3D Breast Tumor Models for Radiobiology Applications
Abstract
:Simple Summary
Abstract
1. Introduction
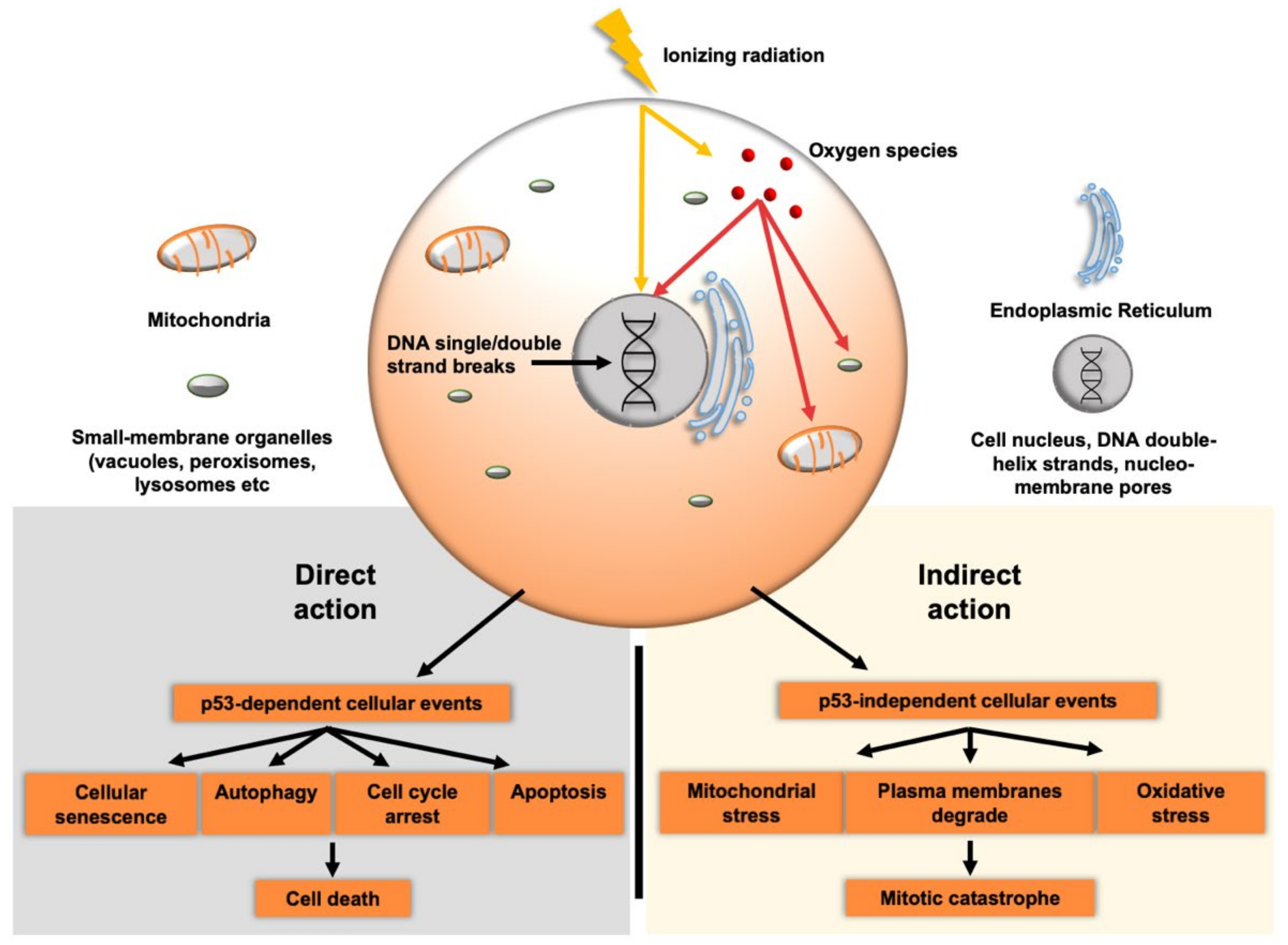
2. Radiation Induced Cell-Death
2.1. Direct Effects
2.2. Indirect Effects
3. Clinical Issues of Radiotherapy and Associated Phenomena
4. Current Preclinical Tools to Evaluate Effects of Radiation Therapy
4.1. Animal Models
4.2. 2D Models
4.3. 3D Models
5. Future Directions in 3D Breast Cancer Radiobiology Models
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acheva, A.; Aerts, A.; Rombouts, C.; Baatout, S.; Salomaa, S.; Manda, K.; Hildebrandt, G.; Kämäräinen, M. Human 3-D tissue models in radiation biology: Current status and future perspectives. Int. J. Radiat. Res. 2014, 12, 81–98. [Google Scholar]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef]
- Lawrence, T.S.; Rosenberg, S.A.; DePinho, R.A.; Weinberg, R.A. Cancer: Principles & Practice of Oncology. Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- Baskar, R.; Dai, J.; Wenlong, N.; Yeo, R.; Yeoh, K.-W. Biological response of cancer cells to radiation treatment. Front. Mol. Biosci. 2014, 1, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barcellos-Hoff, M.H.; Park, C.; Wright, E.G. Radiation and the microenvironment–tumorigenesis and therapy. Nat. Rev. Cancer 2005, 5, 867–875. [Google Scholar] [CrossRef] [Green Version]
- Ceccaldi, R.; Rondinelli, B.; D’Andrea, A.D. Repair Pathway Choices and Consequences at the Double-Strand Break. Trends Cell Biol. 2016, 26, 52–64. [Google Scholar] [CrossRef] [Green Version]
- Rodgers, K.; McVey, M. Error-prone repair of DNA double-strand breaks. J. Cell. Physiol. 2016, 231, 15–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamarche, B.J.; Orazio, N.I.; Weitzman, M.D. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett. 2010, 584, 3682–3695. [Google Scholar] [CrossRef] [Green Version]
- Sishc, B.J.; Davis, A.J. The role of the core non-homologous end joining factors in carcinogenesis and cancer. Cancers 2017, 9, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.H.; Pannunzio, N.R.; Adachi, N.; Lieber, M.R. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat. Rev. Mol. Cell Biol. 2017, 18, 495–506. [Google Scholar] [CrossRef]
- Lai, P.B.; Chi, T.-Y.; Chen, G.G. Different levels of p53 induced either apoptosis or cell cycle arrest in a doxycycline-regulated hepatocellular carcinoma cell line in vitro. Apoptosis 2007, 12, 387–393. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [Green Version]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Baldacchino, G.; Brun, E.; Denden, I.; Bouhadoun, S.; Roux, R.; Khodja, H.; Sicard-Roselli, C. Importance of radiolytic reactions during high-LET irradiation modalities: LET effect, role of O2 and radiosensitization by nanoparticles. Cancer Nanotechnol. 2019, 10, 3. [Google Scholar] [CrossRef]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef]
- Tulard, A.; Hoffschir, F.; de Boisferon, F.H.; Luccioni, C.; Bravard, A. Persistent oxidative stress after ionizing radiation is involved in inherited radiosensitivity. Free Radic. Biol. Med. 2003, 35, 68–77. [Google Scholar] [CrossRef]
- Shimura, T. ATM-Mediated Mitochondrial Radiation Responses of Human Fibroblasts. Genes 2021, 12, 1015. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.; Seo, D.; Kim, D.; Kim, K.; Kim, E.; Kang, J.; Seong, K.M.; Youn, H.; Youn, B. Cellular stress responses in radiotherapy. Cells 2019, 8, 1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, J.F. DNA damage produced by ionizing radiation in mammalian cells: Identities, mechanisms of formation, and reparability. Prog. Nucl. Acid Res. Mol. Biol. 1988, 35, 95–125. [Google Scholar]
- Poli, G.; Schaur, R.J.; Siems, W.a.; Leonarduzzi, G. 4-Hydroxynonenal: A membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2008, 28, 569–631. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Korzets, Y.; Fyles, A.; Shepshelovich, D.; Amir, E.; Goldvaser, H. Toxicity and clinical outcomes of partial breast irradiation compared to whole breast irradiation for early-stage breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2019, 175, 531–545. [Google Scholar] [CrossRef]
- Halle, M.; Gabrielsen, A.; Paulsson-Berne, G.; Gahm, C.; Agardh, H.E.; Farnebo, F.; Tornvall, P. Sustained inflammation due to nuclear factor-kappa B activation in irradiated human arteries. J. Am. Coll. Cardiol. 2010, 55, 1227–1236. [Google Scholar] [CrossRef] [Green Version]
- Hooning, M.J.; Aleman, B.M.; van Rosmalen, A.J.; Kuenen, M.A.; Klijn, J.G.; van Leeuwen, F.E. Cause-specific mortality in long-term survivors of breast cancer: A 25-year follow-up study. Int. J. Radiat. Oncol. Biol. Phys. 2006, 64, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Marinko, T. Pericardial disease after breast cancer radiotherapy. Radiol. Oncol. 2019, 53, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fajardo, L.; Stewart, J.; Cohn, K. Morphology of radiation-induced heart disease. Arch. Pathol. 1968, 86, 512–519. [Google Scholar] [PubMed]
- Najafi, M.; Fardid, R.; Hadadi, G.; Fardid, M. The mechanisms of radiation-induced bystander effect. J. Biomed. Phys. Eng. 2014, 4, 163. [Google Scholar]
