cells. J. Neuroimmunol. 2014, 267, 105–110. [Google Scholar] [CrossRef]
- Rutella, S.; Bonanno, G.; Procoli, A.; Mariotti, A.; de Ritis, D.G.; Curti, A.; Danese, S.; Pessina, G.; Pandolfi, S.; Natoni, F.; et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 2006, 108, 218–227. [Google Scholar] [CrossRef]
- Molnarfi, N.; Benkhoucha, M.; Bjarnadóttir, K.; Juillard, C.; Lalive, P.H. Interferon–β Induces Hepatocyte Growth Factor in Monocytes of Multiple Sclerosis Patients. PLoS ONE 2012, 7, e49882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benkhoucha, M.; Senoner, I.; Lalive, P.H. C-Met is expressed by highly autoreactive encephalitogenic CD8+ cells. J. Neuroinflammation 2020, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Komarowska, I.; Coe, D.; Wang, G.; Haas, R.; Mauro, C.; Kishore, M.; Cooper, D.; Nadkarni, S.; Fu, H.; Steinbruchel, D.A.; et al. Hepatocyte Growth Factor Receptor c-Met Instructs T Cell Cardiotropism and Promotes T Cell Migration to the Heart via Autocrine Chemokine Release. Immunity 2015, 42, 1087–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galimi, F.; Cottone, E.; Vigna, E.; Arena, N.; Boccaccio, C.; Giordano, S.; Naldini, L.; Comoglio, P.M. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J. Immunol. 2001, 166, 1241–1247. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wu, Y.; Xie, X.-D.; Chu, Y.-F.; Li, J.-Q.; Zheng, L. c-Met identifies a population of matrix metalloproteinase 9-producing monocytes in peritumoural stroma of hepatocellular carcinoma. J. Pathol. 2015, 237, 319–329. [Google Scholar] [CrossRef]
- Moghul, A.; Lin, L.; Beedle, A.; Kanbour-Shakir, A.; DeFrances, M.C.; Liu, Y.; Zarnegar, R. Modulation of c-MET proto-oncogene (HGF receptor) mRNA abundance by cytokines and hormones: Evidence for rapid decay of the 8 kb c-MET transcript. Oncogene 1994, 9, 2045–2052. [Google Scholar]
- Boccaccio, C.; Gaudino, G.; Gambarotta, G.; Galimi, F.; Comoglio, P.M. Hepatocyte growth factor (HGF) receptor expression is inducible and is part of the delayed-early response to HGF. J. Biol. Chem. 1994, 269, 12846–12851. [Google Scholar] [CrossRef]
- Gayyed, M.F.; El-Maqsoud, N.M.R.A.; El-Heeny, A.A.E.H.; Mohammed, M.F. c-MET expression in colorectal adenomas and primary carcinomas with its corresponding metastases. J. Gastrointest. Oncol. 2015, 6, 618–627. [Google Scholar] [CrossRef]
- Chen, P.-M.; Liu, K.-J.; Hsu, P.-J.; Wei, C.-F.; Bai, C.-H.; Ho, L.-J.; Sytwu, H.-K.; Yen, B.L. Induction of immunomodulatory monocytes by human mesenchymal stem cell-derived hepatocyte growth factor through ERK1/2. J. Leukoc. Biol. 2014, 96, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Mougiakakos, D.; Choudhury, A.; Lladser, A.; Kiessling, R.; Johansson, C.C. Regulatory T cells in cancer. Adv. Cancer Res. 2010, 107, 57–117. [Google Scholar] [CrossRef]
- Kaga, T.; Kawano, H.; Sakaguchi, M.; Nakazawa, T.; Taniyama, Y.; Morishita, R. Hepatocyte growth factor stimulated angiogenesis without inflammation: Differential actions between hepatocyte growth factor, vascular endothelial growth factor and basic fibroblast growth factor. Vascul. Pharmacol. 2012, 57, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.W.; Su, Y.; Volpert, O.V.; Woude, G.F.V. Hepatocyte growth factor/scatter factor mediates angiogenesis through positive VEGF and negative thrombospondin 1 regulation. Proc. Natl. Acad. Sci. USA 2003, 100, 12718–12723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerritsen, M.E.; Tomlinson, J.E.; Zlot, C.; Ziman, M.; Hwang, S. Using gene expression profiling to identify the molecular basis of the synergistic actions of hepatocyte growth factor and vascular endothelial growth factor in human endothelial cells. Br. J. Pharmacol. 2003, 140, 595–610. [Google Scholar] [CrossRef] [Green Version]
- Kopetz, S.; Hoff, P.M.; Morris, J.S.; Wolff, R.A.; Eng, C.; Glover, K.Y.; Adinin, R.; Overman, M.J.; Valero, V.; Wen, S.; et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: Efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J. Clin. Oncol. 2010, 28, 453–459. [Google Scholar] [CrossRef]
- Patel, M.B.; Pothula, S.P.; Xu, Z.; Lee, A.K.; Goldstein, D.; Pirola, R.C.; Apte, M.V.; Wilson, J.S. The role of the hepatocyte growth factor/c-MET pathway in pancreatic stellate cell-endothelial cell interactions: Anti-angiogenic implications in pancreatic cancer. Carcinogenesis 2014, 35, 1891–1900. [Google Scholar] [CrossRef]
- Abounader, R.; Lal, B.; Luddy, C.; Koe, G.; Davidson, B.; Rosen, E.M.; Laterra, J. In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. FASEB J. 2002, 16, 108–110. [Google Scholar] [CrossRef]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef]
