Influence of TP53 Mutation on Survival of Diffuse Large B-Cell Lymphoma in the CAR T-Cell Era
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Patients
3.2. Pathohistological Characteristics of the CAR T Cell Cohort
3.3. Pathohistological Characteristics of the Control Group
3.4. TP53 Mutations
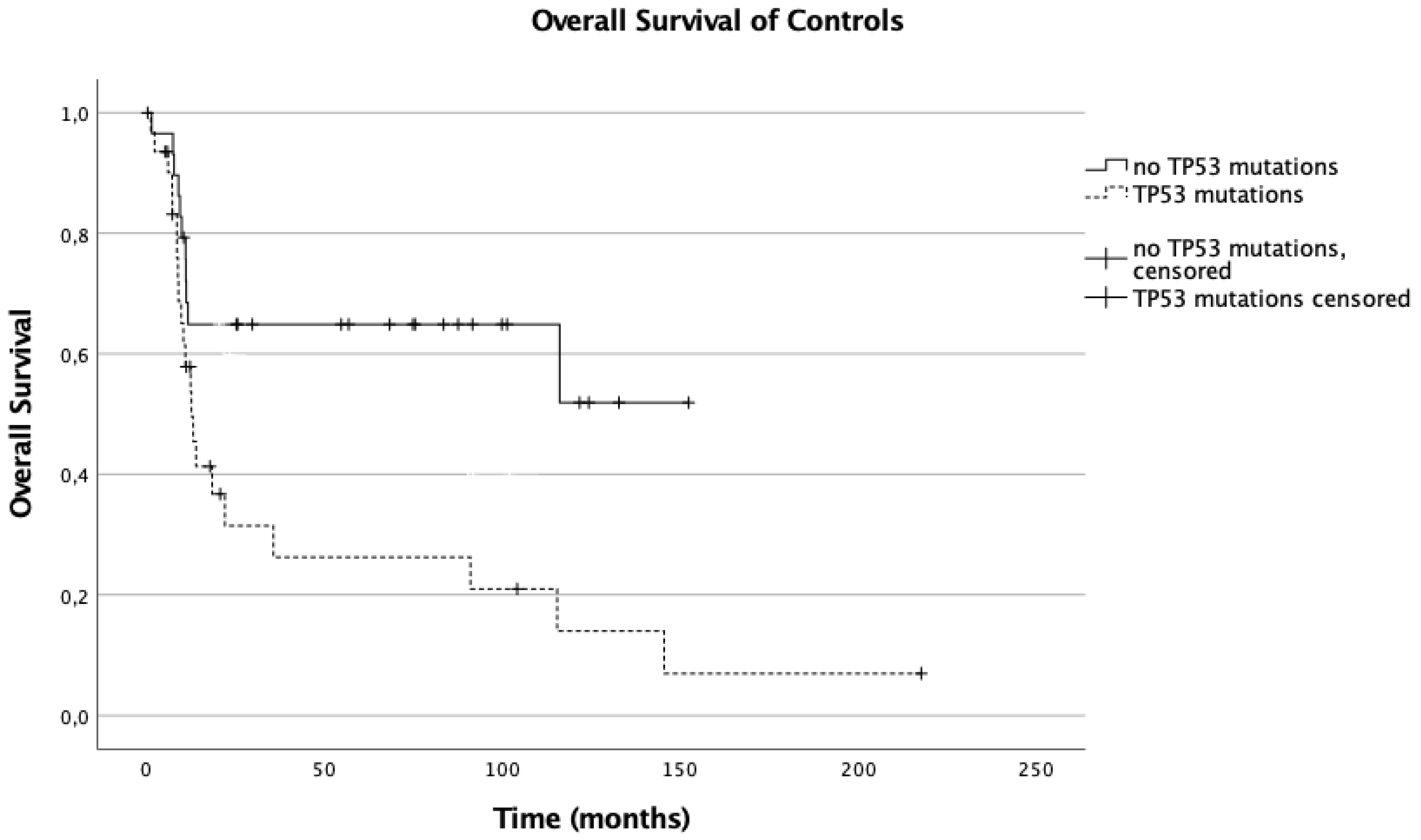
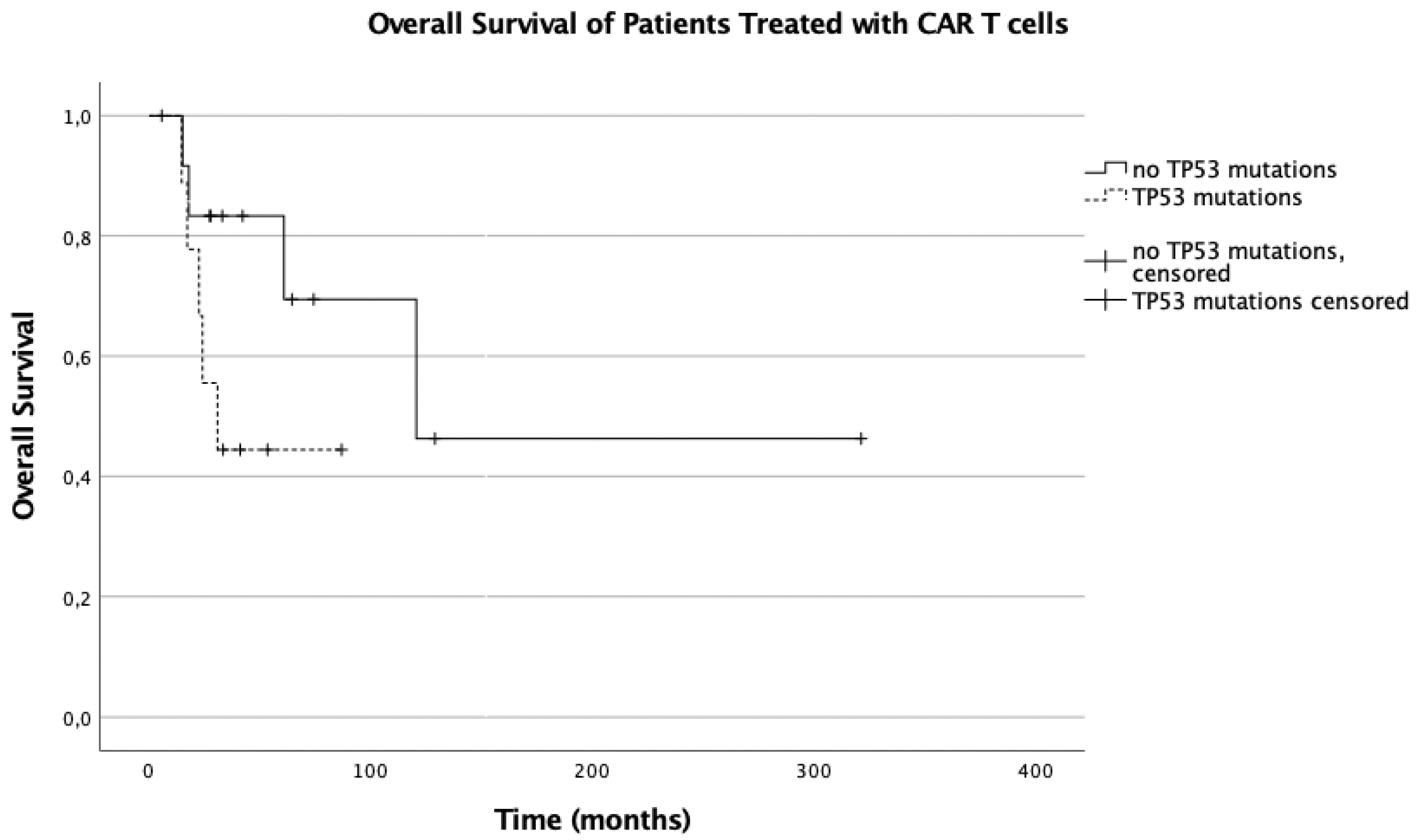
3.5. Influence of TP53 Mutations on Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schmitz, R.; Wright, G.W.; Huang, D.W.; Johnson, C.A.; Phelan, J.D.; Wang, J.Q.; Roulland, S.; Kasbekar, M.; Young, R.M.; Shaffer, A.L.; et al. Genetics and Pathogenesis of Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1396–1407. [Google Scholar] [CrossRef]
- Miao, Y.; Medeiros, L.J.; Li, Y.; Li, J.; Young, K.H. Genetic alterations and their clinical implications in DLBCL. Nat. Rev. Clin. Oncol. 2019, 16, 634–652. [Google Scholar] [CrossRef]
- Reddy, A.; Zhang, J.; Davis, N.S.; Moffitt, A.B.; Love, C.L.; Waldrop, A.; Leppa, S.; Pasanen, A.; Meriranta, L.; Karjalainen-Lindsberg, M.L.; et al. Genetic and Functional Drivers of Diffuse Large B Cell Lymphoma. Cell 2017, 171, 481–494.e415. [Google Scholar] [CrossRef] [Green Version]
- Chapuy, B.; Stewart, C.; Dunford, A.J.; Kim, J.; Kamburov, A.; Redd, R.A.; Lawrence, M.S.; Roemer, M.G.M.; Li, A.J.; Ziepert, M.; et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat. Med. 2018, 24, 679–690. [Google Scholar] [CrossRef]
- Bernard, E.; Nannya, Y.; Hasserjian, R.P.; Devlin, S.M.; Tuechler, H.; Medina-Martinez, J.S.; Yoshizato, T.; Shiozawa, Y.; Saiki, R.; Malcovati, L.; et al. Implications of TP53 allelic state for genome stability, clinical presentation and outcomes in myelodysplastic syndromes. Nat. Med. 2020, 26, 1549–1556. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Chen, S.; Yang, X.; Lei, Y.Y.; Xu, X.Y.; Liu, Y.X.; Guo, Y.H.; Pan, Y.; Wang, X.H.; Zhang, H.L.; et al. [Prognostic evaluation of P53 and BCL2 proteins in MYC/BCL2 double expression DLBCL]. Zhonghua Xue Ye Xue Za Zhi 2019, 40, 589–593. [Google Scholar] [CrossRef] [PubMed]
- Pascual, M.; Mena-Varas, M.; Robles, E.F.; Garcia-Barchino, M.J.; Panizo, C.; Hervas-Stubbs, S.; Alignani, D.; Sagardoy, A.; Martinez-Ferrandis, J.I.; Bunting, K.L.; et al. PD-1/PD-L1 immune checkpoint and p53 loss facilitate tumor progression in activated B-cell diffuse large B-cell lymphomas. Blood 2019, 133, 2401–2412. [Google Scholar] [CrossRef] [PubMed]