- Choi, J.; Yoon, Y.N.; Kim, N.; Park, C.S.; Seol, H.; Park, I.-C.; Kim, H.-A.; Noh, W.C.; Kim, J.-S.; Seong, M.-K. Predicting radiation resistance in breast cancer with expression status of phosphorylated S6K1. Sci. Rep. 2020, 10, 641. [Google Scholar] [CrossRef] [Green Version]
- Dracham, C.B.; Shankar, A.; Madan, R. Radiation induced secondary malignancies: A review article. Radiat. Oncol. J. 2018, 36, 85. [Google Scholar] [CrossRef]
- Grantzau, T.; Overgaard, J. Risk of second non-breast cancer after radiotherapy for breast cancer: A systematic review and meta-analysis of 762,468 patients. Radiother. Oncol. 2015, 114, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Prochazka, M.; Hall, P.; Gagliardi, G.; Granath, F.; Nilsson, B.N.; Shields, P.G.; Tennis, M.; Czene, K. Ionizing radiation and tobacco use increases the risk of a subsequent lung carcinoma in women with breast cancer: Case-only design. J. Clin. Oncol. 2005, 23, 7467–7474. [Google Scholar] [CrossRef] [PubMed]
- Varszegi, D.; Duga, B.; Melegh, B.I.; Sumegi, K.; Kisfali, P.; Maasz, A.; Melegh, B. Hodgkin disease therapy induced second malignancy susceptibility 6q21 functional variants in roma and hungarian population samples. Pathol. Oncol. Res. 2014, 20, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Von Essen, C. Radiation enhancement of metastasis: A review. Clin. Exp. Metastasis 1991, 9, 77–104. [Google Scholar] [CrossRef] [PubMed]
- Vilalta, M.; Rafat, M.; Giaccia, A.J.; Graves, E.E. Recruitment of Circulating Breast Cancer Cells Is Stimulated by Radiotherapy. Cell Rep. 2014, 8, 402–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilalta, M.; Rafat, M.; Graves, E.E. Effects of radiation on metastasis and tumor cell migration. Cell. Mol. Life Sci. 2016, 73, 2999–3007. [Google Scholar] [CrossRef] [Green Version]
- Coleman, C.N.; Higgins, G.S.; Brown, J.M.; Baumann, M.; Kirsch, D.G.; Willers, H.; Prasanna, P.G.S.; Dewhirst, M.W.; Bernhard, E.J.; Ahmed, M.M. Improving the Predictive Value of Preclinical Studies in Support of Radiotherapy Clinical Trials. J. Clin. Cancer Res. 2016, 22, 3138–3147. [Google Scholar] [CrossRef] [Green Version]
- Blakely, E.A.; Chang, P.Y. Preclinical Radiobiology and Predictive Assays. In Ion Beam Therapy: Fundamentals, Technology, Clinical Applications; Linz, U., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 135–145. [Google Scholar]
- Xie, G.; Liu, Y.; Yao, Q.; Zheng, R.; Zhang, L.; Lin, J.; Guo, Z.; Du, S.; Ren, C.; Yuan, Q.; et al. Hypoxia-induced angiotensin II by the lactate-chymase-dependent mechanism mediates radioresistance of hypoxic tumor cells. Sci. Rep. 2017, 7, 42396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Jiang, H.; Van De Gucht, M.; De Ridder, M. Hypoxic Radioresistance: Can ROS Be the Key to Overcome It? Cancers 2019, 11, 112. [Google Scholar] [CrossRef] [Green Version]
- Chan, N.; Koritzinsky, M.; Zhao, H.; Bindra, R.; Glazer, P.M.; Powell, S.; Belmaaza, A.; Wouters, B.; Bristow, R.G. Chronic Hypoxia Decreases Synthesis of Homologous Recombination Proteins to Offset Chemoresistance and Radioresistance. Cancer Res. 2008, 68, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Lin, S.H.; Ye, R.; Wang, Y. Preclinical Strategies for Testing of Targeted Radiosensitizers. In Molecular Targeted Radiosensitizers: Opportunities and Challenges; Willers, H., Eke, I., Eds.; Springer International Publishing: Cham, Swizterland, 2020; pp. 97–114. [Google Scholar]
- Brix, N.; Tiefenthaller, A.; Anders, H.; Belka, C.; Lauber, K. Abscopal, immunological effects of radiotherapy: Narrowing the gap between clinical and preclinical experiences. Immunol. Rev. 2017, 280, 249–279. [Google Scholar] [CrossRef]
- Zhang, L.; Bochkur Dratver, M.; Yazal, T.; Dong, K.; Nguyen, A.; Yu, G.; Dao, A.; Bochkur Dratver, M.; Duhachek-Muggy, S.; Bhat, K.; et al. Mebendazole Potentiates Radiation Therapy in Triple-Negative Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 195–207. [Google Scholar] [CrossRef]
- Atkinson, R.L.; Zhang, M.; Diagaradjane, P.; Peddibhotla, S.; Contreras, A.; Hilsenbeck, S.G.; Woodward, W.A.; Krishnan, S.; Chang, J.C.; Rosen, J.M. Thermal Enhancement with Optically Activated Gold Nanoshells Sensitizes Breast Cancer Stem Cells to Radiation Therapy. J. Sci. Transl. Med. 2010, 2, 55ra79. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Xu, S.; Hu, W.; Xun, X.; Zheng, L.; Su, M. Tumor targeted, stealthy and degradable bismuth nanoparticles for enhanced X-ray radiation therapy of breast cancer. Biomaterials 2018, 154, 24–33. [Google Scholar] [CrossRef]
- Steer, A.; Cordes, N.; Jendrossek, V.; Klein, D. Impact of Cancer-Associated Fibroblast on the Radiation-Response of Solid Xenograft Tumors. Front. Mol. Biosci. 2019, 6, 70. [Google Scholar] [CrossRef] [Green Version]
- Marciscano, A.E.; Haimovitz-Friedman, A.; Lee, P.; Tran, P.T.; Tomé, W.A.; Guha, C.; Spring Kong, F.M.; Sahgal, A.; El Naqa, I.; Rimner, A.; et al. Immunomodulatory Effects of Stereotactic Body Radiation Therapy: Preclinical Insights and Clinical Opportunities. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Schlaak, R.A.; SenthilKumar, G.; Boerma, M.; Bergom, C. Advances in Preclinical Research Models of Radiation-Induced Cardiac Toxicity. Cancers 2020, 12, 415. [Google Scholar] [CrossRef] [Green Version]
