Gastric Cancers Missed at Upper Endoscopy in Central Norway 2007 to 2016—A Population-Based Study
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
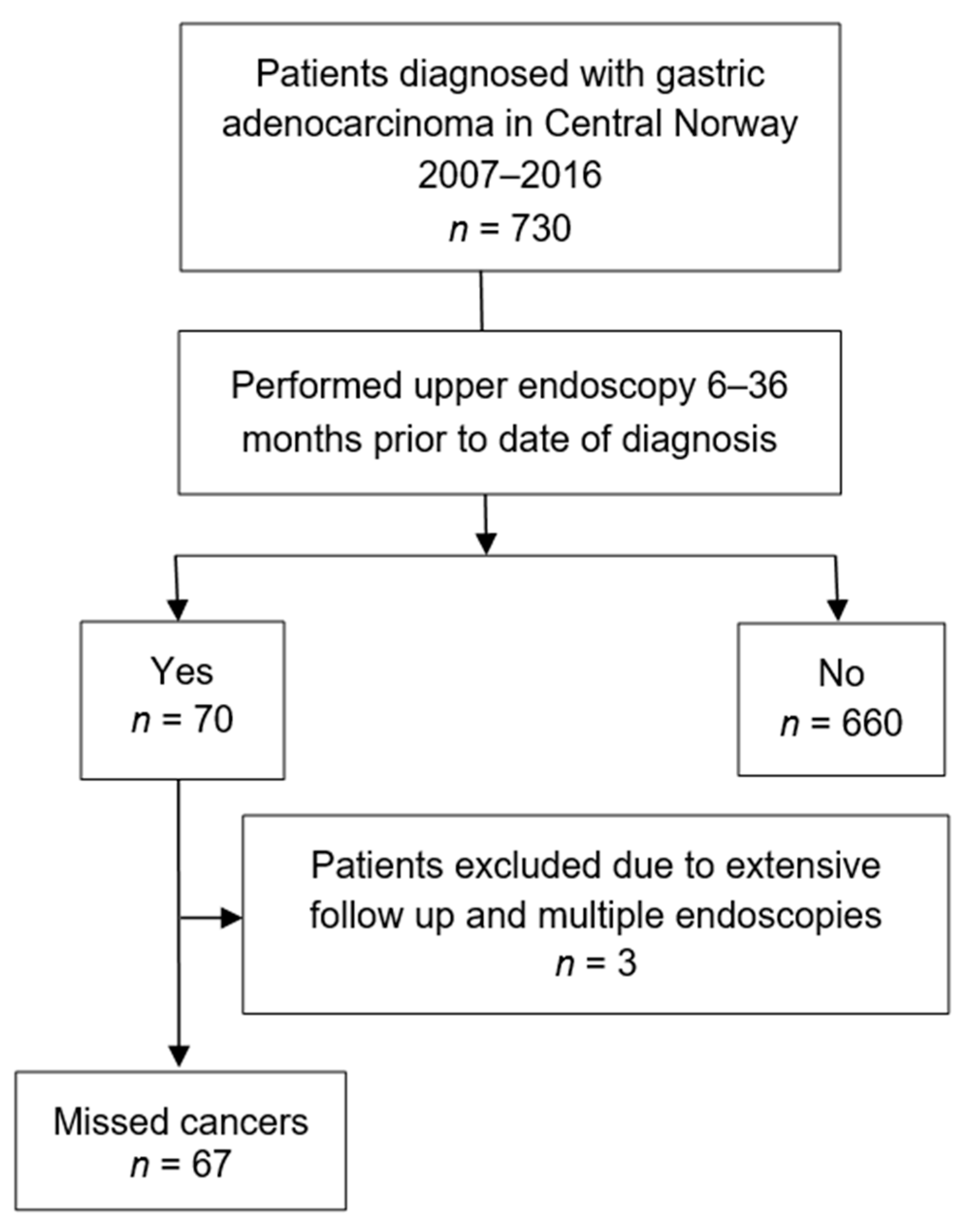
2.1. Study Design and Data Source
2.2. Definitions
2.3. Variables
2.4. Data Collection
2.5. Statistical Analysis
2.6. Ethics Approval
3. Results
3.1. Patient and Characteristics
3.2. Tumour Localization and Stage
3.3. Lauren Classification
3.4. Previous Billroth-2-Anastomosis
3.5. Definitely Missed versus Probably Missed Cancers
3.6. Indications for Upper Endoscopies Prior to Diagnosis versus at Diagnosis
3.7. Biopsy Sampling of Ulcerations
3.8. Endoscopist Experience
3.9. Survival
4. Discussion
4.1. MGC Rate
4.2. Risk Factors of MGC
4.3. Survival
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO International Agency for Research on Cancer. Fact Sheets by Cancer. Available online: http://globocan.iarc.fr/Pages/factsheetscancer.aspx (accessed on 1 June 2021).