- Schiefer, A.I.; Kornauth, C.; Simonitsch-Klupp, I.; Skrabs, C.; Masel, E.K.; Streubel, B.; Vanura, K.; Walter, K.; Migschitz, B.; Stoiber, D.; et al. Impact of Single or Combined Genomic Alterations of TP53, MYC, and BCL2 on Survival of Patients With Diffuse Large B-Cell Lymphomas: A Retrospective Cohort Study. Medicine 2015, 94, e2388. [Google Scholar] [CrossRef] [PubMed]
- Xu-Monette, Z.Y.; Wu, L.; Visco, C.; Tai, Y.C.; Tzankov, A.; Liu, W.M.; Montes-Moreno, S.; Dybkaer, K.; Chiu, A.; Orazi, A.; et al. Mutational profile and prognostic significance of TP53 in diffuse large B-cell lymphoma patients treated with R-CHOP: Report from an International DLBCL Rituximab-CHOP Consortium Program Study. Blood 2012, 120, 3986–3996. [Google Scholar] [CrossRef]
- Morin, R.D.; Assouline, S.; Alcaide, M.; Mohajeri, A.; Johnston, R.L.; Chong, L.; Grewal, J.; Yu, S.; Fornika, D.; Bushell, K.; et al. Genetic Landscapes of Relapsed and Refractory Diffuse Large B-Cell Lymphomas. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2016, 22, 2290–2300. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Sadée, W. Membrane transporters and channels in chemoresistance and -sensitivity of tumor cells. Cancer Lett. 2006, 239, 168–182. [Google Scholar] [CrossRef]
- Marin, J.J.; Romero, M.R.; Martinez-Becerra, P.; Herraez, E.; Briz, O. Overview of the molecular bases of resistance to chemotherapy in liver and gastrointestinal tumours. Curr. Mol. Med. 2009, 9, 1108–1129. [Google Scholar] [CrossRef]
- Hientz, K.; Mohr, A.; Bhakta-Guha, D.; Efferth, T. The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 2017, 8, 8921–8946. [Google Scholar] [CrossRef] [Green Version]
- Intlekofer, A.M.; Joffe, E.; Batlevi, C.L.; Hilden, P.; He, J.; Seshan, V.E.; Zelenetz, A.D.; Palomba, M.L.; Moskowitz, C.H.; Portlock, C.; et al. Integrated DNA/RNA targeted genomic profiling of diffuse large B-cell lymphoma using a clinical assay. Blood Cancer J. 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Preudhomme, C.; Fenaux, P. The clinical significance of mutations of the P53 tumour suppressor gene in haematological malignancies. Br. J. Haematol. 1997, 98, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Simonitsch-Klupp, I.; Hauser, I.; Ott, G.; Drach, J.; Ackermann, J.; Kaufmann, J.; Weltermann, A.; Greinix, H.T.; Skrabs, C.; Dittrich, C.; et al. Diffuse large B-cell lymphomas with plasmablastic/plasmacytoid features are associated with TP53 deletions and poor clinical outcome. Leukemia 2004, 18, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zenz, T.; Kreuz, M.; Fuge, M.; Klapper, W.; Horn, H.; Staiger, A.M.; Winter, D.; Helfrich, H.; Huellein, J.; Hansmann, M.L.; et al. TP53 mutation and