- Ghita, M.; Dunne, V.; Hanna, G.G.; Prise, K.M.; Williams, J.P.; Butterworth, K.T. Preclinical models of radiation-induced lung damage: Challenges and opportunities for small animal radiotherapy. Br. J. Radiol. 2019, 92, 20180473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koontz, B.F.; Verhaegen, F.; Ruysscher, D.D. Tumour and normal tissue radiobiology in mouse models: How close are mice to mini-humans? Br. J. Radiol. 2017, 90, 20160441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, F.; Zhang, X.H.; Hu, X.D.; Liu, P.D.; Zhang, H.Q. The effects of combined selenium nanoparticles and radiation therapy on breast cancer cells in vitro. Artif. Cells Nanomed. Biotechnol. 2018, 46, 937–948. [Google Scholar] [CrossRef] [Green Version]
- Schilling, D.; Herold, B.; Combs, S.E.; Schmid, T.E. Selenium does not affect radiosensitivity of breast cancer cell lines. Radiat. Environ. Biophys. 2019, 58, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Hullo, M.; Grall, R.; Perrot, Y.; Mathe, C.; Menard, V.; Yang, X.M.; Lacombe, S.; Porcel, E.; Villagrasa, C.; Chevillard, S.; et al. Radiation Enhancer Effect of Platinum Nanoparticles in Breast Cancer Cell Lines: In Vitro and In Silico Analyses. Int. J. Mol. Sci. 2021, 22, 4436. [Google Scholar] [CrossRef] [PubMed]
- Darwesh, R.; Aboushoushah, S.F.; Almarhabi, S.; Aldahlawi, A.M.; Elbialy, N.S. TurboBeads magnetic nanoparticles functionalized with gold as a promising nano-radiosensitizer for potential breast cancer radiotherapy: In vitro study. Inorg. Chem. Commun. 2020, 123, 108348. [Google Scholar] [CrossRef]
- Islamian, J.P.; Hatamian, M.; Aval, N.A.; Rashidi, M.R.; Mesbahi, A.; Mohammadzadeh, M.; Jafarabadi, M.A. Targeted superparamagnetic nanoparticles coated with 2-deoxy-D-gloucose and doxorubicin more sensitize breast cancer cells to ionizing radiation. Breast 2017, 33, 97–103. [Google Scholar] [CrossRef]
- Zhou, K.X.; Xie, L.H.; Peng, X.; Guo, Q.M.; Wu, Q.Y.; Wang, W.H.; Zhang, G.L.; Wu, J.F.; Zhang, G.J.; Du, C.W. CXCR4 antagonist AMD3100 enhances the response of MDA-MB-231 triple-negative breast cancer cells to ionizing radiation. Cancer Lett. 2018, 418, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Marvaso, G.; Barone, A.; Amodio, N.; Raimondi, L.; Agosti, V.; Altomare, E.; Scotti, V.; Lombardi, A.; Bianco, R.; Bianco, C.; et al. Sphingosine analog fingolimod (FTY720) increases radiation sensitivity of human breast cancer cells in vitro. Cancer Biol. 2014, 15, 797–805. [Google Scholar] [CrossRef] [Green Version]
- Parsyan, A.; Cruickshank, J.; Hodgson, K.; Wakeham, D.; Pellizzari, S.; Bhat, V.; Cescon, D.W. Anticancer effects of radiation therapy combined with Polo-Like Kinase 4 (PLK4) inhibitor CFI-40 0945 in triple negative breast cancer. Breast 2021, 58, 6–9. [Google Scholar] [CrossRef]
- Da Costa Araldi, I.C.; Bordin, F.P.R.; Cadoná, F.C.; Barbisan, F.; Azzolin, V.F.; Teixeira, C.F.; Baumhardt, T.; da Cruz, I.B.M.; Duarte, M.M.M.F.; Bauermann, L.d.F. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem.-Biol. Interact. 2018, 282, 85–92. [Google Scholar] [CrossRef]
- Speers, C.; Zhao, S.G.; Chandler, B.; Liu, M.; Wilder-Romans, K.; Olsen, E.; Nyati, S.; Ritter, C.; Alluri, P.G.; Kothari, V.; et al. Androgen receptor as a mediator and biomarker of radioresistance in triple-negative breast cancer. NPJ Breast Cancer 2017, 3, 29. [Google Scholar] [CrossRef]
- Groselj, B.; Sharma, N.L.; Hamdy, F.C.; Kerr, M.; Kiltie, A.E. Histone deacetylase inhibitors as radiosensitisers: Effects on DNA damage signalling and repair. Br. J. Cancer 2013, 108, 748–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarmohamadi, A.; Asadi, J.; Gharaei, R.; Mir, M.; Khoshnazar, A. Valproic acid, a histone deacetylase inhibitor, enhances radiosensitivity in breast cancer cell line. J. Radiat. Cancer Res. 2018, 9, 86–92. [Google Scholar] [CrossRef]
- Banuelos, C.A.; Banáth, J.P.; MacPhail, S.H.; Zhao, J.; Reitsema, T.; Olive, P.L. Radiosensitization by the Histone Deacetylase Inhibitor PCI-24781. Clin. Cancer Res. 2007, 13, 6816–6826. [Google Scholar] [CrossRef] [Green Version]
- Chiu, H.-W.; Yeh, Y.-L.; Ho, S.-Y.; Wu, Y.-H.; Wang, B.-J.; Huang, W.-J.; Ho, Y.-S.; Wang, Y.-J.; Chen, L.-C.; Tu, S.-H. A New Histone Deacetylase Inhibitor Enhances Radiation Sensitivity through the Induction of Misfolded Protein Aggregation and Autophagy in Triple-Negative Breast Cancer. Cancers 2019, 11, 1703. [Google Scholar] [CrossRef] [Green Version]
- Rodman, S.N.; Spence, J.M.; Ronnfeldt, T.J.; Zhu, Y.; Solst, S.R.; O’Neill, R.A.; Allen, B.G.; Guan, X.; Spitz, D.R.; Fath, M.A. Enhancement of Radiation Response in Breast Cancer Stem Cells by Inhibition of Thioredoxin- and Glutathione-Dependent Metabolism. Radiat. Res. 2016, 186, 385–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Bouzakoura, S.; de Mey, S.; Jiang, H.; Law, K.; Dufait, I.; Corbet, C.; Verovski, V.; Gevaert, T.; Feron, O.; et al. Auranofin radiosensitizes tumor cells through targeting thioredoxin reductase and resulting overproduction of reactive oxygen species. Oncotarget 2017, 8, 35728–35742. [Google Scholar] [CrossRef] [Green Version]
