Highly Expressed Progesterone Receptor B Isoform Increases Platinum Sensitivity and Survival of Ovarian High-Grade Serous Carcinoma
Abstract
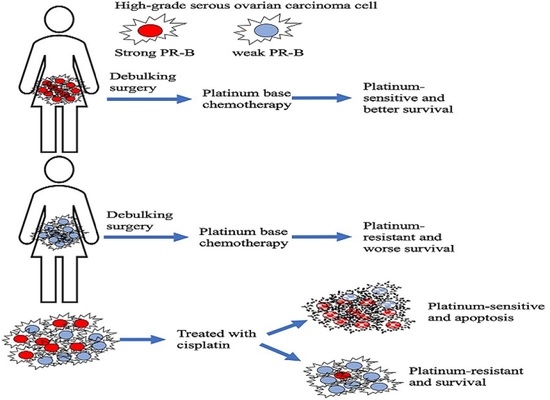
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Patients Enrolled
2.2. Immunohistochemical Staining Analysis and Scoring for PR
2.3. Cell Culture and Generation of Cisplatin-Resistant Ovarian Cancer Cell Lines
2.4. Transfection of Ovarian Cancer Cell Line
2.5. Western Blotting
2.6. Quantitative Real-Time Polymerase Chain Reaction
2.7. Cellular Toxicity via CCK-8 Assay
2.8. Apoptosis Assay
2.9. Statistical Analysis
3. Results
3.1. Clinical Data
3.2. Western Blot Analysis and Quantitative Real-Time Polymerase Chain Reaction on Ovarian HGSC Cells with Different PR-B Expression
3.3. PR-B Expression Promotes Cytotoxic Effects of Cisplatin on Ovarian HGSC Cell
3.4. Effects of Progesterone Treatment on Acquired Cisplatin-Resistant Ovarian HGSC Cells
3.5. Effects of Progesterone Treatment on Ovarian HGSC Cell Survival with Different PR-B Expression
3.6. Apoptosis of Ovarian HGSC Cells during Treatment of Cisplatin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.Y.; Kim, S.; Kim, Y.T.; Lim, M.C.; Lee, B.; Jung, K.W.; Kim, J.W.; Park, S.Y.; Won, Y.J. Changes in ovarian cancer survival during the 20 years before the era of targeted therapy. BMC Cancer 2018, 18, 601. [Google Scholar] [CrossRef]
- Žilovič, D.; Čiurlienė, R.; Sabaliauskaitė, R.; Jarmalaitė, S. Future Screening Prospects for Ovarian Cancer. Cancers 2021, 13, 3840. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, E.; Armengol-Alonso, A.; Muñoz, M.; Seguí-Palmer, M. Current status of hormone therapy in patients with hormone receptor positive (HR+) advanced breast cancer. Breast 2014, 23, 710–720. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.; Barrientos Santillan, N.; Westin, S.N.; Ramirez, P.T. Updates on Conservative Management of Endometrial Cancer. J. Minim. Invasive Gynecol. 2018, 25, 308–313. [Google Scholar] [CrossRef]
- Hurteau, J.A.; Brady, M.F.; Darcy, K.M.; McGuire, W.P.; Edmonds, P.; Pearl, M.L.; Ivanov, I.; Tewari, K.S.; Mannel, R.S.; Zanotti, K.; et al. Randomized phase III trial of tamoxifen versus thalidomide in women with biochemical-recurrent-only epithelial ovarian, fallopian tube or primary peritoneal carcinoma after a complete response to first-line platinum/taxane chemotherapy with an evaluation of serum vascular endothelial growth factor (VEGF): A Gynecologic Oncology Group Study. Gynecol. Oncol. 2010, 119, 444–450. [Google Scholar]
- Kok, P.S.; Beale, P.; O’Connell, R.L.; Grant, P.; Bonaventura, T.; Scurry, J.; Antill, Y.; Goh, J.; Sjoquist, K.; DeFazio, A.; et al. PARAGON (ANZGOG-0903): A phase 2 study of anastrozole in asymptomatic patients with estrogen and progesterone receptor-positive recurrent ovarian cancer and CA125 progression. J. Gynecol. Oncol. 2019, 30, e86. [Google Scholar] [CrossRef]
- Langdon, S.P.; Crew, A.J.; Ritchie, A.A.; Muir, M.; Wakeling, A.; Smyth, J.F.; Miller, W.R. Growth inhibition of oestrogen receptor-positive human ovarian carcinoma by anti-oestrogens in vitro and in a xenograft model. Eur. J. Cancer 1994, 30A, 682–686. [Google Scholar] [CrossRef]