- Henson, D.E.; Dittus, C.; Younes, M.; Nguyen, H.; Albores-Saavedra, J. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973–2000: Increase in the signet ring cell type. Arch. Pathol. Lab. Med. 2004, 128, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Laurén, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. APMIS 6: 209–222. Acta Pathol. Microbiol. Scand. 1965, 6, 31–49. [Google Scholar] [CrossRef]
- Anderson, W.F.; Rabkin, C.S.; Turner, N.; Fraumeni, J.F., Jr.; Rosenberg, P.S.; Camargo, M.C. The Changing Face of Noncardia Gastric Cancer Incidence Among US Non-Hispanic Whites. J. Natl. Cancer Inst. 2018, 110, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Bringeland, E.A.; Wasmuth, H.H.; Mjones, P.; Myklebust, T.A.; Gronbech, J.E. A population-based study on incidence rates, Lauren distribution, stage distribution, treatment, and long-term outcomes for gastric adenocarcinoma in Central Norway 2001–2011. Acta Oncol. 2017, 56, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Leung, W.K.; Wu, M.S.; Kakugawa, Y.; Kim, J.J.; Yeoh, K.G.; Goh, K.L.; Wu, K.C.; Wu, D.C.; Sollano, J.; Kachintorn, U.; et al. Screening for gastric cancer in Asia: Current evidence and practice. Lancet Oncol. 2008, 9, 279–287. [Google Scholar] [CrossRef]
- Shinozaki, S.; Osawa, H.; Hayashi, Y.; Lefor, A.K.; Yamamoto, H. Linked color imaging for the detection of early gastrointestinal neoplasms. Ther. Adv. Gastroenterol. 2019, 12, 1756284819885246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capelle, L.G.; Haringsma, J.; de Vries, A.C.; Steyerberg, E.W.; Biermann, K.; van Dekken, H.; Kuipers, E.J. Narrow band imaging for the detection of gastric intestinal metaplasia and dysplasia during surveillance endoscopy. Dig. Dis. Sci. 2010, 55, 3442–3448. [Google Scholar] [CrossRef] [Green Version]
- Park, J.M.; Huo, S.M.; Lee, H.H.; Lee, B.I.; Song, H.J.; Choi, M.G. Longer Observation Time Increases Proportion of Neoplasms Detected by Esophagogastroduodenoscopy. Gastroenterology 2017, 153, 460–469.e1. [Google Scholar] [CrossRef] [PubMed]
- Hernanz, N.; Rodriguez de Santiago, E.; Marcos Prieto, H.M.; Jorge Turrion, M.A.; Barreiro Alonso, E.; Rodriguez Escaja, C.; Jimenez Jurado, A.; Sierra, M.; Perez Valle, I.; Volpato, N.; et al. Characteristics and consequences of missed gastric cancer: A multicentric cohort study. Dig. Liver Dis. 2019, 51, 894–900. [Google Scholar] [CrossRef]
- Chadwick, G.; Groene, O.; Riley, S.; Hardwick, R.; Crosby, T.; Hoare, J.; Hanna, G.B.; Greenaway, K.; Cromwell, D.A. Gastric Cancers Missed During Endoscopy in England. Clin. Gastroenterol. Hepatol. 2015, 13, 1264–1270.e1. [Google Scholar] [CrossRef]
- Yalamarthi, S.; Witherspoon, P.; McCole, D.; Auld, C.D. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy 2004, 36, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Pimenta-Melo, A.R.; Monteiro-Soares, M.; Libanio, D.; Dinis-Ribeiro, M. Missing rate for gastric cancer during upper gastrointestinal endoscopy: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2016, 28, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Sandø, A.F.; Fougner, R.; Grønbech, J.E.; Bringeland, E.A. The value of restaging CT following neoadjuvant chemotherapy for resectable gastric cancer. A population based study. World J. Surg. Oncol. 2021, 19, 1–9, in press. [Google Scholar] [CrossRef]
- Bringeland, E.A.; Wasmuth, H.H.; Fougner, R.; Mjones, P.; Gronbech, J.E. Impact of perioperative chemotherapy on oncological outcomes after gastric cancer surgery. Br. J. Surg. 2014, 101, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Loftus, E.V., Jr.; Judge, T.A.; Peikin, S.R. Rate and Predictors of Interval Esophageal and Gastric Cancers after Esophagogastroduodenoscopy in the United States. Digestion 2016, 94, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Raftopoulos, S.C.; Segarajasingam, D.S.; Burke, V.; Ee, H.C.; Yusoff, I.F. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am. J. Gastroenterol. 2010, 105, 1292–1297. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef]
- NOMESCO, N.M.-S.C. NOMESCO Classification of Surgical Procedures (NCSP), Version 1.14. Available online: https://www.diva-portal.org/smash/get/diva2:970548/FULLTEXT01.pdf (accessed on 1 June 2021).