- de Mey, S.; Dufait, I.; Jiang, H.; Corbet, C.; Wang, H.; Van De Gucht, M.; Kerkhove, L.; Law, K.L.; Vandenplas, H.; Gevaert, T.; et al. Dichloroacetate Radiosensitizes Hypoxic Breast Cancer Cells. Int. J. Mol. Sci. 2020, 21, 9367. [Google Scholar] [CrossRef]
- Martinel Lamas, D.J.; Cortina, J.E.; Ventura, C.; Sterle, H.A.; Valli, E.; Balestrasse, K.B.; Blanco, H.; Cremaschi, G.A.; Rivera, E.S.; Medina, V.A. Enhancement of ionizing radiation response by histamine in vitro and in vivo in human breast cancer. Cancer Biol. 2015, 16, 137–148. [Google Scholar] [CrossRef] [Green Version]
- Luby, A.O.; Subramanian, C.; Buchman, L.K.; Lynn, J.V.; Urlaub, K.M.; Nelson, N.S.; Donneys, A.; Cohen, M.S.; Buchman, S.R. Amifostine Prophylaxis in Irradiated Breast Reconstruction A Study of Oncologic Safety In Vitro. Ann. Plastic Surg. 2020, 85, 424–429. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Kumar, A.; Laskar, S.; Pandey, B.N. Cytokine profile of conditioned medium from human tumor cell lines after acute and fractionated doses of gamma radiation and its effect on survival of bystander tumor cells. Cytokine 2013, 61, 54–62. [Google Scholar] [CrossRef]
- Jabbari, N.; Nawaz, M.; Rezaie, J. Bystander effects of ionizing radiation: Conditioned media from X-ray irradiated MCF-7 cells increases the angiogenic ability of endothelial cells. Cell Commun. Signal. 2019, 17, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabbari, N.; Nawaz, M.; Rezaie, J. Ionizing Radiation Increases the Activity of Exosomal Secretory Pathway in MCF-7 Human Breast Cancer Cells: A Possible Way to Communicate Resistance against Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speers, C.; Zhao, S.; Liu, M.; Bartelink, H.; Pierce, L.J.; Feng, F.Y. Development and Validation of a Novel Radiosensitivity Signature in Human Breast Cancer. Clin. Cancer Res. 2015, 21, 3667–3677. [Google Scholar] [CrossRef] [Green Version]
- Smith, L.; Qutob, O.; Watson, M.B.; Beavis, A.W.; Potts, D.; Welham, K.J.; Garimella, V.; Lind, M.J.; Drew, P.J.; Cawkwell, L. Proteomic identification of putative biomarkers of radiotherapy resistance: A possible role for the 26S proteasome? Neoplasia 2009, 11, 1194–1207. [Google Scholar] [CrossRef] [Green Version]
- Langlands, F.E.; Horgan, K.; Dodwell, D.D.; Smith, L. Breast cancer subtypes: Response to radiotherapy and potential radiosensitisation. Br. J. Radiol. 2013, 86, 20120601. [Google Scholar] [CrossRef]
- Bravatà, V.; Cava, C.; Minafra, L.; Cammarata, F.P.; Russo, G.; Gilardi, M.C.; Castiglioni, I.; Forte, G.I. Radiation-Induced Gene Expression Changes in High and Low Grade Breast Cancer Cell Types. Int. J. Mol. Sci. 2018, 19, 1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Duan, Q.; Wu, N.; Xu, B. A heterogeneous cellular response to ionizing radiation revealed by single cell transcriptome sequencing. Am. J. Cancer Res. 2021, 11, 513–529. [Google Scholar]
- Speers, C.; Pierce, L.J. Molecular Signatures of Radiation Response in Breast Cancer: Towards Personalized Decision-Making in Radiation Treatment. Int. J. Breast Cancer 2017, 2017, 4279724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costard, L.S.; Hosn, R.R.; Ramanayake, H.; O’Brien, F.J.; Curtin, C.M. Influences of the 3D microenvironment on cancer cell behaviour and treatment responsiveness: A recent update on lung, breast and prostate cancer models. Acta Biomater. 2021, 132, 360–378. [Google Scholar] [CrossRef]
- Krasny, L.; Shimony, N.; Tzukert, K.; Gorodetsky, R.; Lecht, S.; Nettelbeck, D.M.; Haviv, Y.S. An in-vitro tumour microenvironment model using adhesion to type I collagen reveals Akt-dependent radiation resistance in renal cancer cells. Nephrol. Dial. Transpl. 2010, 25, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordes, N.; Blaese, M.A.; Plasswilm, L.; Rodemann, H.P.; Van Beuningen, D. Fibronectin and laminin increase resistance to ionizing radiation and the cytotoxic drug Ukrain in human tumour and normal cells in vitro. Int. J. Radiat. Biol. 2003, 79, 709–720. [Google Scholar] [CrossRef]
- Cordes, N.; Meineke, V. Cell Adhesion-Mediated Radioresistance (CAM-RR) Extracellular Matrix-Dependent Improvement of Cell Survival in Human Tumor and Normal Cells in Vitro. Strahlenther. Onkol. 2003, 179, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Krisnawan, V.E.; Stanley, J.A.; Schwarz, J.K.; DeNardo, D.G. Tumor Microenvironment as a Regulator of Radiation Therapy: New Insights into Stromal-Mediated Radioresistance. Cancers 2020, 12, 2916. [Google Scholar] [CrossRef]
- Babel, L.; Grunewald, M.; Lehn, R.; Langhans, M.; Meckel, T. Direct evidence for cell adhesion-mediated radioresistance (CAM-RR) on the level of individual integrin β1 clusters. Sci. Rep. 2017, 7, 3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, K.M.; Zhang, H.; Park, C.C. NF-κB regulates radioresistance mediated by β1-integrin in three-dimensional culture of breast cancer cells. Cancer Res. 2013, 73, 3737–3748. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.M.; Onodera, Y.; Bissell, M.J.; Park, C.C. Breast cancer cells in three-dimensional culture display an enhanced radioresponse after coordinate targeting of integrin alpha5beta1 and fibronectin. Cancer Res. 2010, 70, 5238–5248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, T.; Zhou, R.; Zhao, Y.; Wu, G. Integrin α6/Akt/Erk signaling is essential for human breast cancer resistance to radiotherapy. Sci. Rep. 2016, 6, 33376. [Google Scholar] [CrossRef] [PubMed]