survival in aggressive B cell lymphoma. Int. J. Cancer 2017, 141, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sermer, D.; Batlevi, C.; Palomba, M.L.; Shah, G.; Lin, R.J.; Perales, M.A.; Scordo, M.; Dahi, P.; Pennisi, M.; Afuye, A.; et al. Outcomes in patients with DLBCL treated with commercial CAR T cells compared with alternate therapies. Blood Adv. 2020, 4, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Sermer, D.; Brentjens, R. CAR T-cell therapy: Full speed ahead. Hematol. Oncol. 2019, 37, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1-2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Hans, C.P.; Weisenburger, D.D.; Greiner, T.C.; Gascoyne, R.D.; Delabie, J.; Ott, G.; Müller-Hermelink, H.K.; Campo, E.; Braziel, R.M.; Jaffe, E.S.; et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood 2004, 103, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, T.M.; Young, K.H.; Visco, C.; Xu-Monette, Z.Y.; Orazi, A.; Go, R.S.; Nielsen, O.; Gadeberg, O.V.; Mourits-Andersen, T.; Frederiksen, M.; et al. Immunohistochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 3460–3467. [Google Scholar] [CrossRef] [PubMed]
- Bouaoun, L.; Sonkin, D.; Ardin, M.; Hollstein, M.; Byrnes, G.; Zavadil, J.; Olivier, M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum. Mutat. 2016, 37, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Aas, T.; Børresen, A.L.; Geisler, S.; Smith-Sørensen, B.; Johnsen, H.; Varhaug, J.E.; Akslen, L.A.; Lønning, P.E. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nat. Med. 1996, 2, 811–814. [Google Scholar] [CrossRef] [PubMed]
- Knappskog, S.; Chrisanthar, R.; Løkkevik, E.; Anker, G.; Østenstad, B.; Lundgren, S.; Risberg, T.; Mjaaland, I.; Leirvaag, B.; Miletic, H.; et al. Low expression levels of ATM may substitute for CHEK2/TP53 mutations predicting resistance towards anthracycline and mitomycin chemotherapy in breast cancer. Breast Cancer Res. BCR 2012, 14, R47. [Google Scholar] [CrossRef] [Green Version]
- Geisler, S.; Lønning, P.E.; Aas, T.; Johnsen, H.; Fluge, O.; Haugen, D.F.; Lillehaug, J.R.; Akslen, L.A.; Børresen-Dale, A.L. Influence of TP53 gene alterations and c-erbB-2 expression on the response to treatment with doxorubicin in locally advanced breast cancer. Cancer Res. 2001, 61, 2505–2512. [Google Scholar]
- Varna, M.; Bousquet, G.; Plassa, L.F.; Bertheau, P.; Janin, A. TP53 status and response to treatment in breast cancers. J. Biomed. Biotechnol. 2011, 2011, 284584. [Google Scholar] [CrossRef] [Green Version]