- Shirey, D.R.; Kavanagh, J.J., Jr.; Gershenson, D.M.; Freedman, R.S.; Copeland, L.J.; Jones, L.A. Tamoxifen therapy of epithelial ovarian cancer. Obstet. Gynecol. 1985, 66, 575–578. [Google Scholar]
- Papadimitriou, C.A.; Markaki, S.; Siapkaras, J.; Vlachos, G.; Efstathiou, E.; Grimani, I.; Hamilos, G.; Zorzou, M.; Dimopoulos, M.A. Hormonal therapy with letrozole for relapsed epithelial ovarian cancer. Long-term results of a phase II study. Oncology 2004, 66, 112–117. [Google Scholar] [CrossRef]
- Gwinn, M.L.; Lee, N.C.; Rhodes, P.H.; Layde, P.M.; Rubin, G.L. Pregnancy, breast feeding, and oral contraceptives and the risk of epithelial ovarian cancer. J. Clin. Epidemiol. 1990, 43, 559–568. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Li, S.; Zhao, M.; Sheng, B.; Zhu, H.; Zhu, X. Prognostic value of progesterone receptor expression in ovarian cancer: A meta-analysis. Oncotarget 2017, 8, 36845–36856. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, G.C.; Turbov, J.; Rosales, R.; Yoo, J.; Hunn, J.; Zappia, K.J.; Lund, K.; Barry, C.P.; Rodriguez, I.V.; Pike, J.W.; et al. Progestins inhibit calcitriol-induced CYP24A1 and synergistically inhibit ovarian cancer cell viability: An opportunity for chemoprevention. Gynecol. Oncol. 2016, 143, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peres, L.C.; Cushing-Haugen, K.L.; Köbel, M.; Harris, H.R.; Berchuck, A.; Rossing, M.A.; Schildkraut, J.M.; Doherty, J.A. Invasive Epithelial Ovarian Cancer Survival by Histotype and Disease Stage. J. Natl. Cancer Inst. 2019, 111, 60–68. [Google Scholar] [CrossRef]
- Sieh, W.; Köbel, M.; Longacre, T.A.; Bowtell, D.D.; deFazio, A.; Goodman, M.T.; Høgdall, E.; Deen, S.; Wentzensen, N.; Moysich, K.B.; et al. Hormone-receptor expression and ovarian cancer survival: An Ovarian Tumor Tissue Analysis consortium study. Lancet Oncol. 2013, 14, 853–862. [Google Scholar] [CrossRef] [Green Version]
- Le Page, C.; Rahimi, K.; Mes-Masson, A.M.; Köbel, M. Prognostic value of progesterone receptor expression in tubo-ovarian high-grade serous carcinoma of the COEUR cohort. Histopathology 2019, 74, 663–666. [Google Scholar] [CrossRef]
- Feng, Z.; Wen, H.; Bi, R.; Ju, X.; Chen, X.; Yang, W.; Wu, X. A clinically applicable molecular classification for high-grade serous ovarian cancer based on hormone receptor expression. Sci. Rep. 2016, 6, 25408. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Dai, X.; Gao, Y.; Shen, F.; Ding, J.; Chen, Q. The positivity of estrogen receptor and progesterone receptor may not be associated with metastasis and recurrence in epithelial ovarian cancer. Sci. Rep. 2017, 7, 16922. [Google Scholar] [CrossRef] [Green Version]
- Rojas, P.A.; May, M.; Sequeira, G.R.; Elia, A.; Alvarez, M.; Martínez, P.; Gonzalez, P.; Hewitt, S.; He, X.; Perou, C.M.; et al. Progesterone Receptor Isoform Ratio: A Breast Cancer Prognostic and Predictive Factor for Antiprogestin Responsiveness. J. Natl. Cancer Inst. 2017, 109, djw317. [Google Scholar] [CrossRef] [Green Version]
