Dysregulation of Microtubule Nucleating Proteins in Cancer Cells
Abstract
Simple Summary
Abstract
1. Introduction
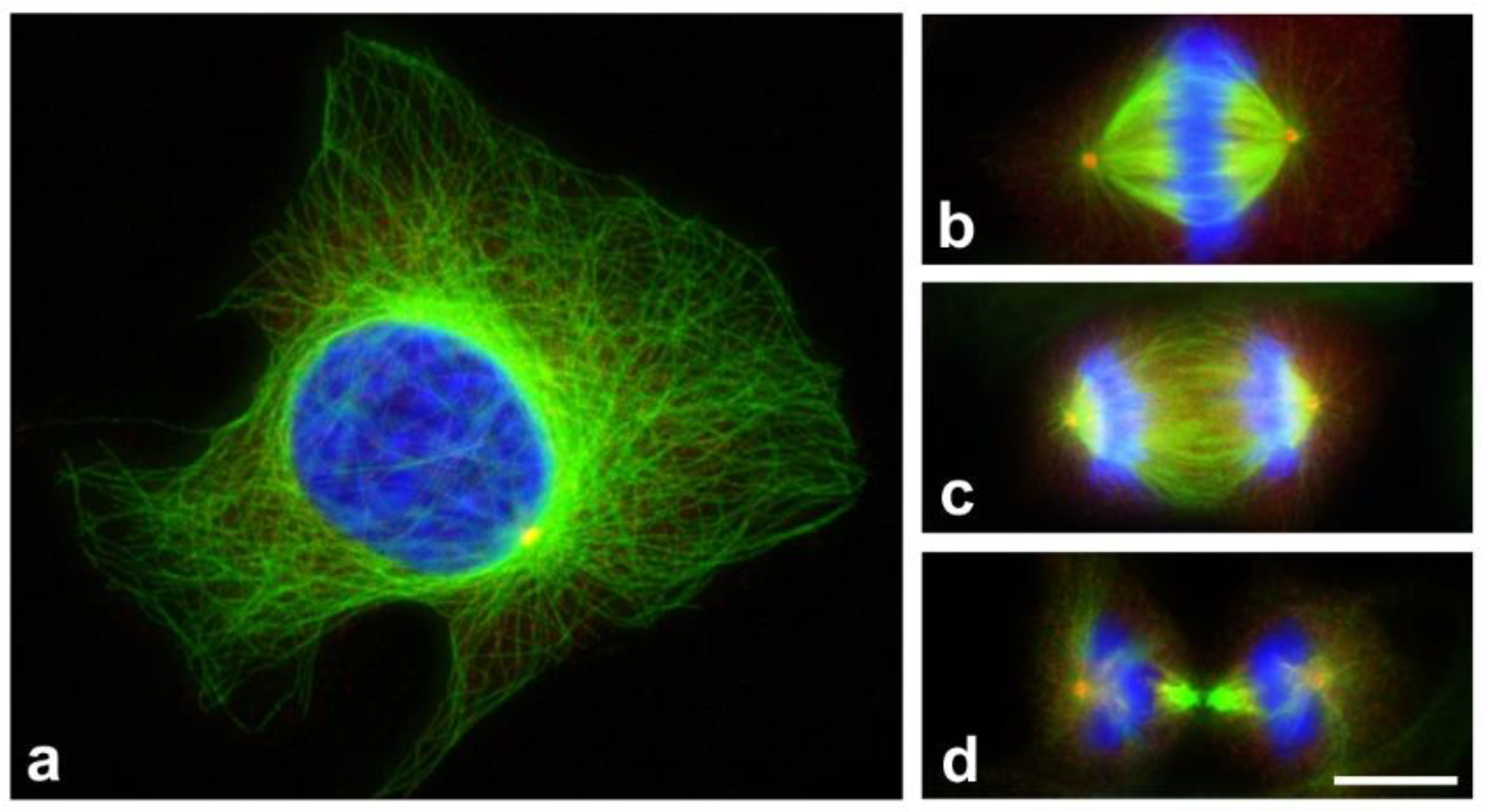
2. Microtubules as Targets in Cancer Chemotherapy
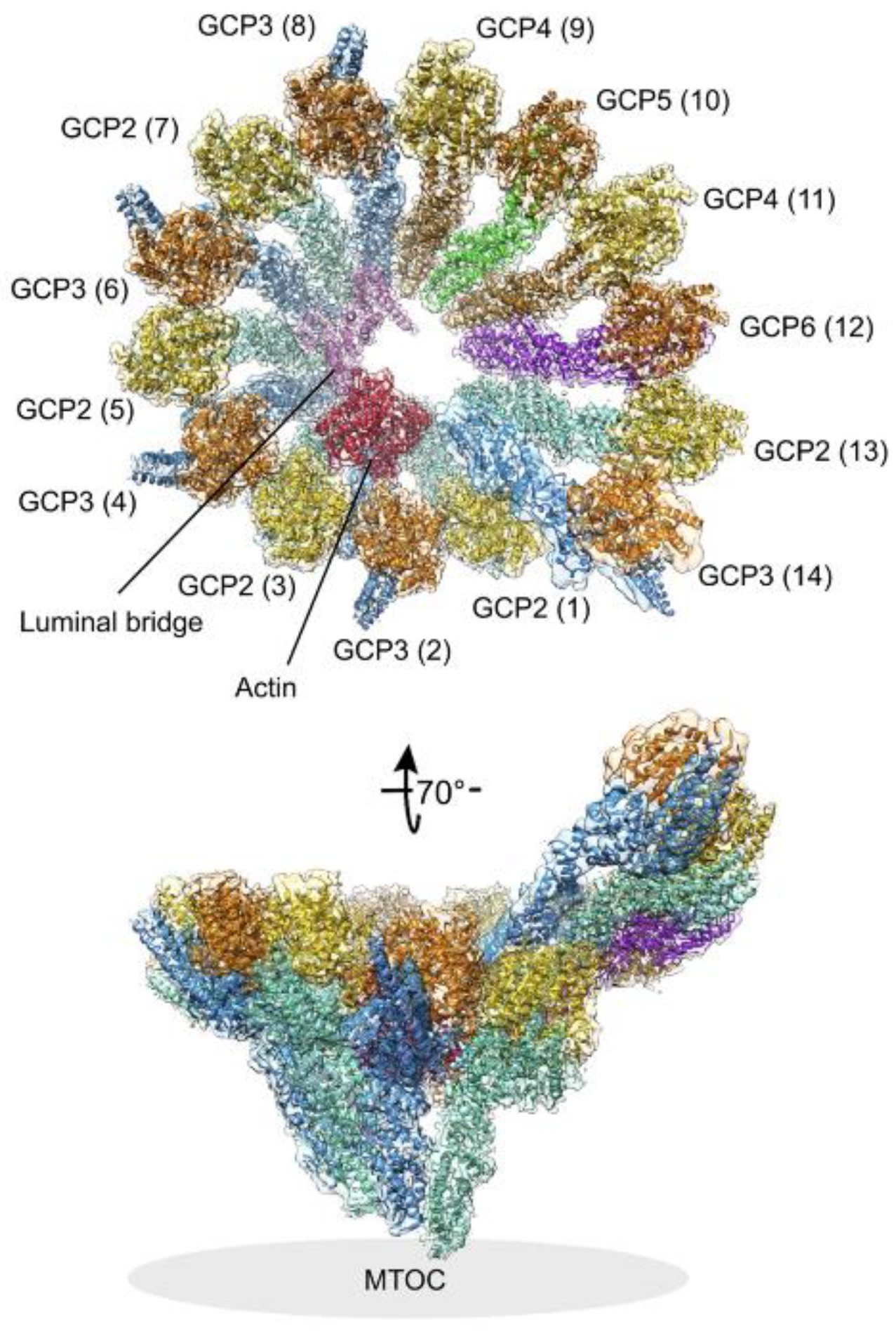
3. γ-Tubulin and Microtubule Nucleation by γ-Tubulin Complexes
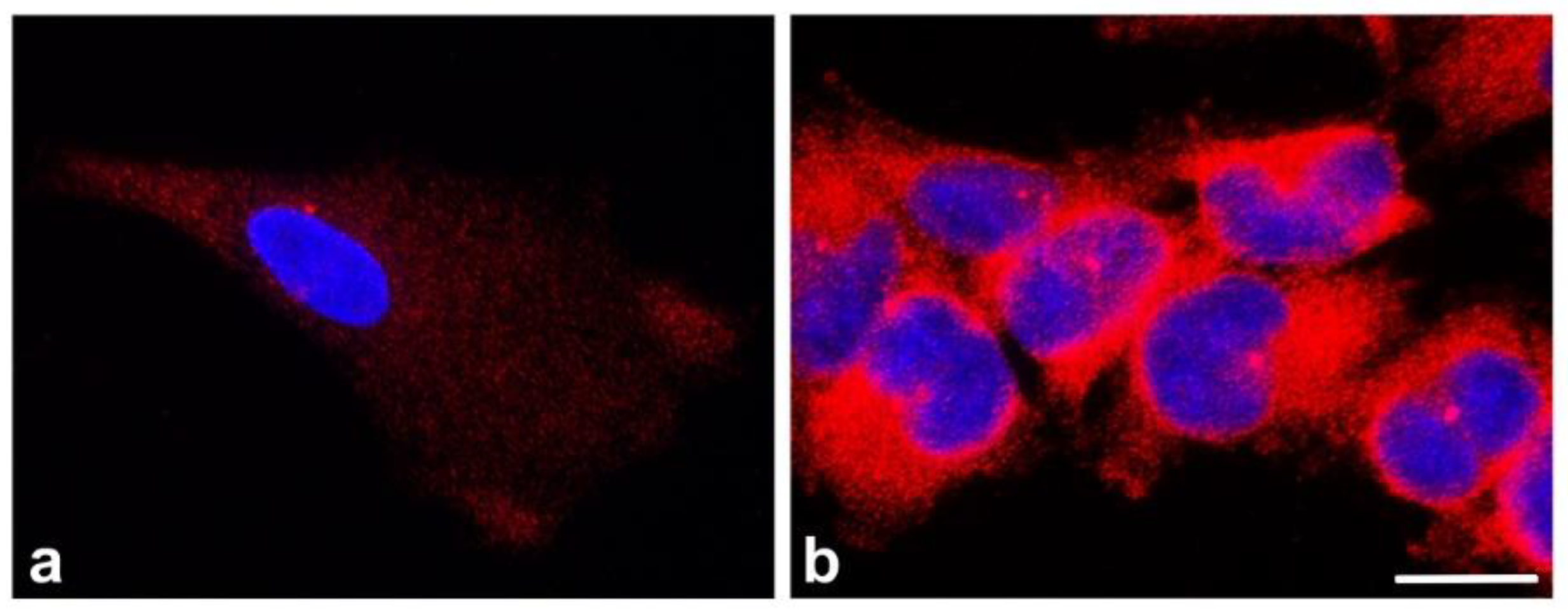
4. Dysregulation of γ-Tubulin in Cancer Cells
5. Dysregulation of the Other γ-TuRC Building Components
6. Therapeutic Potential of γ-Tubulin Targeting
7. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKAP9 | A-kinase anchor protein 9 |
| BRCA1 | breast cancer type 1 susceptibility protein |
| CDK5RAP2 | cyclin-dependent kinase 5 regulatory subunit-asociated protein 2 |
| CDK5RAP3 | cyclin-dependent kinase 5 regulatory subunit-associated protein 3 |
| CDA | citral dimethyl acetal |
| Cep192 | centrosomal protein 192 |
| GBM | glioblastoma multiforme |
| GCP | γ-tubulin complex protein |
| GRIP | γ-tubulin ring protein |
| MAP | microtubule-associated protein |
| MDA | microtubule destabilizing agent |
| MTA | microtubule targeting agent |
| MTOC | microtubule organizing center |
| MSA | microtubule stabilizing agent |
| MZT | mitotic spindle organizing protein |
| NEDD1 | neural precursor cell expressed developmentally down-regulated protein 1 |
| NHA | normal human astrocytes |
| NME7 | nucleoside diphosphate kinase |
| NSCLC | non-small cell lung cancer |
| PCNA | proliferating cell nuclear antigen |
| PTM | post-translational modification |
| RB1 | retinoblastoma transcriptional corepressor 1 |
| γ-TuRC | γ-tubulin ring complex |
| γ-TuSC | γ-tubulin small complex |
References
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Nogales, E.; Wang, H.W. Structural mechanisms underlying nucleotide-dependent self-assembly of tubulin and its relatives. Curr. Opin. Struct. Biol. 2006, 16, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Akhmanova, A.; Steinmetz, M.O. Control of microtubule organization and dynamics: Two ends in the limelight. Nat. Rev. Mol. Cell Biol. 2015, 16, 711–726. [Google Scholar] [CrossRef] [PubMed]
- Dráber, P.; Dráberová, E. Microtubules. In Cytoskeleton and Human Disease; Kavallaris, M., Ed.; Humana Press: New York, NY, USA, 2012; pp. 29–54. [Google Scholar]
- Woodruff, J.B.; Wueseke, O.; Hyman, A.A. Pericentriolar material structure and dynamics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130459. [Google Scholar] [CrossRef]
- Fry, A.M.; Sampson, J.; Shak, C.; Shackleton, S. Recent advances in pericentriolar material organization: Ordered layers and scaffolding gels. F1000Research 2017, 6, 1622. [Google Scholar] [CrossRef] [PubMed]
- Arquint, C.; Gabryjonczyk, A.M.; Nigg, E.A. Centrosomes as signalling centres. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130464. [Google Scholar] [CrossRef]
- Farina, F.; Gaillard, J.; Guerin, C.; Coute, Y.; Sillibourne, J.; Blanchoin, L.; Théry, M. The centrosome is an actin-organizing centre. Nat. Cell Biol. 2016, 18, 65–75. [Google Scholar] [CrossRef]
- Inoue, D.; Obino, D.; Pineau, J.; Farina, F.; Gaillard, J.; Guerin, C.; Blanchoin, L.; Lennon-Dumenil, A.M.; Théry, M. Actin filaments regulate microtubule growth at the centrosome. EMBO J. 2019, 38, e99630. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.R.; Paolillo, V.; Zheng, Y. γ-Tubulin complexes in microtubule nucleation and beyond. Mol. Biol. Cell 2015, 26, 2957–2962. [Google Scholar] [CrossRef] [PubMed]
- Petry, S.; Vale, R.D. Microtubule nucleation at the centrosome and beyond. Nat. Cell Biol. 2015, 17, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Sulimenko, V.; Hájková, Z.; Klebanovych, A.; Dráber, P. Regulation of microtubule nucleation mediated by γ-tubulin complexes. Protoplasma 2017, 254, 1187–1199. [Google Scholar] [CrossRef]
- Wu, J.; Akhmanova, A. Microtubule-organizing centers. Annu. Rev. Cell Dev. Biol. 2017, 33, 51–75. [Google Scholar] [CrossRef] [PubMed]
- Nigg, E.A. Centrosome aberrations: Cause or consequence of cancer progression? Nat. Rev. Cancer 2002, 27, 1554–1561. [Google Scholar] [CrossRef] [PubMed]
