1. Introduction
Cachexia is a syndrome associated with several neoplastic diseases. Cancer cachexia is characterized by the progressive loss of lean body mass, with or without the loss of adipose tissue that can be partially but not totally reversed by diet support [
1], and the skeletal muscle is the main tissue of protein loss [
2]. Cachexia contributes to the reduction in functional capacity and quality of life [
2], and these comorbidities account for approximately 20% of cancer deaths [
3].
Cancer cachexia, one of the main manifestations of the damage that neoplastic diseases cause, causes muscle atrophy, which is the result of an imbalance between protein synthesis and degradation in skeletal muscle [
3]. The reasons for cancer-reduced skeletal muscle mass, cellular mechanism and function are still poorly understood. Additionally, one of the possible mechanisms is through an altered expression of microRNAs in skeletal muscle [
4]. MicroRNAs are small non-coding RNAs that negatively regulate their target mRNAs by either promoting messenger decay or by dampening translation [
5]. MyomiRs are a set of microRNAs exclusively expressed in skeletal and cardiac muscle, that have been identified as physiological regulators of myogenesis, muscle fiber-type composition, muscle growth and cellular homeostasis [
6]. In pathological processes, myomiRs also influence myogenesis and muscle damage repair preventing skeletal muscle injuries and dysfunction [
7].
Accumulating evidence shows that the expression of microRNAs in skeletal muscle modifies cancer cachexia in experimental animals and humans [
8]. The microRNAs-147-3p, -299a-3p, 1933-3p, 511-3p, 3473d, 233-3p, 431-5p, 665-3p and 205-3p were found to be differently expressed in skeletal muscle in mice with lung cancer [
8]. These microRNAs are related to cell survival pathways, inflammatory response, cell cycle, cell development and cell morphology [
8]. Narasimhan et al. [
9] analyzed small RNAome profiling from skeletal muscle of the cachectic and non-cachectic patients with pancreas or colon cancer. The expression of the microRNAs-let-7d-3p, -3184-3p and -1296-5p were increased in the cachectic patients. These microRNAs were selected for in silico analysis, and target prediction and putative functional annotation revealed targets genes related to adipogenesis, myogenesis, inflammation and immune response [
9]. When considered together, these findings highlight that microRNAs are responsible for the development and maintenance of skeletal muscle mass [
6,
9]. It has been reported that skeletal muscle injury provokes microRNA release from the muscle cells to the plasma. This response suggests circulating microRNAs as biomarkers of physiological or pathological conditions [
10]. Furthermore, extracellular microRNAs can have a direct biologic influence in other tissues. For example, microRNA-206, which is a specific skeletal muscle microRNA, was found markedly decreased in estrogen receptors (ER)α positive human breast cancer tissue, and antagomiR-206 treatment inhibits tumor growth in estrogen-dependent breast cancer [
11].
Evidence shows that exercise training provokes remarkable adaptations in skeletal muscle in healthy and pathological processes. One of the most marked effects is recovering the muscle protein balance [
12,
13] that preserves the myofibrillar and sarcoplasmic proteins probably mediated by intracellular mechanisms involving microRNAs’ regulation. Aerobic exercise training (AET) plays an important role in regulating the expression of the microRNAs in skeletal muscle and circulation. AET has therapeutic potential modulating the expression of several altered microRNAs in chronic diseases [
14,
15,
16]. However, whether there is a change in myomiR’s profile in the cancer cachexia is still under-investigated, and the underlying molecular pathways remain to be elucidated.
There is no evidence about the effects of AET on the expression of myomiRs in the cachectic and non-cachectic state associated with cancer. Therefore, our goals are to investigate the profile of myomiRs expressed in CT26 colon cancer cachectic and MMTV-PyMT mammary cancer non-cachectic mice. In addition, to investigate the effects of AET on the myomiRs and skeletal muscle phenotype and function, as well as evaluate whether cancer cachexia and AET are modulating the expression of circulating myomiRs.