- Manda, K.; Juerß, D.; Fischer, P.; Schröder, A.; Koenen, A.; Hildebrandt, G. Simvastatin treatment varies the radiation response of human breast cells in 2D or 3D culture. Investig. New Drugs 2016, 6, 33376. [Google Scholar] [CrossRef]
- Gomez-Roman, N.; Chong, M.Y.; Chahal, S.K.; Caragher, S.P.; Jackson, M.R.; Stevenson, K.H.; Dongre, S.A.; Chalmers, A.J. Radiation Responses of 2D and 3D Glioblastoma Cells: A Novel, 3D-specific Radioprotective Role of VEGF/Akt Signaling through Functional Activation of NHEJ. Mol. Cancer Ther. 2020, 19, 575–589. [Google Scholar] [CrossRef]
- Jiguet Jiglaire, C.; Baeza-Kallee, N.; Denicolaï, E.; Barets, D.; Metellus, P.; Padovani, L.; Chinot, O.; Figarella-Branger, D.; Fernandez, C. Ex vivo cultures of glioblastoma in three-dimensional hydrogel maintain the original tumor growth behavior and are suitable for preclinical drug and radiation sensitivity screening. Exp. Cell Res. 2014, 321, 99–108. [Google Scholar] [CrossRef]
- Simon, K.A.; Mosadegh, B.; Minn, K.T.; Lockett, M.R.; Mohammady, M.R.; Boucher, D.M.; Hall, A.B.; Hillier, S.M.; Udagawa, T.; Eustace, B.K.; et al. Metabolic response of lung cancer cells to radiation in a paper-based 3D cell culture system. Biomaterials 2016, 95, 47–59. [Google Scholar] [CrossRef] [Green Version]
- El-Ashmawy, M.; Coquelin, M.; Luitel, K.; Batten, K.; Shay, J.W. Organotypic culture in three dimensions prevents radiation-induced transformation in human lung epithelial cells. Sci. Rep. 2016, 6, 31669. [Google Scholar] [CrossRef]
- Bodgi, L.; Bahmad, H.F.; Araji, T.; Al Choboq, J.; Bou-Gharios, J.; Cheaito, K.; Zeidan, Y.H.; Eid, T.; Geara, F.; Abou-Kheir, W. Assessing Radiosensitivity of Bladder Cancer in vitro: A 2D vs. 3D Approach. Front. Oncol. 2019, 9, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamdi, D.H.; Barbieri, S.; Chevalier, F.; Groetz, J.-E.; Legendre, F.; Demoor, M.; Galera, P.; Lefaix, J.-L.; Saintigny, Y. In vitro engineering of human 3D chondrosarcoma: A preclinical model relevant for investigations of radiation quality impact. BMC Cancer 2015, 15, 579. [Google Scholar] [CrossRef] [Green Version]
- Vincent-Chong, V.K.; Seshadri, M. Development and Radiation Response Assessment in A Novel Syngeneic Mouse Model of Tongue Cancer: 2D Culture, 3D Organoids and Orthotopic Allografts. Cancers 2020, 12, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Rose, B.; Lee, C.S.; Hong, A.M. In vitro 3-dimensional tumor model for radiosensitivity of HPV positive OSCC cell lines. Cancer Biol. 2015, 16, 1231–1240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivascu, A.; Kubbies, M. Rapid generation of single-tumor spheroids for high-throughput cell function and toxicity analysis. J. Biomol. Screen 2006, 11, 922–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Feky, S.E.; Ghany Megahed, M.A.; Abd El Moneim, N.A.; Zaher, E.R.; Khamis, S.A.; Ali, L.M.A. Cytotoxic, chemosensitizing and radiosensitizing effects of curcumin based on thioredoxin system inhibition in breast cancer cells: 2D vs. 3D cell culture system. Exp. Ther. Med. 2021, 21, 506. [Google Scholar] [CrossRef]
- Xue, G.; Ren, Z.; Grabham, P.W.; Chen, Y.; Zhu, J.; Du, Y.; Pan, D.; Li, X.; Hu, B. Reprogramming mediated radio-resistance of 3D-grown cancer cells. J. Radiat. Res. 2015, 56, 656–662. [Google Scholar] [CrossRef] [Green Version]
- Sowa, M.B.; Chrisler, W.B.; Zens, K.D.; Ashjian, E.J.; Opresko, L.K. Three-dimensional culture conditions lead to decreased radiation induced cytotoxicity in human mammary epithelial cells. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2010, 687, 78–83. [Google Scholar] [CrossRef]
- Dubois, C.; Martin, F.; Hassel, C.; Magnier, F.; Daumar, P.; Aubel, C.; Guerder, S.; Mounetou, E.; Penault-Lorca, F.; Bamdad, M. Low-Dose and Long-Term Olaparib Treatment Sensitizes MDA-MB-231 and SUM1315 Triple-Negative Breast Cancers Spheroids to Fractioned Radiotherapy. J. Clin. Med. 2020, 9, 64. [Google Scholar] [CrossRef] [Green Version]
- Debeb, B.G.; Xu, W.; Mok, H.; Li, L.; Robertson, F.; Ueno, N.T.; Reuben, J.; Lucci, A.; Cristofanilli, M.; Woodward, W.A. Differential radiosensitizing effect of valproic acid in differentiation versus self-renewal promoting culture conditions. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 889–895. [Google Scholar] [CrossRef] [Green Version]
- Anastasov, N.; Höfig, I.; Radulović, V.; Ströbel, S.; Salomon, M.; Lichtenberg, J.; Rothenaigner, I.; Hadian, K.; Kelm, J.M.; Thirion, C.; et al. A 3D-microtissue-based phenotypic screening of radiation resistant tumor cells with synchronized chemotherapeutic treatment. BMC Cancer 2015, 15, 466. [Google Scholar] [CrossRef] [Green Version]
- Falkenberg, N.; Höfig, I.; Rosemann, M.; Szumielewski, J.; Richter, S.; Schorpp, K.; Hadian, K.; Aubele, M.; Atkinson, M.J.; Anastasov, N. Three-dimensional microtissues essentially contribute to preclinical validations of therapeutic targets in breast cancer. Cancer Med. 2016, 5, 703–710. [Google Scholar] [CrossRef] [Green Version]