- Godinho, S.A.; Picone, R.; Burute, M.; Dagher, R.; Su, Y.; Leung, C.T.; Polyak, K.; Brugge, J.S.; Théry, M.; Pellman, D. Oncogene-like induction of cellular invasion from centrosome amplification. Nature 2014, 510, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Marteil, G.; Guerrero, A.; Vieira, A.F.; de Almeida, B.P.; Machado, P.; Mendonca, S.; Mesquita, M.; Villarreal, B.; Fonseca, I.; Francia, M.E.; et al. Over-elongation of centrioles in cancer promotes centriole amplification and chromosome missegregation. Nat. Commun. 2018, 9, 1258. [Google Scholar] [CrossRef]
- Mittal, K.; Kaur, J.; Jaczko, M.; Wei, G.; Toss, M.S.; Rakha, E.A.; Janssen, E.A.M.; Søiland, H.; Kucuk, O.; Reid, M.D.; et al. Centrosome amplification: A quantifiable cancer cell trait with prognostic value in solid malignancies. Cancer Metastasis Rev. 2021, 40, 319–339. [Google Scholar] [CrossRef] [PubMed]
- Goundiam, O.; Basto, R. Centrosomes in disease: How the same music can sound so different? Curr. Opin. Struct. Biol. 2021, 66, 74–82. [Google Scholar] [CrossRef]
- Nováková, M.; Dráberová, E.; Schürmann, W.; Czihak, G.; Viklický, V.; Dráber, P. γ-Tubulin redistribution in taxol-treated mitotic cells probed by monoclonal antibodies. Cell Motil. Cytoskel. 1996, 33, 38–51. [Google Scholar] [CrossRef]
- Ludueña, R.F. Are tubulin isotypes functionally significant? Mol. Biol. Cell 1993, 4, 445–457. [Google Scholar] [CrossRef]
- Ludueña, R.F. A hypothesis on the origin and evolution of tubulin. Int. Rev. Cell. Mol. Biol. 2013, 302, 41–185. [Google Scholar] [CrossRef]
- Roll-Mecak, A. The tubulin code in microtubule dynamics and information encoding. Dev. Cell 2020, 54, 7–20. [Google Scholar] [CrossRef]
- Gadadhar, S.; Bodakuntla, S.; Natarajan, K.; Janke, C. The tubulin code at a glance. J. Cell Sci. 2017, 130, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Janke, C.; Magiera, M.M. The tubulin code and its role in controlling microtubule properties and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 307–326. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Denoulet, P.; Jeantet, C. High level of tubulin microheterogeneity in the mouse brain. Neurosci. Lett. 1982, 31, 323–328. [Google Scholar] [CrossRef]
- Linhartová, I.; Dráber, P.; Dráberová, E.; Viklický, V. Immunological discrimination of b-tubulin isoforms in developing mouse brain. Posttranslational modification of non-class III β-tubulins. Biochem. J. 1992, 288, 919–924. [Google Scholar] [CrossRef] [PubMed]
- Bodakuntla, S.; Jijumon, A.S.; Villablanca, C.; Gonzalez-Billault, C.; Janke, C. Microtubule-associated proteins: Structuring the cytoskeleton. Trends Cell Biol. 2019, 29, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Portran, D.; Schaedel, L.; Xu, Z.; Thery, M.; Nachury, M.V. Tubulin acetylation protects long-lived microtubules against mechanical ageing. Nat. Cell Biol. 2017, 19, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Valenstein, M.L.; Roll-Mecak, A. Graded control of microtubule severing by tubulin glutamylation. Cell 2016, 164, 911–921. [Google Scholar] [CrossRef]
- Kavallaris, M. Microtubules and resistance to tubulin-binding agents. Nat. Rev. Cancer 2010, 10, 194–204. [Google Scholar] [CrossRef]
- Ludueña, R.F.; Banerjee, A. The isotypes of tubulin: Distribution and functional significance. In The Role of Microtubules in Cell Biology, Neurobiology and Oncology; Fojo, T., Ed.; Humana Press: Totowa, NJ, USA, 2008; pp. 123–175. [Google Scholar]
- Kanakkanthara, A.; Miller, J.H. βIII-tubulin overexpression in cancer: Causes, consequences, and potential therapies. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188607. [Google Scholar] [CrossRef]
- Kops, G.J.; Weaver, B.A.; Cleveland, D.W. On the road to cancer: Aneuploidy and the mitotic checkpoint. Nat. Rev. Cancer 2005, 5, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; Dráber, P. Tubulins as therapeutic targets in cancer: From bench to bedside. Curr. Pharm. Design 2012, 18, 2778–2792. [Google Scholar] [CrossRef]