- Bergamaschi, D.; Gasco, M.; Hiller, L.; Sullivan, A.; Syed, N.; Trigiante, G.; Yulug, I.; Merlano, M.; Numico, G.; Comino, A.; et al. p53 polymorphism influences response in cancer chemotherapy via modulation of p73-dependent apoptosis. Cancer Cell 2003, 3, 387–402. [Google Scholar] [CrossRef] [Green Version]
- Minotti, G.; Menna, P.; Salvatorelli, E.; Cairo, G.; Gianni, L. Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacol. Rev. 2004, 56, 185–229. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Howell, S.B. DNA mismatch repair and p53 function are major determinants of the rate of development of cisplatin resistance. Mol. Cancer Ther. 2006, 5, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Gadducci, A.; Cosio, S.; Muraca, S.; Genazzani, A.R. Molecular mechanisms of apoptosis and chemosensitivity to platinum and paclitaxel in ovarian cancer: Biological data and clinical implications. Eur. J. Gynaecol. Oncol. 2002, 23, 390–396. [Google Scholar] [PubMed]
- Rusch, V.; Klimstra, D.; Venkatraman, E.; Oliver, J.; Martini, N.; Gralla, R.; Kris, M.; Dmitrovsky, E. Aberrant p53 expression predicts clinical resistance to cisplatin-based chemotherapy in locally advanced non-small cell lung cancer. Cancer Res. 1995, 55, 5038–5042. [Google Scholar] [PubMed]
- Tung, M.C.; Lin, P.L.; Wang, Y.C.; He, T.Y.; Lee, M.C.; Yeh, S.D.; Chen, C.Y.; Lee, H. Mutant p53 confers chemoresistance in non-small cell lung cancer by upregulating Nrf2. Oncotarget 2015, 6, 41692–41705. [Google Scholar] [CrossRef] [PubMed]
- Dhayat, S.A.; Mardin, W.A.; Seggewiß, J.; Ströse, A.J.; Matuszcak, C.; Hummel, R.; Senninger, N.; Mees, S.T.; Haier, J. MicroRNA Profiling Implies New Markers of Gemcitabine Chemoresistance in Mutant p53 Pancreatic Ductal Adenocarcinoma. PLoS ONE 2015, 10, e0143755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiorini, C.; Cordani, M.; Padroni, C.; Blandino, G.; Di Agostino, S.; Donadelli, M. Mutant p53 stimulates chemoresistance of pancreatic adenocarcinoma cells to gemcitabine. Biochim. Biophys. Acta 2015, 1853, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Galmarini, C.M.; Clarke, M.L.; Falette, N.; Puisieux, A.; Mackey, J.R.; Dumontet, C. Expression of a non-functional p53 affects the sensitivity of cancer cells to gemcitabine. Int. J. Cancer 2002, 97, 439–445. [Google Scholar] [CrossRef] [Green Version]
- Sarkozy, C.; Sehn, L.H. Management of relapsed/refractory DLBCL. Best Pract. Res. Clin. Haematol. 2018, 31, 209–216. [Google Scholar] [CrossRef]