- Lapeyre-Prost, A.; Terme, M.; Pernot, S.; Pointet, A.-L.; Voron, T.; Tartour, E.; Taieb, J. Immunomodulatory Activity of VEGF in Cancer. Int. Rev. Cell Mol. Biol. 2017, 330, 295–342. [Google Scholar] [CrossRef]
- FYakes, M.; Chen, J.; Tan, J.; Yamaguchi, K.; Shi, Y.; Yu, P.; Qian, F.; Chu, F.; Bentzien, F.; Cancilla, B.; et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol. Cancer Ther. 2011, 10, 2298–2308. [Google Scholar] [CrossRef] [Green Version]
- Kwilas, A.R.; Ardiani, A.; Donahue, R.N.; Aftab, D.T.; Hodge, J.W. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J. Transl. Med. 2014, 12, 294. [Google Scholar] [CrossRef] [Green Version]
- Apolo, A.B.; Nadal, R.; Tomita, Y.; Davarpanah, N.N.; Cordes, L.M.; Steinberg, S.M.; Cao, L.; Parnes, H.L.; Costello, R.; Merino, M.J.; et al. Cabozantinib in patients with platinum-refractory metastatic urothelial carcinoma: An open-label, single-centre, phase 2 trial. Lancet Oncol. 2020, 21, 1099–1109. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Bourlon, M.T.; Zurawski, B.; Juárez, V.M.O.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; Suarez, C.; et al. 696O_PR Nivolumab + cabozantinib vs sunitinib in first-line treatment for advanced renal cell carcinoma: First results from the randomized phase III CheckMate 9ER trial. Ann. Oncol. 2020, 31, S1159. [Google Scholar] [CrossRef]
- Powles, T. Recent eUpdate to the ESMO Clinical Practice Guidelines on renal cell carcinoma on cabozantinib and nivolumab for first-line clear cell renal cancer: Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up 1. Ann. Oncol. 2021, 32, 422–423. [Google Scholar] [CrossRef]
| N = 80 | N (%) |
|---|---|
| Age (years) | |
| median | 65 |
| Sex | |
| male | 56 (70) |
| female | 24 (30) |
| Localization | |
| lower esophagus | 4 (5) |
| esogastric junction | 17 (21.5) |
| gastric body | 57 (71) |
| missing data | 2 (2.5) |
| Differentiation | |
| well | 8 (10) |
| moderate | 28 (35) |
| poor | 28 (35) |
| missing data | 16 (20) |
| Linitis | |
| yes | 14 (17.5) |
| no | 45 (56) |
| mixed | 11 (14) |
| missing data | 10 (12.5) |
| Disease status | |
| local/locally advanced | 28 (35) |
| metastatic | 52 (65) |
| Tumor burden | |
| yes | 70 (87.5) |
| no (post-surgery patients) | 10 (12.5) |
| HER2 status | |
| positive | 11 (13.75) |
| negative | 61 (76.25) |
| missing data | 8 (10) |
| N = 80 | N (%) |
|---|---|
| Age (years) | |
| median | 65 |
| Sex | |
| male | 56 (70) |
| female | 24 (30) |
| Localization | |
| lower esophagus | 4 (5) |
| esogastric junction | 17 (21.5) |
| gastric body | 57 (71) |
| missing data | 2 (2.5) |
| Differentiation | |
| well | 8 (10) |
| moderate | 28 (35) |
| poor | 28 (35) |
| missing data | 16 (20) |
| Linitis/Diffuse type (Lauren’s classification) | |
| yes | 14 (17.5) |
| no | 45 (56) |
| mixed | 11 (14) |
| missing data | 10 (12.5) |
| Disease status | |
| local/locally advanced | 28 (35) |
| metastatic | 52 (65) |
| Tumor burden | |
| yes | 70 (87.5) |
| no (post-surgery patients) | 10 (12.5) |
| HER2 status | |
| positive | 11 (13.75) |
| negative | 61 (76.25) |
| missing data | 8 (10) |
| chemotherapy received by metastatic patients | |
| FOLFOX | 25 (48) |
| FOLFIRI | 6 (11.5) |
| FOLFOX HERCEPTIN | 3 (5.8) |
| OTHER | 8 (15.4) |
| NONE | 7 (13.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palle, J.; Hirsch, L.; Lapeyre-Prost, A.; Malka, D.; Bourhis, M.; Pernot, S.; Marcheteau, E.; Voron, T.; Castan, F.; Lacotte, A.; et al. Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients. Cancers 2021, 13, 5562. https://doi.org/10.3390/cancers13215562
Palle J, Hirsch L, Lapeyre-Prost A, Malka D, Bourhis M, Pernot S, Marcheteau E, Voron T, Castan F, Lacotte A, et al. Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients. Cancers. 2021; 13(21):5562. https://doi.org/10.3390/cancers13215562
Chicago/Turabian StylePalle, Juliette, Laure Hirsch, Alexandra Lapeyre-Prost, David Malka, Morgane Bourhis, Simon Pernot, Elie Marcheteau, Thibault Voron, Florence Castan, Ariane Lacotte, and et al. 2021. "Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients" Cancers 13, no. 21: 5562. https://doi.org/10.3390/cancers13215562
APA StylePalle, J., Hirsch, L., Lapeyre-Prost, A., Malka, D., Bourhis, M., Pernot, S., Marcheteau, E., Voron, T., Castan, F., Lacotte, A., Benhamouda, N., Tanchot, C., François, E., Ghiringhelli, F., de la Fouchardière, C., Zaanan, A., Tartour, E., Taieb, J., & Terme, M. (2021). Targeting HGF/c-Met Axis Decreases Circulating Regulatory T Cells Accumulation in Gastric Cancer Patients. Cancers, 13(21), 5562. https://doi.org/10.3390/cancers13215562