- Dumontet, C.; Jordan, M.A. Microtubule-binding agents: A dynamic field of cancer therapeutics. Nat. Rev. Drug Discov. 2010, 9, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, M.O.; Prota, A.E. Microtubule-targeting agents: Strategies to hijack the cytoskeleton. Trends Cell Biol. 2018, 28, 776–792. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, E.; Adhami, V.M.; Mukhtar, H. Targeting microtubules by natural agents for cancer therapy. Mol. Cancer Ther. 2014, 13, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Teo, W.S.; McCarroll, J.A.; Kavallaris, M. An emerging role for tubulin isotypes in modulating cancer biology and chemotherapy resistance. Int. J. Mol. Sci. 2017, 18, 1434. [Google Scholar] [CrossRef] [PubMed]
- Oakley, C.E.; Oakley, B.R. Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans. Nature 1989, 338, 662–664. [Google Scholar] [CrossRef]
- Stearns, T.; Evans, L.; Kirschner, M. γ-Tubulin is highly conserved component of the centrosome. Cell 1991, 65, 825–836. [Google Scholar] [CrossRef]
- Dráberová, E.; Sulimenko, V.; Vinopal, S.; Sulimenko, T.; Sládková, V.; D’Agostino, L.; Sobol, M.; Hozák, P.; Křen, L.; Katsetos, C.D.; et al. Differential expression of human γ-tubulin isotypes during neuronal development and oxidative stress points to a γ-tubulin-2 prosurvival function. FASEB J. 2017, 31, 1828–1846. [Google Scholar] [CrossRef]
- Ohashi, T.; Yamamoto, T.; Yamanashi, Y.; Ohsugi, M. Human TUBG2 gene is expressed as two splice variant mRNA and involved in cell growth. FEBS Lett. 2016, 590, 1053–1063. [Google Scholar] [CrossRef]
- Wise, D.O.; Krahe, R.; Oakley, B.R. The γ-tubulin gene family in humans. Genomics 2000, 67, 164–170. [Google Scholar] [CrossRef]
- Yuba-Kubo, A.; Kubo, A.; Hata, M.; Tsukita, S. Gene knockout analysis of two γ-tubulin isoforms in mice. Dev. Biol. 2005, 282, 361–373. [Google Scholar] [CrossRef]
- Vinopal, S.; Černohorská, M.; Sulimenko, V.; Sulimenko, T.; Vosecká, V.; Flemr, M.; Dráberová, E.; Dráber, P. γ-Tubulin 2 nucleates microtubules and is downregulated in mouse early embryogenesis. PLoS ONE 2012, 7, e29919. [Google Scholar] [CrossRef]
- Gombos, L.; Neuner, A.; Berynskyy, M.; Fava, L.L.; Wade, R.C.; Sachse, C.; Schiebel, E. GTP regulates the microtubule nucleation activity of γ-tubulin. Nat. Cell Biol. 2013, 15, 1317–1327. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Kristensson, M.; Rodriguez, M.J.; Silio, V.; Valpuesta, J.M.; Carrera, A.C. SADB phosphorylation of γ-tubulin regulates centrosome duplication. Nat. Cell Biol. 2009, 11, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Starita, L.M.; Groen, A.C.; Ko, M.J.; Parvin, J.D. Centrosomal microtubule nucleation activity is inhibited by BRCA1-dependent ubiquitination. Mol. Cell Biol. 2005, 25, 8656–8668. [Google Scholar] [CrossRef]
- Yin, C.; Lui, E.S.W.; Jiang, T.; Qi, R.Z. Proteolysis of γ-tubulin small complex proteins is mediated by the ubiquitin-proteasome system. FEBS Lett. 2021, 595, 1987–1996. [Google Scholar] [CrossRef]
- Moritz, M.; Braunfeld, M.B.; Sedat, J.W.; Alberts, B.; Agard, D.A. Microtubule nucleation by γ-tubulin-containing rings in the centrosome. Nature 1995, 378, 638–640. [Google Scholar] [CrossRef]
- Zheng, Y.; Alberts, B.; Mitchison, T. Nucleation of microtubule assembly by a γ-tubulin-containing ring complex. Nature 1995, 378, 578–583. [Google Scholar] [CrossRef]
- Kollman, J.M.; Polka, J.K.; Zelter, A.; Davis, T.N.; Agard, D.A. Microtubule nucleating γ-TuSC assembles structures with 13-fold microtubule-like symmetry. Nature 2010, 466, 879–882. [Google Scholar] [CrossRef]
- Kollman, J.M.; Merdes, A.; Mourey, L.; Agard, D.A. Microtubule nucleation by γ-tubulin complexes. Nat. Rev. Mol. Cell Biol. 2011, 12, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Gunawardane, R.N.; Martin, O.C.; Cao, K.; Zhang, L.; Dej, K.; Iwamatsu, A.; Zheng, Y. Characterization and reconstitution of Drosophila γ-tubulin ring complex subunits. J. Cell Biol. 2000, 151, 1513–1524. [Google Scholar] [CrossRef]
- Consolati, T.; Locke, J.; Roostalu, J.; Chen, Z.A.; Gannon, J.; Asthana, J.; Lim, W.M.; Martino, F.; Cvetkovic, M.A.; Rappsilber, J.; et al. Microtubule nucleation properties of single human γTuRCs explained by their cryo-EM structure. Dev. Cell 2020, 53, 603–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zupa, E.; Neuner, A.; Böhler, A.; Loerke, J.; Flemming, D.; Ruppert, T.; Rudack, T.; Peter, C.; Spahn, C.; et al. Insights into the assembly and activation of the microtubule nucleator γ-TuRC. Nature 2020, 578, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Urnavicius, L.; Ti, S.C.; Molloy, K.R.; Chait, B.T.; Kapoor, T.M. Asymmetric molecular architecture of the human γ-tubulin ring complex. Cell 2020, 180, 165–175. [Google Scholar] [CrossRef]