- Gisselbrecht, C.; Van Den Neste, E. How I manage patients with relapsed/refractory diffuse large B cell lymphoma. Br. J. Haematol. 2018, 182, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Avanzi, M.P.; Yeku, O.; Li, X.; Wijewarnasuriya, D.P.; van Leeuwen, D.G.; Cheung, K.; Park, H.; Purdon, T.J.; Daniyan, A.F.; Spitzer, M.H.; et al. Engineered Tumor-Targeted T Cells Mediate Enhanced Anti-Tumor Efficacy Both Directly and through Activation of the Endogenous Immune System. Cell Rep. 2018, 23, 2130–2141. [Google Scholar] [CrossRef]
- Yeku, O.O.; Purdon, T.J.; Koneru, M.; Spriggs, D.; Brentjens, R.J. Armored CAR T cells enhance antitumor efficacy and overcome the tumor microenvironment. Sci. Rep. 2017, 7, 10541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kochenderfer, J.N.; Somerville, R.P.T.; Lu, T.; Shi, V.; Bot, A.; Rossi, J.; Xue, A.; Goff, S.L.; Yang, J.C.; Sherry, R.M.; et al. Lymphoma Remissions Caused by Anti-CD19 Chimeric Antigen Receptor T Cells Are Associated With High Serum Interleukin-15 Levels. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2017, 35, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, X.A.; Yang, J.; Zhang, G.; Li, J.; Song, L.; Su, Y.; Shi, Y.; Zhang, M.; He, J.; et al. Efficacy and safety of anti-CD19 CAR T-cell therapy in 110 patients with B-cell acute lymphoblastic leukemia with high-risk features. Blood Adv. 2020, 4, 2325–2338. [Google Scholar] [CrossRef]
- Pan, J.; Tan, Y.; Deng, B.; Tong, C.; Hua, L.; Ling, Z.; Song, W.; Xu, J.; Duan, J.; Wang, Z.; et al. Frequent occurrence of CD19-negative relapse after CD19 CAR T and consolidation therapy in 14 TP53-mutated r/r B-ALL children. Leukemia 2020, 34, 3382–3387. [Google Scholar] [CrossRef]
- Pan, J.; Niu, Q.; Deng, B.; Liu, S.; Wu, T.; Gao, Z.; Liu, Z.; Zhang, Y.; Qu, X.; Zhang, Y.; et al. CD22 CAR T-cell therapy in refractory or relapsed B acute lymphoblastic leukemia. Leukemia 2019, 33, 2854–2866. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Yang, J.F.; Deng, B.P.; Zhao, X.J.; Zhang, X.; Lin, Y.H.; Wu, Y.N.; Deng, Z.L.; Zhang, Y.L.; Liu, S.H.; et al. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia 2017, 31, 2587–2593. [Google Scholar] [CrossRef]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef]
| Characteristics | Whole Cohort | CAR T cells | Controls | ||||
|---|---|---|---|---|---|---|---|
| No | % | No | % | No | % | p-Value | |
| 170 | 100 | 29 | 17.1 | 141 | 82.9 | ||
| Gender | |||||||
| Female | 72 | 42.4 | 13 | 44.8 | 59 | 41.8 | 0.838 |
| Male | 98 | 57.6 | 16 | 55.2 | 82 | 58.2 | 0.838 |
| Median age at diagnosis | 56.99 | (19–95) | 54.83 | (31–79) | 59.76 | (19–95) | 0.123 |
| Stage = III–IV | 107 | 62.9 | 10 | 34.5 | 97 | 68.8 | <0.001 |