- Miyamoto, T.; Watanabe, J.; Hata, H.; Jobo, T.; Kawaguchi, M.; Hattori, M.; Saito, M.; Kuramoto, H. Significance of progesterone receptor-A and -B expressions in endometrial adenocarcinoma. J. Steroid Biochem. Mol. Biol. 2004, 92, 111–118. [Google Scholar] [CrossRef]
- Mutch, D.G.; Prat, J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol. Oncol. 2014, 133, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Markman, M.; Rothman, R.; Hakes, T.; Reichman, B.; Hoskins, W.; Rubin, S.; Jones, W.; Almadrones, L.; Lewis, J.L., Jr. Second-line platinum therapy in patients with ovarian cancer previously treated with cisplatin. J. Clin. Oncol. 1991, 9, 389–393. [Google Scholar] [CrossRef]
- Van Kruchten, M.; de Vries, E.F.; Arts, H.J.; Jager, N.M.; Bongaerts, A.H.; Glaudemans, A.W.; Hollema, H.; de Vries, E.G.; Hospers, G.A.; Reyners, A.K. Assessment of estrogen receptor expression in epithelial ovarian cancer patients using 16α-18F-fluoro-17β-estradiol PET/CT. J. Nucl. Med. 2015, 56, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Su, S.; Blackwelder, A.J.; Grossman, G.; Minges, J.T.; Yuan, L.; Young, S.L.; Wilson, E.M. Primate-specific melanoma antigen-A11 regulates isoform-specific human progesterone receptor-B transactivation. J. Biol. Chem. 2012, 287, 34809–34824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruopp, M.D.; Perkins, N.J.; Whitcomb, B.W.; Schisterman, E.F. Youden Index and optimal cut-point estimated from observations affected by a lower limit of detection. Biom. J. 2008, 50, 419–430. [Google Scholar] [CrossRef] [Green Version]
- Tkalia, I.G.; Vorobyova, L.I.; Svintsitsky, V.S.; Nespryadko, S.V.; Goncharuk, I.V.; Lukyanova, N.Y.; Chekhun, V.F. Clinical significance of hormonal receptor status of malignant ovarian tumors. Exp. Oncol. 2014, 36, 125–133. [Google Scholar] [PubMed]
- Matsuo, K.; Sheridan, T.B.; Mabuchi, S.; Yoshino, K.; Hasegawa, K.; Studeman, K.D.; Im, D.D.; Rosenshein, N.B.; Roman, L.D.; Sood, A.K. Estrogen receptor expression and increased risk of lymphovascular space invasion in high-grade serous ovarian carcinoma. Gynecol. Oncol. 2014, 133, 473–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peluso, J.J.; Gawkowska, A.; Liu, X.; Shioda, T.; Pru, J.K. Progesterone receptor membrane component-1 regulates the development and Cisplatin sensitivity of human ovarian tumors in athymic nude mice. Endocrinology 2009, 150, 4846–4854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.; Syed, V. Progesterone inhibits growth and induces apoptosis in cancer cells through modulation of reactive oxygen species. Gynecol. Endocrinol. 2011, 27, 830–836. [Google Scholar] [CrossRef]
- Pedernera, E.; Gómora, M.J.; Morales-Vásquez, F.; Pérez-Montiel, D.; Mendez, C. Progesterone reduces cell survival in primary cultures of endometrioid ovarian cancer. J. Ovarian Res. 2019, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Syed, V.; Mukherjee, K.; Godoy-Tundidor, S.; Ho, S.M. Progesterone induces apoptosis in TRAIL-resistant ovarian cancer cells by circumventing c-FLIPL overexpression. J. Cell Biochem. 2007, 102, 442–452. [Google Scholar] [CrossRef]