- Zimmermann, F.; Serna, M.; Ezquerra, A.; Fernandez-Leiro, R.; Llorca, O.; Lüders, J. Assembly of the asymmetric human γ-tubulin ring complex by RUVBL1-RUVBL2 AAA ATPase. Sci. Adv. 2020, 6, eabe0894. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Huang, T.L.; Urnavicius, L.; Hsia, K.C.; Kapoor, T.M. MZT proteins form multi-faceted structural modules in the γ-tubulin ring complex. Cell Rep. 2020, 31, 107791. [Google Scholar] [CrossRef]
- Zupa, E.; Liu, P.; Würtz, M.; Schiebel, E.; Pfeffer, S. The structure of the γ-TuRC: A 25-years-old molecular puzzle. Curr. Opin. Struct. Biol. 2021, 66, 15–21. [Google Scholar] [CrossRef]
- Kollman, J.M.; Greenberg, C.H.; Li, S.; Moritz, M.; Zelter, A.; Fong, K.K.; Fernandez, J.J.; Sali, A.; Kilmartin, J.; Davis, T.N.; et al. Ring closure activates yeast γTuRC for species-specific microtubule nucleation. Nat. Struct. Mol. Biol. 2015, 22, 132–137. [Google Scholar] [CrossRef]
- Choi, Y.K.; Liu, P.; Sze, S.K.; Dai, C.; Qi, R.Z. CDK5RAP2 stimulates microtubule nucleation by the γ-tubulin ring complex. J. Cell Biol. 2010, 191, 1089–1095. [Google Scholar] [CrossRef]
- Liu, P.; Choi, Y.K.; Qi, R.Z. NME7 is a functional component of the γ-tubulin ring complex. Mol. Biol. Cell 2014, 25, 2017–2025. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, T.; Shi, L.; Zhang, L.; Zheng, W.; Qu, J.Y.; Niu, R.; Qi, R.Z. Conserved motif of CDK5RAP2 mediates its localization to centrosomes and the Golgi complex. J. Biol. Chem. 2010, 285, 22658–22665. [Google Scholar] [CrossRef]
- Lüders, J.; Patel, U.K.; Stearns, T. GCP-WD is a γ-tubulin targeting factor required for centrosomal and chromatin mediated microtubule nucleation. Nat. Cell Biol. 2006, 8, 137–147. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Q.; Feng, J.; Hou, J.; Yang, F.; Liu, J.; Jiang, Q.; Zhang, C. Sequential phosphorylation of Nedd1 by Cdk1 and Plk1 is required for targeting of the γTuRC to the centrosome. J. Cell Sci. 2009, 122, 2240–2251. [Google Scholar] [CrossRef]
- Takahashi, M.; Yamagiwa, A.; Nishimura, T.; Mukai, H.; Ono, Y. Centrosomal proteins CG-NAP and kendrin provide microtubule nucleation sites by anchoring γ-tubulin ring complex. Mol. Biol. Cell 2002, 13, 3235–3245. [Google Scholar] [CrossRef]
- Zimmerman, W.C.; Sillibourne, J.; Rosa, J.; Doxsey, S.J. Mitosis-specific anchoring of γ-tubulin complexes by pericentrin controls spindle organization and mitotic entry. Mol. Biol. Cell 2004, 15, 3642–3657. [Google Scholar] [CrossRef] [PubMed]
- Delgehyr, N.; Sillibourne, J.; Bornens, M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J. Cell Sci. 2005, 118, 1565–1575. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Ferreria, M.A.; Rath, U.; Buster, D.W.; Chanda, S.K.; Caldwell, J.S.; Rines, D.R.; Sharp, D.J. Human Cep192 is required for mitotic centrosome and spindle assembly. Curr. Biol. 2007, 17, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Thomas, G.E.; Gireesh, K.K.; Manna, T.K. TACC3 protein regulates microtubule nucleation by affecting γ-tubulin ring complexes. J. Biol. Chem. 2014, 289, 31719–31735. [Google Scholar] [CrossRef] [PubMed]
- Rajeev, R.; Singh, P.; Asmita, A.; Anand, U.; Manna, T.K. Aurora A site specific TACC3 phosphorylation regulates astral microtubule assembly by stabilizing γ-tubulin ring complex. BMC Mol. Cell Biol. 2019, 20, 58. [Google Scholar] [CrossRef]
- Tovey, C.A.; Tubman, C.E.; Hamrud, E.; Zhu, Z.; Dyas, A.E.; Butterfield, A.N.; Fyfe, A.; Johnson, E.; Conduit, P.T. γ-TuRC heterogeneity revealed by analysis of Mozart1. Curr. Biol. 2018, 28, 2314–2323. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, N.; Lüders, J. From tip to toe-dressing centrioles in γTuRC. J. Cell Sci. 2021, 134, jcs258397. [Google Scholar] [CrossRef] [PubMed]
- Bouissou, A.; Verollet, C.; Sousa, A.; Sampaio, P.; Wright, M.; Sunkel, C.E.; Merdes, A.; Raynaud-Messina, B. γ-Tubulin ring complexes regulate microtubule plus end dynamics. J. Cell Biol. 2009, 187, 327–334. [Google Scholar] [CrossRef]