| Extranodal | 109 | 64.1 | 10 | 34.5 | 99 | 70.2 | <0.001 |
| Transformed | 21 | 12.4 | 5 | 17.2 | 16 | 11.3 | 0.553 |
| COO | |||||||
| Non-GCB | 63 | 36.8 | 16 | 55.2 | 47 | 33.3 | 0.035 |
| GCB | 93 | 55 | 11 | 37.9 | 82 | 58.2 | 0.064 |
| NA | 14 | 8.2 | 2 | 6.9 | 12 | 8.5 | 0.999 |
| DEL | 71 | 41.8 | 19 | 65.5 | 52 | 36.9 | 0.003 |
| TP53+ | 41 | 24.1 | 10 | 34.5 | 31 | 22.0 | 0.804 |
| MYC+ | 72 | 42.4 | 8 | 27.6 | 64 | 45.4 | 0.055 |
| BCL2+ | 41 | 24.1 | 4 | 13.8 | 37 | 26.2 | 0.121 |
| DHL/THL | 35 | 20.6 | 5 | 17.2 | 30 | 21.3 | 0.628 |
| Primary refractory DLBCL | 56 | 32.9 | 9 | 31.0 | 47 | 33.3 | 0.833 |
| Relapsed DLBCL | 114 | 67.1 | 20 | 69.0 | 94 | 66.7 | 0.833 |
| Death | 90 | 52.9 | 11 | 37.9 | 79 | 56.0 | 0.102 |
| Diagnosis | Cohort | Sequencing | Mutation | Exon | Effect | TA Class |
|---|---|---|---|---|---|---|
| DLBCL | Control | Sanger | G266V | Exon8 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | Y205C | Exon6 | Missense | Nonfunctional |
| DHL | Control | Sanger | M237I | Exon7 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | G245A | Exon7 | Missense | Nonfunctional |
| DHL | Control | Sanger | F134L | Exon5 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | V143E | Exon5 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | R248Q | Exon7 | Missense | Nonfunctional |
| R267W | Exon8 | Missense | Nonfunctional | |||
| DHL | Control | Sanger | R248Q | Exon7 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | R280S | Exon8 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | L194P | Exon6 | Missense | Nonfunctional |
| M246V | Exon7 | Missense | Nonfunctional | |||
| DHL | Control | Sanger | R248Q | Exon7 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | R248Q | Exon7 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | G245S | Exon7 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | Y234S | Exon7 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | R273H | Exon8 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | Q331X | Exon9 | Nonsense | Na |
| DLBCL | Control | Sanger | Y220C | Exon6 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | Y236H | Exon7 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | G245S | Exon7 | Missense | Nonfunctional |
| DHL | Control | Sanger | W53L | Exon4 | Missense | Functional |
| DHL | Control | Sanger | E286G | Exon8 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | R175H | Exon5 | Missense | Nonfunctional |
| G262V | Exon8 | Missense | Nonfunctional | |||
| DLBCL | Control | Sanger | N239D | Exon7 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | H179Y | Exon5 | Missense | Partially Functional |