- Cheung, D.; Menon, S.; Hoare, J.; Dhar, A.; Trudgill, N. Factors Associated with Upper Gastrointestinal Cancer Occurrence After Endoscopy that Did Not Diagnose Cancer. Dig. Dis. Sci. 2016, 61, 2674–2684. [Google Scholar] [CrossRef]
- Fujita, S. Biology of early gastric carcinoma. Pathol. Res. Pract. 1978, 163, 297–309. [Google Scholar] [CrossRef]
- Kohli, Y.; Kawai, K.; Fujita, S. Analytical studies on growth of human gastric cancer. J. Clin. Gastroenterol. 1981, 3, 129–133. [Google Scholar] [CrossRef]
- Park, M.S.; Yoon, J.Y.; Chung, H.S.; Lee, H.; Park, J.C.; Shin, S.K.; Lee, S.K.; Lee, Y.C. Clinicopathologic characteristics of interval gastric cancer in Korea. Gut Liver 2015, 9, 166–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeno, S.; Hashimoto, T.; Maki, K.; Shibata, R.; Shiwaku, H.; Yamana, I.; Yamashita, R.; Yamashita, Y. Gastric cancer arising from the remnant stomach after distal gastrectomy: A review. World J. Gastroenterol. 2014, 20, 13734–13740. [Google Scholar] [CrossRef] [PubMed]
- Iida, T.; Yamashita, K.; Ohwada, S.; Ohkubo, Y.; Hirano, T.; Miyake, T.; Onodera, K.; Kubo, T.; Yamano, H.; Nakase, H. Natural history of gastric cancer from a retrospective review of endoscopic images of older patients with interval gastric cancer. Geriatr. Gerontol. Int. 2018, 18, 997–1002. [Google Scholar] [CrossRef] [PubMed]

| Variable | Entire Cohort | Missed Cancers | Control Group | p Value |
|---|---|---|---|---|
| Patients, n (%) | 730 (100.0) | 67 (9.2) | 663 (90.8) | |
| Age at diagnosis, years | 0.619 | |||
| Median (range) | 73.8 (21.1–98.5) | 73.0 (46.0–94.5) | 73.8 (21.1–98.5) | |
| Sex, n (%) | 0.728 | |||
| Male | 461 (63.2) | 41 (61.2) | 420 (63.3) | |
| Cancer localization, n (%) | 0.009 | |||
| Cardia | 225 (30.8) | 15 (22.4) | 210 (31.7) | |
| Corpus | 198 (27.1) | 30 (44.8) | 168 (25.3) | |
| Antrum | 220 (30.1) | 16 (23.9) | 204 (30.8) | |
| Diffuse | 84 (11.5) | 6 (9.0) | 78 (11.8) | |
| T stage, n (%) | 0.620 | |||
| T0 + Tis | 29 (4.0) | 3 (4.5) | 26 (3.9) | |
| T1 | 68 (9.3) | 10 (14.9) | 58 (8.7) | |
| T2 | 35 (4.8) | 3 (4.5) | 32 (4.8) | |
| T3 | 114 (15.6) | 10 (14.9) | 104 (15.7) | |
| T4a | 103 (14.1) | 12 (17.9) | 91 (13.7) | |
| T4b | 33 (4.5) | 2 (3.0) | 31 (4.7) | |
| Tx | 344 (47.1) | 27 (40.3) | 317 (47.8) | |
| TNM stage, n (%) | 0.224 | |||
| Stage 0 + 1 | 108 (14.8) | 15 (22.4) | 93 (14.0) | |
| Stage 2 | 107 (14.7) | 12 (17.9) | 95 (14.3) | |
| Stage 3 | 111 (15.3) | 11 (16.4) | 100 (15.1) | |
| Stage 4 | 310 (42.5) | 21 (31.3) | 289 (43.6) | |
| Stage X | 94 (12.9) | 8 (11.9) | 86 (13.0) | |
| Lauren classification, n (%) | 0.028 | |||
| Diffuse | 214 (29.3) | 25 (37.3) | 189 (28.5) | |
| Intestinal | 311 (42.6) | 21 (31.3) | 290 (43.7) | |
| Mixed diffuse/intestinal | 78 (10.7) | 5 (7.5) | 73 (11.0) | |
| Cancer NUD | 101 (13.8) | 16 (23.9) | 85 (12.8) | |
| No malignant biopsy | 14 (1.9) | - | 14 (2.1) | |
| No biopsy | 12 (1.6) | - | 12 (1.8) | |
| Previous B2-operation, n (%) | 41 (5.6) | 10 (14.9) | 31 (4.7) | 0.001 |
| Variable | Missed Cancers (6–36 mo.) | Definitely Missed (6–12 mo.) | Potentially Missed (12–36 mo.) | p Value |