- Hendrickson, T.W.; Yao, J.; Bhadury, S.; Corbett, A.H.; Joshi, H.C. Conditional mutations in γ-tubulin reveal its involvement in chromosome segregation and cytokinesis. Mol. Biol. Cell 2001, 12, 2469–2481. [Google Scholar] [CrossRef]
- Nayak, T.; Edgerton-Morgan, H.; Horio, T.; Xiong, Y.; De Souza, C.P.; Osmani, S.A.; Oakley, B.R. γ-Tubulin regulates the anaphase-promoting complex/cyclosome during interphase. J. Cell Biol. 2010, 190, 317–330. [Google Scholar] [CrossRef]
- Hořejší, B.; Vinopal, S.; Sládková, V.; Dráberová, E.; Sulimenko, V.; Sulimenko, T.; Vosecká, V.; Philimonenko, A.; Hozák, P.; Katsetos, C.D.; et al. Nuclear γ-tubulin associates with nucleoli and interacts with tumor suppressor protein C53. J. Cell. Physiol. 2012, 227, 367–382. [Google Scholar] [CrossRef]
- Höög, G.; Zarrizi, R.; von Stedingk, K.; Jonsson, K.; Alvarado-Kristensson, M. Nuclear localization of γ-tubulin affects E2F transcriptional activity and S-phase progression. FASEB J. 2011, 25, 3815–3827. [Google Scholar] [CrossRef] [PubMed]
- Kállai, B.M.; Kourová, H.; Chumová, J.; Papdi, C.; Trögelová, L.; Kofroňová, O.; Hozák, P.; Filimonenko, V.; Mészáros, T.; Magyar, Z.; et al. γ-Tubulin interacts with E2F transcription factors to regulate proliferation and endocycling in Arabidopsis. J. Exp. Bot. 2020, 71, 1265–1277. [Google Scholar] [CrossRef] [PubMed]
- Lesca, C.; Germanier, M.; Raynaud-Messina, B.; Pichereaux, C.; Etievant, C.; Emond, S.; Burlet-Schiltz, O.; Monsarrat, B.; Wright, M.; Defais, M. DNA damage induce γ-tubulin-RAD51 nuclear complexes in mammalian cells. Oncogene 2005, 24, 5165–5172. [Google Scholar] [CrossRef]
- Hubert, T.; Vandekerckhove, J.; Gettemans, J. Cdk1 and BRCA1 target γ-tubulin to microtubule domains. Biochem. Biophys. Res. Commun. 2011, 414, 240–245. [Google Scholar] [CrossRef]
- Zhang, S.; Hernmerich, P.; Grosse, F. Centrosomal localization of DNA damage checkpoint proteins. J. Cell. Biochem. 2007, 101, 451–465. [Google Scholar] [CrossRef]
- Sulimenko, V.; Sulimenko, T.; Poznanovic, S.; Nechiporuk-Zloy, V.; Böhm, J.K.; Macurek, L.; Unger, E.; Dráber, P. Association of brain γ-tubulins with αβ-tubulin dimers. Biochem. J. 2002, 365, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Chumová, J.; Trögelová, L.; Kourová, H.; Volc, J.; Sulimenko, V.; Halada, P.; Kučera, O.; Benada, O.; Kuchařová, A.; Klebanovych, A.; et al. γ-Tubulin has a conserved intrinsic property of self-polymerization into double stranded filaments and fibrillar networks. BBA Mol. Cell Res. 2018, 1865, 734–748. [Google Scholar] [CrossRef]
- Lindström, L.; Li, T.; Malycheva, D.; Kancharla, A.; Nilsson, H.; Vishnu, N.; Mulder, H.; Johansson, M.; Rosselló, C.A.; Alvarado-Kristensson, M. The GTPase domain of gamma-tubulin is required for normal mitochondrial function and spatial organization. Commun. Biol. 2018, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Chumová, J.; Kourová, H.; Trögelová, L.; Halada, P.; Binarová, P. Microtubular and nuclear functions of γ-tubulin: Are they LINCed? Cells 2019, 8, 259. [Google Scholar] [CrossRef]
- Rosselló, C.A.; Lindström, L.; Eklund, G.; Corvaisier, M.; Alvarado-Kristensson, M.A. γ-Tubulin-γ-tubulin interactions as the basis for the formation of a meshwork. Int. J. Mol. Sci. 2018, 19, 3245. [Google Scholar] [CrossRef] [PubMed]
- Chabin-Brion, K.; Marceiller, J.; Perez, F.; Settegrana, C.; Drechou, A.; Durand, G.; Pous, C. The Golgi complex is a microtubule-organizing organelle. Mol. Biol. Cell 2001, 12, 2047–2060. [Google Scholar] [CrossRef]
- Hehnly, H.; Doxsey, S. Rab11 endosomes contribute to mitotic spindle organization and orientation. Dev. Cell 2014, 28, 497–507. [Google Scholar] [CrossRef]
- Katsetos, C.D.; Dráberová, E.; Legido, A.; Dráber, P. Tubulin targets in the pathobiology and therapy of glioblastoma multiforme. II. g-Tubulin. J. Cell. Physiol. 2009, 221, 514–520. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 2005, 64, 479–489. [Google Scholar] [CrossRef]
- Rickman, D.S.; Bobek, M.P.; Misek, D.E.; Kuick, R.; Blaivas, M.; Kurnit, D.M.; Taylor, J.; Hanash, S.M. Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer Res. 2001, 61, 6885–6891. [Google Scholar] [PubMed]