| DHL | Control | NGS | R273H | Exon8 | Missense | Nonfunctional |
| THL | Control | NGS | R175H | Exon5 | Missense | Nonfunctional |
| DLBCL | Control | NGS | H214R | Exon6 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | G187S | Exon5 | Missense | Functional |
| DLBCL | Control | Sanger | S315F | Exon9 | Missense | Functional |
| DHL | Control | Sanger | V272E | Exon8 | Missense | Nonfunctional |
| DLBCL | Control | Sanger | R282W | Exon8 | Missense | Nonfunctional |
| THL | CAR T | NGS | V157G | Exon5 | Missense | Nonfunctional |
| DLBCL | CAR T | NGS | C242S | Exon7 | Missense | Nonfunctional |
| THL | CAR T | NGS | C137W | Exon5 | Missense | Partially Functional |
| DLBCL | CAR T | NGS | R248Q | Exon7 | Missense | Nonfunctional |
| DLBCL | CAR T | NGS | C176* | Exon5 | Nonsense | na |
| DHL | CAR T | NGS | Y234C | Exon7 | Missense | Nonfunctional |
| DLBCL | CAR T | NGS | Q167* | Exon5 | Nonsense | na |
| DLBCL | CAR T | NGS | R273H | Exon8 | Missense | Nonfunctional |
| DLBCL | CAR T | NGS | F113del | Exon4 | Na | Na |
| DLBCL | CAR T | NGS | Q331* | Exon9 | Nonsense | Na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porpaczy, E.; Wohlfarth, P.; Königsbrügge, O.; Rabitsch, W.; Skrabs, C.; Staber, P.; Worel, N.; Müllauer, L.; Simonitsch-Klupp, I.; Kornauth, C.; et al. Influence of TP53 Mutation on Survival of Diffuse Large B-Cell Lymphoma in the CAR T-Cell Era. Cancers 2021, 13, 5592. https://doi.org/10.3390/cancers13225592
Porpaczy E, Wohlfarth P, Königsbrügge O, Rabitsch W, Skrabs C, Staber P, Worel N, Müllauer L, Simonitsch-Klupp I, Kornauth C, et al. Influence of TP53 Mutation on Survival of Diffuse Large B-Cell Lymphoma in the CAR T-Cell Era. Cancers. 2021; 13(22):5592. https://doi.org/10.3390/cancers13225592
Chicago/Turabian StylePorpaczy, Edit, Philipp Wohlfarth, Oliver Königsbrügge, Werner Rabitsch, Cathrin Skrabs, Philipp Staber, Nina Worel, Leonhard Müllauer, Ingrid Simonitsch-Klupp, Christoph Kornauth, and et al. 2021. "Influence of TP53 Mutation on Survival of Diffuse Large B-Cell Lymphoma in the CAR T-Cell Era" Cancers 13, no. 22: 5592. https://doi.org/10.3390/cancers13225592
APA StylePorpaczy, E., Wohlfarth, P., Königsbrügge, O., Rabitsch, W., Skrabs, C., Staber, P., Worel, N., Müllauer, L., Simonitsch-Klupp, I., Kornauth, C., Rohrbeck, J., Jaeger, U., & Schiefer, A.-I. (2021). Influence of TP53 Mutation on Survival of Diffuse Large B-Cell Lymphoma in the CAR T-Cell Era. Cancers, 13(22), 5592. https://doi.org/10.3390/cancers13225592