- Igaz, N.; Szőke, K.; Kovács, D.; Buhala, A.; Varga, Z.; Bélteky, P.; Rázga, Z.; Tiszlavicz, L.; Vizler, C.; Hideghéty, K.; et al. Synergistic Radiosensitization by Gold Nanoparticles and the Histone Deacetylase Inhibitor SAHA in 2D and 3D Cancer Cell Cultures. Nanomaterials 2020, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Sethi, P.; Jyoti, A.; Swindell, E.P.; Chan, R.; Langner, U.W.; Feddock, J.M.; Nagarajan, R.; O’Halloran, T.V.; Upreti, M. 3D tumor tissue analogs and their orthotopic implants for understanding tumor-targeting of microenvironment-responsive nanosized chemotherapy and radiation. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 2013–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upreti, M.; Jamshidi-Parsian, A.; Koonce, N.A.; Webber, J.S.; Sharma, S.K.; Asea, A.A.; Mader, M.J.; Griffin, R.J. Tumor-Endothelial Cell Three-dimensional Spheroids: New Aspects to Enhance Radiation and Drug Therapeutics. Transl. Oncol. 2011, 4, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Plava, J.; Cihova, M.; Burikova, M.; Matuskova, M.; Kucerova, L.; Miklikova, S. Recent advances in understanding tumor stroma-mediated chemoresistance in breast cancer. Mol. Cancer 2019, 18, 67. [Google Scholar] [CrossRef] [PubMed]
- Boelens, M.C.; Wu, T.J.; Nabet, B.Y.; Xu, B.; Qiu, Y.; Yoon, T.; Azzam, D.J.; Twyman-Saint Victor, C.; Wiemann, B.Z.; Ishwaran, H.; et al. Exosome Transfer from Stromal to Breast Cancer Cells Regulates Therapy Resistance Pathways. Cell 2014, 159, 499–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan Harati, M.; Rodemann, H.P.; Toulany, M. Nanog Signaling Mediates Radioresistance in ALDH-Positive Breast Cancer Cells. Int. J. Mol. Sci. 2019, 20, 1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sedelnikova, O.A.; Nakamura, A.; Kovalchuk, O.; Koturbash, I.; Mitchell, S.A.; Marino, S.A.; Brenner, D.J.; Bonner, W.M. DNA Double-Strand Breaks Form in Bystander Cells after Microbeam Irradiation of Three-dimensional Human Tissue Models. Cancer Res. 2007, 67, 4295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acheva, A.; Ghita, M.; Patel, G.; Prise, K.M.; Schettino, G. Mechanisms of DNA Damage Response to Targeted Irradiation in Organotypic 3D Skin Cultures. PLoS ONE 2014, 9, e86092. [Google Scholar] [CrossRef] [Green Version]
- Park, C.C.; Zhang, H.; Pallavicini, M.; Gray, J.W.; Baehner, F.; Park, C.J.; Bissell, M.J. Beta1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth, and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 2006, 66, 1526–1535. [Google Scholar] [CrossRef] [Green Version]
- Heldin, C.-H.; Rubin, K.; Pietras, K.; Östman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef]
- Nathanson, S.D.; Nelson, L. Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann. Surg. Oncol. 1994, 1, 333–338. [Google Scholar] [CrossRef]
- Milosevic, M.; Fyles, A.; Hedley, D.; Pintilie, M.; Levin, W.; Manchul, L.; Hill, R. Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor oxygen measurements. Cancer Res. 2001, 61, 6400–6405. [Google Scholar]
- Roh, H.D.; Boucher, Y.; Kalnicki, S.; Buchsbaum, R.; Bloomer, W.D.; Jain, R.K. Interstitial Hypertension in Carcinoma of Uterine Cervix in Patients: Possible Correlation with Tumor Oxygenation and Radiation Response. Cancer Res. 1991, 51, 6695–6698. [Google Scholar] [PubMed]
- Qin, X.; Li, J.; Sun, J.; Liu, L.; Chen, D.; Liu, Y. Low shear stress induces ERK nuclear localization and YAP activation to control the proliferation of breast cancer cells. Biochem. Biophys. Res. Commun. 2019, 510, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Triantafillu, U.L.; Park, S.; Klaassen, N.L.; Raddatz, A.D.; Kim, Y. Fluid shear stress induces cancer stem cell-like phenotype in MCF7 breast cancer cell line without inducing epithelial to mesenchymal transition. Int. J. Oncol. 2017, 50, 993–1001. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Diaz, M.F.; Price, K.M.; Ozuna, J.A.; Zhang, S.; Sevick-Muraca, E.M.; Hagan, J.P.; Wenzel, P.L. Fluid shear stress activates YAP1 to promote cancer cell motility. Nat. Commun. 2017, 8, 14122. [Google Scholar] [CrossRef] [Green Version]
- Triantafillu, U.L.; Park, S.; Kim, Y. Fluid Shear Stress Induces Drug Resistance to Doxorubicin and Paclitaxel in the Breast Cancer Cell Line MCF7. Adv. Ther. 2019, 2, 1800112. [Google Scholar] [CrossRef]
- Marshall, L.E.; Goliwas, K.F.; Miller, L.M.; Penman, A.D.; Frost, A.R.; Berry, J.L. Flow-perfusion bioreactor system for engineered breast cancer surrogates to be used in preclinical testing. J. Tissue Eng. Regen. Med. 2017, 11, 1242–1250. [Google Scholar] [CrossRef]
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous Chemotaxis as a Mechanism of Tumor Cell Homing to Lymphatics via Interstitial Flow and Autocrine CCR7 Signaling. Cancer Cell 2007, 11, 526–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, C.M.; Horst, E.N.; Taylor, C.C.; Liu, C.Z.; Mehta, G. Fluid shear stress stimulates breast cancer cells to display invasive and chemoresistant phenotypes while upregulating PLAU in a 3D bioreactor. Biotechnol. Bioeng. 2019, 116, 3084–3097. [Google Scholar] [CrossRef] [PubMed]
- Azimi, T.; Loizidou, M.; Dwek, M.V. Cancer cells grown in 3D under fluid flow exhibit an aggressive phenotype and reduced responsiveness to the anti-cancer treatment doxorubicin. Sci. Rep. 2020, 10, 12020. [Google Scholar] [CrossRef] [PubMed]