- Katsetos, C.D.; Reddy, G.; Dráberová, E.; Šmejkalová, B.; Del Valle, L.; Ashraf, Q.; Tradevosyan, A.; Yelin, K.; Maraziotis, T.; Mishra, O.P.; et al. Altered cellular distribution and subcellular sorting of γ-tubulin in diffuse astrocytic gliomas and human glioblastoma cell lines. J. Neuropathol. Exp. Neurol. 2006, 65, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Katsetos, C.D.; Dráberová, E.; Šmejkalová, B.; Reddy, G.; Bertrand, L.; de Chadarévian, J.P.; Legido, A.; Nissanov, J.; Baas, P.W.; Dráber, P. Class III β-tubulin and γ-tubulin are co-expressed and form complexes in human glioblastoma cells. Neurochem. Res. 2007, 32, 1387–1398. [Google Scholar] [CrossRef]
- Loh, J.K.; Lieu, A.S.; Chou, C.H.; Lin, F.Y.; Wu, C.H.; Howng, S.L.; Chio, C.C.; Hong, Y.R. Differential expression of centrosomal proteins at different stages of human glioma. BMC Cancer 2010, 10, 268. [Google Scholar] [CrossRef]
- Loh, J.K.; Lieu, A.S.; Chou, C.H.; Lin, C.C.; Yang, M.C.; Lin, F.Y.; Hong, Y.R.; Howng, S.L. Differential expression of centrosome-associated proteins in human brain tumors: A possible role of hNinein isoform 6 in cell differentiation. Biofactors 2012, 38, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.P.; Tsai, C.Y.; Lieu, A.S.; Chai, C.Y.; Kwan, A.L.; Howng, S.L.; Loh, J.K. Association of Aurora A and γ-tubulin expression in astrocytomas and patient survival. Neurol. Res. 2014, 36, 746–751. [Google Scholar] [CrossRef]
- Caracciolo, V.; D’Agostino, L.; Dráberová, E.; Sládková, V.; Crozier-Fitzgerald, C.; Agamanolis, D.P.; de Chadarévian, J.P.; Legido, A.; Giordano, A.; Dráber, P.; et al. Differential expression and cellular distribution of γ-tubulin and βIII-tubulin in medulloblastomas and human medulloblastoma cell lines. J. Cell. Physiol. 2010, 223, 519–529. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Liu, T.; Niu, Y.; Yu, Y.; Liu, Y.; Zhang, F. Increased γ-tubulin expression and P16INK4A promoter methylation occur together in preinvasive lesions and carcinomas of the breast. Ann. Oncol. 2009, 20, 441–448. [Google Scholar] [CrossRef]
- Niu, Y.; Liu, T.; Tse, G.M.; Sun, B.; Niu, R.; Li, H.M.; Wang, H.; Yang, Y.; Ye, X.; Wang, Y.; et al. Increased expression of centrosomal α, γ-tubulin in atypical ductal hyperplasia and carcinoma of the breast. Cancer Sci. 2009, 100, 580–587. [Google Scholar] [CrossRef]
- Cho, E.H.; Whipple, R.A.; Matrone, M.A.; Balzer, E.M.; Martin, S.S. Delocalization of γ-tubulin due to increased solubility in human breast cancer cell lines. Cancer Biol. Ther. 2010, 9, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, S.; Crone, D.E.; Palazzo, R.E.; Parvin, J.D. BRCA1 regulates γ-tubulin binding to centrosomes. Cancer Biol. Ther. 2007, 6, 1853–1857. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoshino, Y.; Fang, Z.; Qi, H.; Kobayashi, A.; Chiba, N. Dysregulation of the centrosome induced by BRCA1 deficiency contributes to tissue-specific carcinogenesis. Cancer Sci. 2021, 112, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Zarrizi, R.; Menard, J.A.; Belting, M.; Massoumi, R. Deubiquitination of γ-tubulin by BAP1 prevents chromosome instability in breast cancer cells. Cancer Res. 2014, 74, 6499–6508. [Google Scholar] [CrossRef] [PubMed]
- Maounis, N.F.; Dráberová, E.; Mahera, E.; Chorti, M.; Caracciolo, V.; Sulimenko, T.; Riga, D.; Trakas, N.; Emmanouilidou, A.; Giordano, A.; et al. Overexpression of γ-tubulin in non-small cell lung cancer. Histol. Histopathol. 2012, 27, 1183–1194. [Google Scholar] [CrossRef]
- Maounis, N.F.; Dráberová, E.; Trakas, N.; Chorti, M.; Riga, D.; Tzannis, K.; Kanakis, M.; Voralu, K.; Ellina, E.; Mahera, E.; et al. Expression of γ-tubulin in non-small cell lung cancer and effect on patient survival. Histol. Histopathol. 2019, 34, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.I.; Syed, S.; Minty, F.; Harrower, S.; Singh, J.; Chin, A.; McLellan, D.R.; Parkinson, E.K.; Clark, L.J. Gamma tubulin: A promising indicator of recurrence in squamous cell carcinoma of the larynx. Otolaryngol. Head Neck Surg. 2009, 140, 498–504. [Google Scholar] [CrossRef]