- Syed, V.; Ho, S.M. Progesterone-induced apoptosis in immortalized normal and malignant human ovarian surface epithelial cells involves enhanced expression of FasL. Oncogene 2003, 22, 6883–6890. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Han, Y.; Fang, Z.; Wu, W.; Ji, M.; Teng, F.; Zhu, W.; Yang, X.; Jia, X.; Zhang, C. Progesterone protects ovarian cancer cells from cisplatin-induced inhibitory effects through progesterone receptor membrane component 1/2 as well as AKT signaling. Oncol. Rep. 2013, 30, 2488–2494. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Feng, Y. Effect of progesterone combined with chemotherapy on epithelial ovarian cancer. Chin. Med. J. 2003, 116, 388–391. [Google Scholar] [PubMed]
- Kowalik, M.K.; Rekawiecki, R.; Kotwica, J. The putative roles of nuclear and membrane-bound progesterone receptors in the female reproductive tract. Reprod. Biol. 2013, 13, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Akahira, J.; Inoue, T.; Suzuki, T.; Ito, K.; Konno, R.; Sato, S.; Moriya, T.; Okamura, K.; Yajima, A.; Sasano, H. Progesterone receptor isoforms A and B in human epithelial ovarian carcinoma: Immunohistochemical and RT-PCR studies. Br. J. Cancer 2000, 83, 1488–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Parameter | Platinum Sensitive (n = 49) | Platinum Resistant (n = 41) | p-Value |
|---|---|---|---|
| Age (years), mean (SD) | 55.6 (10.4) | 56.4 (11.2) | 0.731 |
| CA-125 (U/mL), mean (SD) | 1708.3 (2678.9) | 2349.4 (3357.4) | 0.093 |
| CEA (ng/mL), mean (SD) | 2.5 (4.3) | 1.6 (1.3) | 0.271 |
| FIGO stage | |||
| I | 7 (14.3%) | 0 (0%) | |
| II | 6 (12.2%) | 3 (7.3%) | |
| III | 32 (65.3%) | 28 (68.3%) | |
| IV | 4 (8.2%) | 10 (24.4%) | 0.017 |
| Optimal debulking | 36 (73.5%) | 12 (29.3%) | <0.001 |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Variable | OR | 95% C.I. | p Value | OR | 95% C.I. | p Value |
| Age ≥ 60 | 1.31 | 0.54–3.15 | 0.550 | |||
| Menopause | 1.48 | 0.62–3.55 | 0.373 | |||
| Stage (III, IV) | 4.57 | 1.20–17.4 | 0.026 | 2.83 | 0.66–12.2 | 0.163 |
| Sub-optimal debulking | 6.69 | 2.66–16.9 | <0.001 | 5.56 | 2.09–14.8 | 0.001 |
| CA125 ≥ 500 U/mL | 2.27 | 0.97–5.29 | 0.059 | |||
| PR-B H-score < 12.5 | 4.02 | 1.43–11.3 | 0.008 | 3.69 | 1.18–11.6 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.; Lan, K.-C.; Ou, Y.-C.; Wu, C.-H.; Kang, H.-Y.; Chuang, I.-C.; Fu, H.-C. Highly Expressed Progesterone Receptor B Isoform Increases Platinum Sensitivity and Survival of Ovarian High-Grade Serous Carcinoma. Cancers 2021, 13, 5578. https://doi.org/10.3390/cancers13215578
Lin H, Lan K-C, Ou Y-C, Wu C-H, Kang H-Y, Chuang I-C, Fu H-C. Highly Expressed Progesterone Receptor B Isoform Increases Platinum Sensitivity and Survival of Ovarian High-Grade Serous Carcinoma. Cancers. 2021; 13(21):5578. https://doi.org/10.3390/cancers13215578
Chicago/Turabian StyleLin, Hao, Kuo-Chung Lan, Yu-Che Ou, Chen-Hsuan Wu, Hong-Yo Kang, I-Chieh Chuang, and Hung-Chun Fu. 2021. "Highly Expressed Progesterone Receptor B Isoform Increases Platinum Sensitivity and Survival of Ovarian High-Grade Serous Carcinoma" Cancers 13, no. 21: 5578. https://doi.org/10.3390/cancers13215578