- Polacheck, W.J.; German, A.E.; Mammoto, A.; Ingber, D.E.; Kamm, R.D. Mechanotransduction of fluid stresses governs 3D cell migration. Proc. Natl. Acad. Sci. USA 2014, 111, 2447–2452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, C.-W.; Wu, C.-C.; Ch’ang, H.-J. Radiation sensitization of tumor cells induced by shear stress: The roles of integrins and FAK. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2014, 1843, 2129–2137. [Google Scholar] [CrossRef] [Green Version]
- Eke, I.; Hehlgans, S.; Sandfort, V.; Cordes, N. 3D matrix-based cell cultures: Automated analysis of tumor cell survival and proliferation. Int. J. Oncol. 2016, 48, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.W.; Lee, S.-Y.; Park, L.; Kang, M.-S.; Kim, M.-H.; Doh, I.; Ryu, G.H.; Nam, D.-H. High-Throughput Clonogenic Analysis of 3D-Cultured Patient-Derived Cells with a Micropillar and Microwell Chip. SLAS Discov. Adv. Sci. Drug Discov. 2017, 22, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pouliliou, S.; Koukourakis, M.I. Gamma histone 2AX (γ-H2AX)as a predictive tool in radiation oncology. Biomarkers 2014, 19, 167–180. [Google Scholar] [CrossRef]
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Ríos-Arrabal, S.; Artacho-Cordón, F.; Torralbo, A.I.; López-Ruiz, E.; Marchal, J.A.; Núñez, M.I. Radiation and Stemness Phenotype May Influence Individual Breast Cancer Outcomes: The Crucial Role of MMPs and Microenvironment. Cancers 2019, 11, 1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andarawewa, K.L.; Costes, S.V.; Fernandez-Garcia, I.; Chou, W.S.; Ravani, S.A.; Park, H.; Barcellos-Hoff, M.H. Lack of radiation dose or quality dependence of epithelial-to-mesenchymal transition (EMT) mediated by transforming growth factor β. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Blondel, D.; Lutolf, M.P. Bioinspired Hydrogels for 3D Organoid Culture. Chimia 2019, 73, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Li, W. 3D scaffolds in breast cancer research. Biomaterials 2016, 81, 135–156. [Google Scholar] [CrossRef]
- Li, Y.; Kumacheva, E. Hydrogel microenvironments for cancer spheroid growth and drug screening. Sci. Adv. 2018, 4, eaas8998. [Google Scholar] [CrossRef] [Green Version]
- Fisher, M.F.; Rao, S.S. Three-dimensional culture models to study drug resistance in breast cancer. Biotechnol. Bioeng. 2020, 117, 2262–2278. [Google Scholar] [CrossRef]
- Desrosiers, M.; DeWerd, L.; Deye, J.; Lindsay, P.; Murphy, M.K.; Mitch, M.; Macchiarini, F.; Stojadinovic, S.; Stone, H. The Importance of Dosimetry Standardization in Radiobiology. J. Res. Natl. Inst. Stand. Technol. 2013, 118, 403–418. [Google Scholar] [CrossRef]
- Kennedy, R.; Kuvshinov, D.; Sdrolia, A.; Kuvshinova, E.; Hilton, K.; Crank, S.; Beavis, A.W.; Green, V.; Greenman, J. A patient tumour-on-a-chip system for personalised investigation of radiotherapy based treatment regimens. Sci. Rep. 2019, 9, 6327. [Google Scholar] [CrossRef] [Green Version]
- Lacombe, J.; Phillips, S.L.; Zenhausern, F. Microfluidics as a new tool in radiation biology. Cancer Lett. 2016, 371, 292–300. [Google Scholar] [CrossRef] [Green Version]
- Quan, Y.; Sun, M.; Tan, Z.; Eijkel, J.C.T.; van den Berg, A.; van der Meer, A.; Xie, Y. Organ-on-a-chip: The next generation platform for risk assessment of radiobiology. RSC Adv. 2020, 10, 39521–39530. [Google Scholar] [CrossRef]
- Bavoux, M.; Kamio, Y.; Vigneux-Foley, E.; Lafontaine, J.; Najyb, O.; Refet-Mollof, E.; Carrier, J.F.; Gervais, T.; Wong, P. X-ray on chip: Quantifying therapeutic synergies between radiotherapy and anticancer drugs using soft tissue sarcoma tumor spheroids. Radiother. Oncol. 2021, 157, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Osei, B.; Osei, E. A review of radiation genomics: Integrating patient radiation response with genomics for personalised and targeted radiation therapy. J. Radiother. Pract. 2019, 18, 198–209. [Google Scholar] [CrossRef]
- Scott, J.G.; Sedor, G.; Ellsworth, P.; Scarborough, J.A.; Ahmed, K.A.; Oliver, D.E.; Eschrich, S.A.; Kattan, M.W.; Torres-Roca, J.F. Pan-cancer prediction of radiotherapy benefit using genomic-adjusted radiation dose (GARD): A cohort-based pooled analysis. Lancet Oncol. 2021, 22, 1221–1229. [Google Scholar] [CrossRef]
- Story, M.D.; Durante, M. Radiogenomics. Med. Phys. 2018, 45, e1111–e1122. [Google Scholar] [CrossRef]
- Chong, Z.X.; Yeap, S.K.; Ho, W.Y. Role of miRNAs in regulating responses to radiotherapy in human breast cancer. Int. J. Radiat. Biol. 2021, 97, 289–301. [Google Scholar] [CrossRef]
- Aristei, C.; Perrucci, E.; Alì, E.; Marazzi, F.; Masiello, V.; Saldi, S.; Ingrosso, G. Personalization in Modern Radiation Oncology: Methods, Results and Pitfalls. Pers. Interv. Breast Cancer 2021, 11, 616042. [Google Scholar] [CrossRef]
- Palumbo, E.; Piotto, C.; Calura, E.; Fasanaro, E.; Groff, E.; Busato, F.; El Khouzai, B.; Rigo, M.; Baggio, L.; Romualdi, C.; et al. Individual Radiosensitivity in Oncological Patients: Linking Adverse Normal Tissue Reactions and Genetic Features. Front. Oncol. 2019, 9, 987. [Google Scholar] [CrossRef]
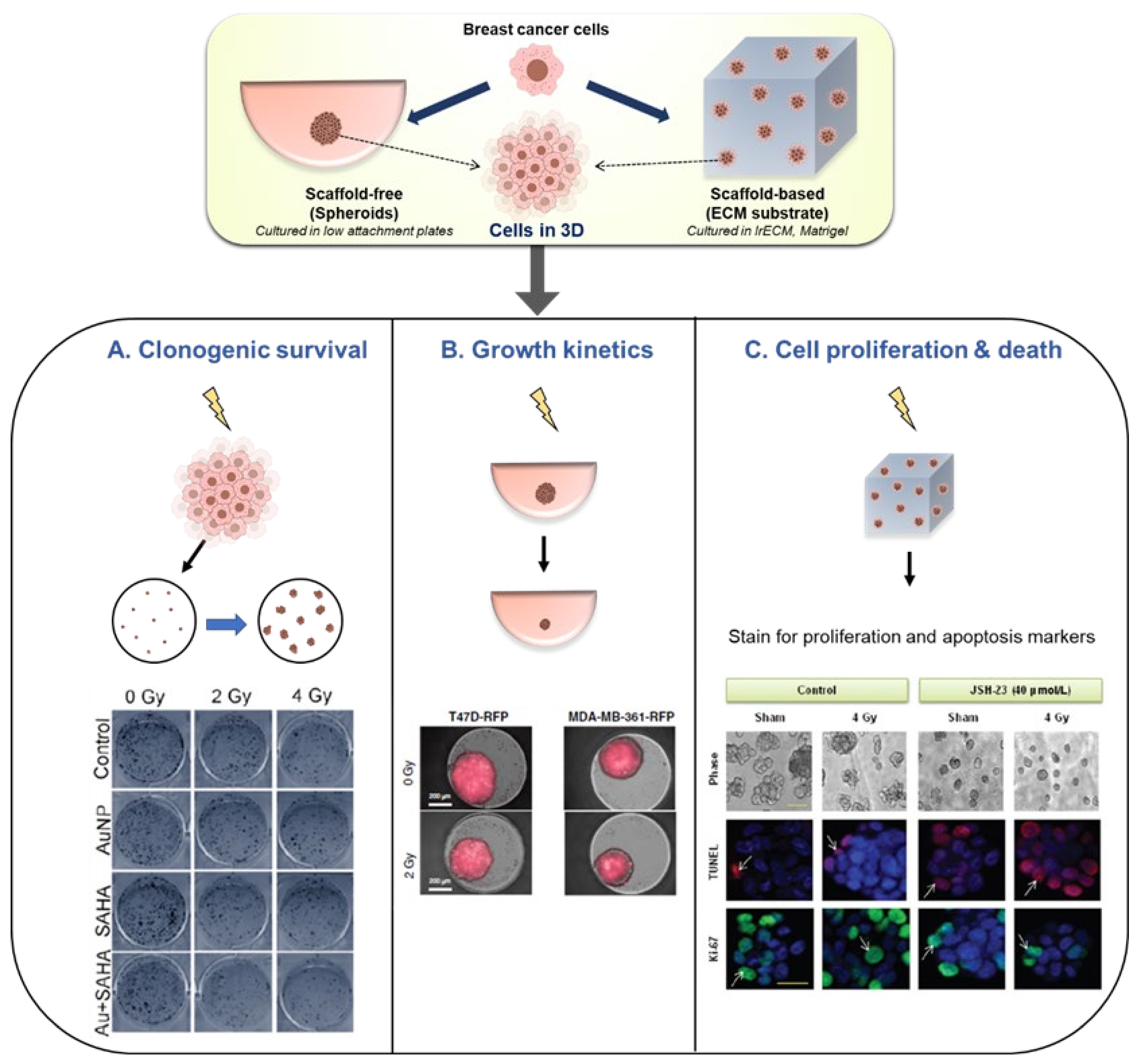
| S No | Cells Matrix (Substrate-Based) | Radiation Dosage | Dose Rate | Observations (Radiation Effects) | Methods of Analysis | Ref. |