- Montero-Conde, C.; Martin-Camposo, J.M.; Lerma, E.; Martinez-Guitarte, J.L.; Combalia, N.; Montaner, D.; Matias-Guiu, X.; Dopazo, J.; de Leiva, A.; Robledo, M.; et al. Molecular profiling related to poor prognosis in thyroid carcinoma. Combining gene expression data and biological information. Oncogene 2008, 27, 1554–1561. [Google Scholar] [CrossRef]
- Hsu, L.C.; Kapali, M.; DeLoia, J.A.; Gallion, H.H. Centrosome abnormalities in ovarian cancer. Int. J. Cancer 2005, 113, 746–751. [Google Scholar] [CrossRef]
- Li, Y.W.; Hussain, M.; Sarkar, S.H.; Eliason, J.; Li, R.; Sarkar, F.H. Gene expression profiling revealed novel mechanism of action of Taxotere and Furtulon in prostate cancer cells. BMC Cancer 2005, 5, 7. [Google Scholar] [CrossRef]
- LoMastro, G.M.; Holland, A.J. The emerging link between centrosome aberrations and metastasis. Dev. Cell 2019, 49, 325–331. [Google Scholar] [CrossRef]
- Dráberová, E.; D’Agostino, L.; Caracciolo, V.; Sládková, V.; Sulimenko, T.; Sulimenko, V.; Sobol, M.; Maounis, N.F.; Tzelepis, E.; Mahera, E.; et al. Overexpression and nucleolar localization of γ-tubulin small complex proteins GCP2 and GCP3 in glioblastoma. J. Neuropathol. Exp. Neurol. 2015, 74, 723–742. [Google Scholar] [CrossRef]
- Huang, S.L.; Chao, C.C. Silencing of taxol-sensitizer genes in cancer cells: Lack of sensitization effects. Cancers 2015, 7, 1052–1071. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, X.; Cheng, Y.; Ren, H.; Hu, Y.; Zhang, Y.; Su, H.; Zou, Z.; Wang, Q.; Liu, Z.; et al. MZT2A promotes NSCLC viability and invasion by increasing Akt phosphorylation via the MOZART2 domain. Cancer Sci. 2021, 112, 2210–2222. [Google Scholar] [CrossRef]
- Liu, P.; Würtz, M.; Zupa, E.; Pfeffer, S.; Schiebel, E. Microtubule nucleation: The waltz between γ-tubulin ring complex and associated proteins. Curr. Opin. Cell Biol. 2021, 68, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Diaz, R.J. The remodelling of actin composition as a hallmark of cancer. Transl. Oncol. 2021, 14, 101051. [Google Scholar] [CrossRef] [PubMed]
- Po’uha, S.T.; Kavallaris, M. Gamma-actin is involved in regulating centrosome function and mitotic progression in cancer cells. Cell Cycle 2015, 14, 3908–3919. [Google Scholar] [CrossRef] [PubMed]
- Nejedlá, M.; Sadi, S.; Sulimenko, V.; de Almeida, F.N.; Blom, H.; Dráber, P.; Aspenström, P.; Karlsson, R. Profilin connects actin assembly with microtubule dynamics. Mol. Biol. Cell 2016, 27, 2381–2393. [Google Scholar] [CrossRef]
- Henty-Ridilla, J.L.; Juanes, M.A.; Goode, B.L. Profilin directly promotes microtubule growth through residues mutated in amyotrophic lateral sclerosis. Curr. Biol. 2017, 27, 3535–3543. [Google Scholar] [CrossRef] [PubMed]
- Nejedlá, M.; Klebanovych, A.; Sulimenko, V.; Sulimenko, T.; Dráberová, E.; Dráber, P.; Karlsson, R. The actin regulator profilin 1 is functionally associated with the mammalian centrosome. Life Sci. Alliance 2021, 4, e202000655. [Google Scholar] [CrossRef]
- Pimm, M.L.; Hotaling, J.; Henty-Ridilla, J.L. Profilin choreographs actin and microtubules in cells and cancer. Int. Rev. Cell Mol. Biol. 2020, 355, 155–204. [Google Scholar] [CrossRef]
- Karlsson, R.; Dráber, P. Profilin-A master coordinator of actin and microtubule organization in mammalian cells. J. Cell. Physiol. 2021, 236, 7256–7265. [Google Scholar] [CrossRef] [PubMed]
- Khodjakov, A.; Rieder, C.L. The sudden recruitment of γ-tubulin to the centrosome at the onset of mitosis and its dynamic exchange throughout the cell cycle, do not require microtubules. J. Cell Biol. 1999, 146, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Kondoh, Y.; Natsume, Y.; Yamanaka, H.; Inoue, M.; Toki, H.; Takagi, R.; Shimizu, T.; Yamori, T.; Osada, H.; et al. A small compound targeting TACC3 revealed its different spatiotemporal contributions for spindle assembly in cancer cells. Oncogene 2014, 33, 4242–4252. [Google Scholar] [CrossRef] [PubMed]