|---|---|---|---|---|
| Patients, n (%) | 67 (100.0) | 22 (32.8) | 45 (67.2) | |
| Time from UE to diagnosis in months, mean (SD) | 17.5 (8.8) | 7.9 (2.0) | 22.2 (6.8) | 0.000 |
| Alarm symptoms, n (%) | 37 (55.2) | 11 (50.0) | 26 (57.8) | 0.584 |
| Findings at UE * | ||||
| Normal, n (%) | 18 (26.9) | 5 (22.7) | 13 (28.9) | 0.593 |
| Gastritis, n (%) | 26 (38.8) | 8 (36.4) | 18 (40.0) | 0.774 |
| Ulceration, n (%) | 17 (25.4) | 9 (40.9) | 8 (17.8) | 0.041 |
| GERD, n (%) | 14 (20.9) | 6 (27.3) | 8 (17.8) | 0.369 |
| Other, n (%) | 16 (23.9) | 5 (22.7) | 11 (24.4) | 0.877 |
| Cancer localization, n (%) | 0.850 | |||
| Cardia | 15 (22.4) | 6 (27.3) | 9 (20.0) | |
| Corpus | 30 (44.8) | 10 (45.5) | 20 (44.4) | |
| Antrum | 16 (23.9) | 4 (18.2) | 12 (26.7) | |
| Diffuse | 6 (9.0) | 2 (9.1) | 4 (8.9) | |
| Lauren classification, n (%) | 0.499 | |||
| Diffuse | 25 (37.3) | 6 (27.3) | 19 (42.2) | |
| Intestinal | 21 (31.3) | 9 (40.9) | 12 (26.7) | |
| Mixed diffuse/intestinal | 5 (7.5) | 1 (4.5) | 4 (8.9) | |
| Cancer NUD | 16 (23.9) | 6 (27.3) | 10 (22.2) |
| Symptom/Indication | Endoscopy Prior to Diagnosis (n = 67) | Endoscopy at Diagnosis (n = 67) | p Value * |
|---|---|---|---|
| GI-bleeding, n (%) | 25 (37.1) | 24 (35.8) | 0.858 |
| Upper abdominal pain, n (%) | 15 (22.4) | 18 (26.9) | 0.545 |
| Nausea/vomiting, n (%) | 7 (10.5) | 8 (11.9) | 0.784 |
| Ulcer follow-up, n (%) | 6 (9.0) | 6 (9.0) | 0.762 |
| Barrett’s follow-up, n (%) | 5 (7.5) | 1 (1.5) | 0.208 |
| B2 follow-up, n (%) | 5 (7.5) | 2 (3.0) | 0.441 |
| GERD/dyspepsia, n (%) | 4 (6.0) | 12 (17.9) | 0.059 |
| Weight loss, n (%) | 4 (6.0) | 18 (26.9) | 0.002 |
| Search for primary cancer, n (%) | 1 (1.5) | 3 (4.5) | 0.619 |
| Gastritis follow-up, n (%) | 1 (1.5) | 1 (1.5) | 1 |
| Search for primary cancer, n (%) | 1 (1.5) | 3 (4.5) | 0.619 |
| Gastric NET follow-up, n (%) | 1 (1.5) | 1 (1.5) | 1 |
| Preoperative before gastric bypass, n (%) | 1 (1.5) | 0 | 1 |
| Jaundice, n (%) | 0 | 1 (1.5) | 1 |
| Polyp follow-up, n (%) | 0 | 2 (3.0) | 0.496 |
| Diarrhea, n (%) | 0 | 1 (1.5) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beck, M.; Bringeland, E.A.; Qvigstad, G.; Fossmark, R. Gastric Cancers Missed at Upper Endoscopy in Central Norway 2007 to 2016—A Population-Based Study. Cancers 2021, 13, 5628. https://doi.org/10.3390/cancers13225628
Beck M, Bringeland EA, Qvigstad G, Fossmark R. Gastric Cancers Missed at Upper Endoscopy in Central Norway 2007 to 2016—A Population-Based Study. Cancers. 2021; 13(22):5628. https://doi.org/10.3390/cancers13225628
Chicago/Turabian StyleBeck, Marianne, Erling A. Bringeland, Gunnar Qvigstad, and Reidar Fossmark. 2021. "Gastric Cancers Missed at Upper Endoscopy in Central Norway 2007 to 2016—A Population-Based Study" Cancers 13, no. 22: 5628. https://doi.org/10.3390/cancers13225628
APA StyleBeck, M., Bringeland, E. A., Qvigstad, G., & Fossmark, R. (2021). Gastric Cancers Missed at Upper Endoscopy in Central Norway 2007 to 2016—A Population-Based Study. Cancers, 13(22), 5628. https://doi.org/10.3390/cancers13225628