|---|---|---|---|---|---|---|
| Irradiator | ||||||
| Spheroid-Based Models | ||||||
| 1 | MDA-MB-231, SUM1315 | 2 Gy x 5 | 400 (MU/min) | Radiosensitizer: Olaparib in low doses with longer exposure team enhances radiosensitivity | Metabolic activity, live/dead analysis | [105] |
| Varian Medical Systems | ||||||
| 2 | MCF-7, primary human breast cancer | 2, 4, 6 Gy | Radiosensitizer: Valproic acid radiosensitizes in 2D and radioprotects in 3D | GelCount Colony Counter | [106] | |
| Cs-137 irradiator | ||||||
| 3 | GFP-4T1 + 2H11 murine endothelial cells | 2 Gy | Co-culture: Presence of endothelial cells sensitized cells to chemotherapy and protected tumor cells from irradiation | 2D replating and survival assay | [111] | |
| Not stated | ||||||
| 4 | SUM159PT, MDA-MB-231 | 4, 8 Gy | 2.789 Gy/min | Radiosensitizer: Mebendazole inhibited IR-induced conversion of TNBC cells into cancer-initiating phenotype | Mammospheres count | [46] |
| X-ray irradiator Gulmay Medical Inc | ||||||
| 5 | T47D, HTB-133, MDA-MB-361, MDA-MB-231 + primary normal human dermal fibroblasts | 2, 4, 6, 8 Gy | 0.5 Gy/min | Radiosensitizer and co-culture: Vinblastine and radiation inhibited cancer cell growth | Image-based analysis of tissue area | [107] |
| Cs-137 irradiator | ||||||
| 6 | T47D, JIMT-1 | 5 Gy | 0.95 Gy/minute | Radiosensitizer: Trastuzumab and radiation inhibited growth in 3D | Image-based analysis of tissue area | [108] |
| Cs-137 irradiator | ||||||
| 7 | 4T1-mCherry tumor cells, C166-GFP endothelial cells, murine embryonic fibroblasts | 3 Gy | 1.018 ± 0.10 Gy/min | Radiation enhances expression of Galectin-1 in endothelial cells that is targeted using nanoparticles carrying arsenic trioxide and cisplatin | Dead cell staining (Sytox blue) | [110] |
| Varian TrueBeam System | ||||||
| 8 | MCF-7 | 2, 4, 6, 8 Gy | 200 MU/min | Increased radiosensitivity in 3D compared to 2D Curcumin enhances radiosensitivity of cancer cells | MTT assay, RT-PCR, ELISA | [102] |
| PRIMUSTM linear accelerator | ||||||
| 9 | A549 lung adenocarcinoma, DU-145 and PC-3 prostate cancer and MCF-7 breast cancer | 0, 2, 4 Gy | Radiosensitizer: Gold nanoparticle, Vorinostat and radiation reduces colony forming ability of cells and enhanced DNA damage | 2D replating and clonogenic survival assay, γH2AX staining | [109] | |
| Primus linear accelerator | ||||||
| Substrate-based Models | ||||||
| 10 | MCF7, MDA-MB-231, SK-BR-3 Matrigel | 2, 6 Gy | Gene expression of CSC depended on radiation dose Radiation had differing effects on expression of MMP, TIMP and HDAc | RT-PCR | [136] | |
| Yxlon Smart Maxishot 200-E | ||||||
| 11 | T4-2 (malignant) GFR BME (laminin-rich ECM) | 2, 4 Gy | IR caused upregulation of integrin leading to increased cell survival Inhibition of integrin induced apoptosis | Immunoblotting, immunofluorescence, TUNEL assay | [90] | |
| Not stated | ||||||
| 12 | 184A1 human MamECs Matrigel | 0.5, 1, 2.5, 5 Gy | 0.16–0.58 Gy/min | Reduced apoptosis in 3D compared to 2D Increased survival in long-term 3D cultures because of growth inhibition in 3D | Trypsinize and count, flow cytometry | [104] |
| Pantak XRAD 320 Cabinet X-ray machine | ||||||
| 13 | MCF10a, 184v human MamECs Matrigel | 0.4–2 Gy | X-ray: 4 Gy/min, 0.03 Gy/min γ: 0.03–2 Gy/min, 56Fe: 0.2–1 Gy/min | E-cadherin was reduced in TGF-β–treated cells irradiated with radiation TGF-β–mediated EMT is not dependent on radiation dose or quality | Immunofluorescence (cryosections) | [137] |
| X-rays: Varian 2300 linear accelerator | ||||||
| 14 | T4-2 (malignant), S1 (non-malignant) GFR BME (laminin-rich ECM) | 0–8 Gy | Integrin induced by exposure to radiation through NFkB–mediated gene activation in 3D | Western blot, RT-PCR, NF-kB DNA-binding assay, immunofluorescence, TUNEL assay | [89] | |
| Not stated | ||||||
| 15 | A549 adenocarcinoma, MCF7, PC3 prostate cancer Matrigel | 0–4 Gy | 0.751 Gy/min | 3D cultures have increased radioresistance | 2D replating and survival assay | [103] |
| Faxitron RX-650 facility | ||||||
| 16 | Py8119, NIH-3T3 Matrigel | 3, 6, 9 Gy | 3 Gy/min | Co-culture: Presence of fibroblasts increased the survival fraction of irradiated cultures | Survival assay and fluorescence | [49] |
| Isovolt-320-X-ray machine | ||||||
| 17 | MCF10A, MCF7 Matrigel | 0.5, 2, 4, 6 Gy | 3.75 Gy/min | Radiosensitizer: Simvastatin tends to radiosensitize in 2D and not in 3D | 3D clonogenic survival assay | [92] |
| Linac Siemens Oncor Expression | ||||||
| Not stated | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravichandran, A.; Clegg, J.; Adams, M.N.; Hampson, M.; Fielding, A.; Bray, L.J. 3D Breast Tumor Models for Radiobiology Applications. Cancers 2021, 13, 5714. https://doi.org/10.3390/cancers13225714
Ravichandran A, Clegg J, Adams MN, Hampson M, Fielding A, Bray LJ. 3D Breast Tumor Models for Radiobiology Applications. Cancers. 2021; 13(22):5714. https://doi.org/10.3390/cancers13225714
Chicago/Turabian StyleRavichandran, Akhilandeshwari, Julien Clegg, Mark N. Adams, Madison Hampson, Andrew Fielding, and Laura J. Bray. 2021. "3D Breast Tumor Models for Radiobiology Applications" Cancers 13, no. 22: 5714. https://doi.org/10.3390/cancers13225714