- Sabat-Pospiech, D.; Fabian-Kolpanowicz, K.; Prior, I.A.; Coulson, J.M.; Fielding, A.B. Targeting centrosome amplification, an Achilles’ heel of cancer. Biochem. Soc. Trans. 2019, 47, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Friesen, D.E.; Barakat, K.H.; Semenchenko, V.; Perez-Pineiro, R.; Fenske, B.W.; Mane, J.; Wishart, D.S.; Tuszynski, J.A. Discovery of small molecule inhibitors that interact with γ-tubulin. Chem. Biol. Drug. Des. 2012, 79, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Aldaz, H.; Rice, L.M.; Stearns, T.; Agard, D.A. Insights into microtubule nucleation from the crystal structure of human γ-tubulin. Nature 2005, 435, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Liu, P.; Shioda, S.; Pagel, J.; Cerikan, B.; Lin, T.C.; Gruss, O.; Hayashi, Y.; Takeno, H.; Shima, T.; et al. The γ-tubulin-specific inhibitor gatastatin reveals temporal requirements of microtubule nucleation during the cell cycle. Nat. Commun. 2015, 6, 8722. [Google Scholar] [CrossRef]
- Rayevsky, A.S.M.; Samofalova, D.; Demchuk, O.; Karpov, P.; Blume, Y. In silico mechanistic model of microtubule assembly inhibition by selective chromone derivatives. J. Mol. Struct. 2021, 1241, 130633. [Google Scholar] [CrossRef]
- Ebisu, H.; Shintani, K.; Chinen, T.; Nagumo, Y.; Shioda, S.; Hatanaka, T.; Sakakura, A.; Hayakawa, I.; Kigoshi, H.; Usui, T. Dual inhibition of γ-tubulin and Plk1 induces mitotic cell death. Front. Pharmacol. 2020, 11, 620185. [Google Scholar] [CrossRef] [PubMed]
- Shintani, K.; Ebisu, H.; Mukaiyama, M.; Hatanaka, T.; Chinen, T.; Takao, D.; Nagumo, Y.; Sakakura, A.; Hayakawa, I.; Usui, T. Structure optimization of gatastatin for the development of γ-tubulin-specific inhibitor. ACS Med. Chem. Lett. 2020, 11, 1125–1129. [Google Scholar] [CrossRef]
- Traversi, G.; Staid, D.S.; Fiore, M.; Percario, Z.; Trisciuoglio, D.; Antonioletti, R.; Morea, V.; Degrassi, F.; Cozzi, R. A novel resveratrol derivative induces mitotic arrest, centrosome fragmentation and cancer cell death by inhibiting γ-tubulin. Cell Div. 2019, 14, 3. [Google Scholar] [CrossRef]
- Naik, P.K.; Santoshi, S.; Rai, A.; Joshi, H.C. Molecular modelling and competition binding study of Br-noscapine and colchicine provide insight into noscapinoid-tubulin binding site. J. Mol. Graph. Model. 2011, 29, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Suri, C.; Naik, P.K. Combined molecular dynamics and continuum solvent approaches (MM-PBSA/GBSA) to predict noscapinoid binding to gamma-tubulin dimer. SAR QSAR Environ. Res. 2015, 26, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Altinoz, M.A.; Topcu, G.; Hacimuftuoglu, A.; Ozpinar, A.; Ozpinar, A.; Hacker, E.; Elmaci, I. Noscapine, a non-addictive opioid and microtubule-inhibitor in potential treatment of glioblastoma. Neurochem. Res. 2019, 44, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; Nambiar, R.; Rosario, S.R.; Smiraglia, D.J.; Goodrich, D.W.; Witkiewicz, A.K. Pan-cancer molecular analysis of the RB tumor suppressor pathway. Commun. Biol. 2020, 3, 158. [Google Scholar] [CrossRef] [PubMed]
- Ehlén, Å.; Rosselló, C.A.; von Stedingk, K.; Höög, G.; Nilsson, E.; Pettersson, H.M.; Jirström, K.; Alvarado-Kristensson, M. Tumors with nonfunctional retinoblastoma protein are killed by reduced γ-tubulin levels. J. Biol. Chem. 2012, 287, 17241–17247. [Google Scholar] [CrossRef]
- Lindström, L.; Villoutreix, B.O.; Lehn, S.; Hellsten, R.; Nilsson, E.; Crneta, E.; Olsson, R.; Alvarado-Kristensson, M. Therapeutic targeting of nuclear γ-tubulin in RB1-negative tumors. Mol. Cancer Res. 2015, 13, 1073–1082. [Google Scholar] [CrossRef]
| γ-TuRC Proteins | Activating Proteins | Targeting Proteins | Anchoring Proteins |
|---|---|---|---|
| γ-tubulin | CDK5RAP2 | CDK5RAP2 | AKAP9 |
| GCP2 | NME7 | NEDD1 | Cep192 |
| GCP3 | Ninein | ||
| GCP4 | Pericentrin | ||
| GCP5 | |||
| GCP6 | |||
| MZT1 | |||
| MZT2 | |||
| Actin |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dráber, P.; Dráberová, E. Dysregulation of Microtubule Nucleating Proteins in Cancer Cells. Cancers 2021, 13, 5638. https://doi.org/10.3390/cancers13225638
Dráber P, Dráberová E. Dysregulation of Microtubule Nucleating Proteins in Cancer Cells. Cancers. 2021; 13(22):5638. https://doi.org/10.3390/cancers13225638
Chicago/Turabian StyleDráber, Pavel, and Eduarda Dráberová. 2021. "Dysregulation of Microtubule Nucleating Proteins in Cancer Cells" Cancers 13, no. 22: 5638. https://doi.org/10.3390/cancers13225638
APA StyleDráber, P., & Dráberová, E. (2021). Dysregulation of Microtubule Nucleating Proteins in Cancer Cells. Cancers, 13(22), 5638. https://doi.org/10.3390/cancers13225638