2. Materials and Methods
2.1. Sampling
Balb/c female mice that represent a cachectic model were injected with 1 × 10
6 of CT26 colon cancer cells (ATCC
® CRL-2638™, Manassas, VA, USA). These mice were randomly assigned in colon cancer sedentary (CT26;
n = 11) and colon cancer trained (CT26 + TR;
n = 8) groups. A control group was also assigned in wild type sedentary (WT;
n = 12) and wild type trained (WT+TR;
n = 5) groups. MMTV (Mouse Mammary Tumor Virus Polyoma Middle T antigen; MMTV-PyMT) c57 female mice that represent non-cachectic transgenic mice with spontaneous tumor appearance were also studied [
17]. They were randomly assigned in mammary cancer sedentary (MMTV;
n = 8) and mammary cancer trained (MMTV+TR;
n = 8) groups. A control group was assigned in wild type sedentary (WT;
n = 7) and wild type trained (WT+TR;
n = 6) groups. The mice were provided from the Medical School of the University of Sao Paulo animal facility. The animals were housed 5 per cage at controlled room temperature (22 °C) with a 12-h dark–light cycle and were fed standard mouse chow and had access to water ad libitum.
2.2. Training Protocol
The training protocol was performed on a treadmill according to the protocol developed by Ferreira et al. [
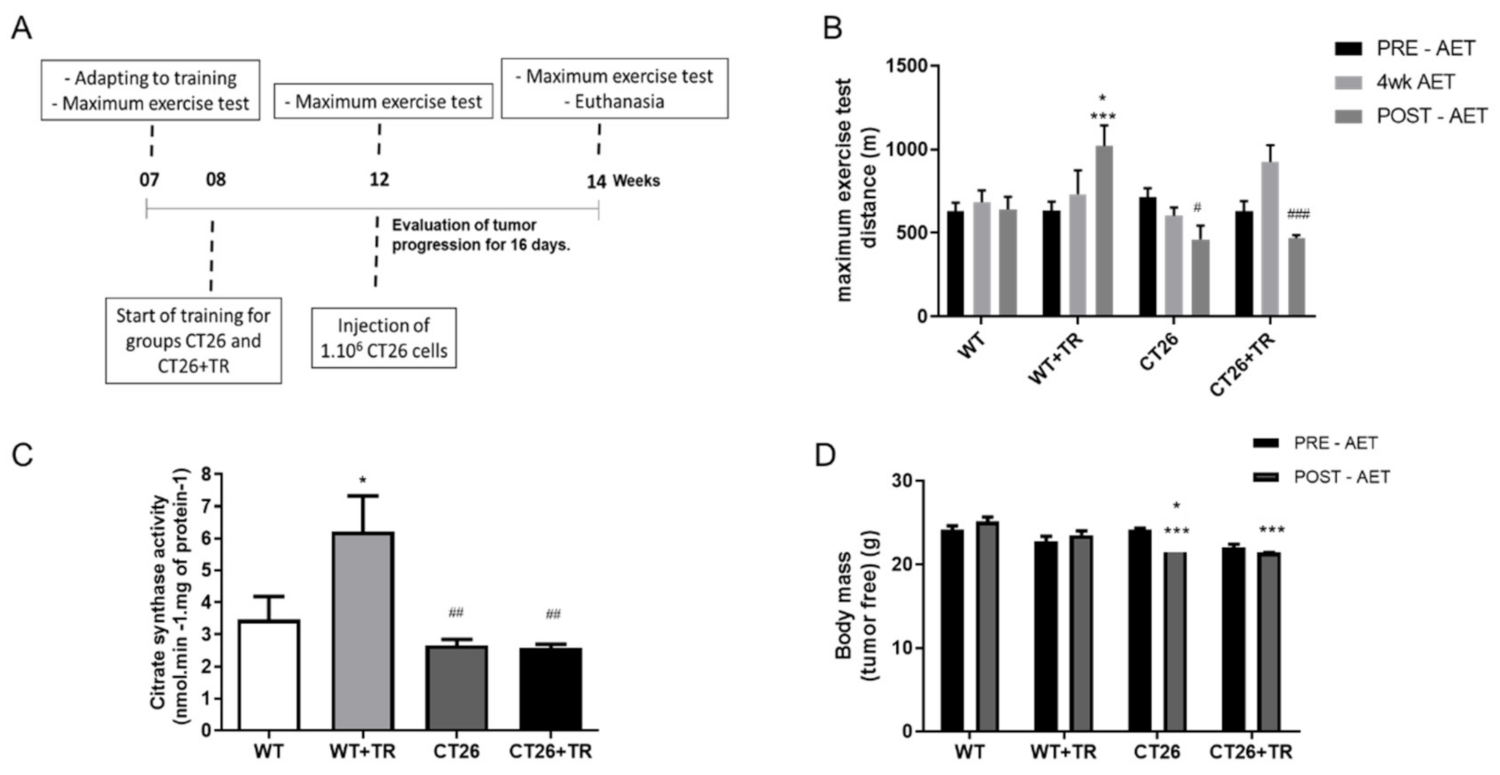
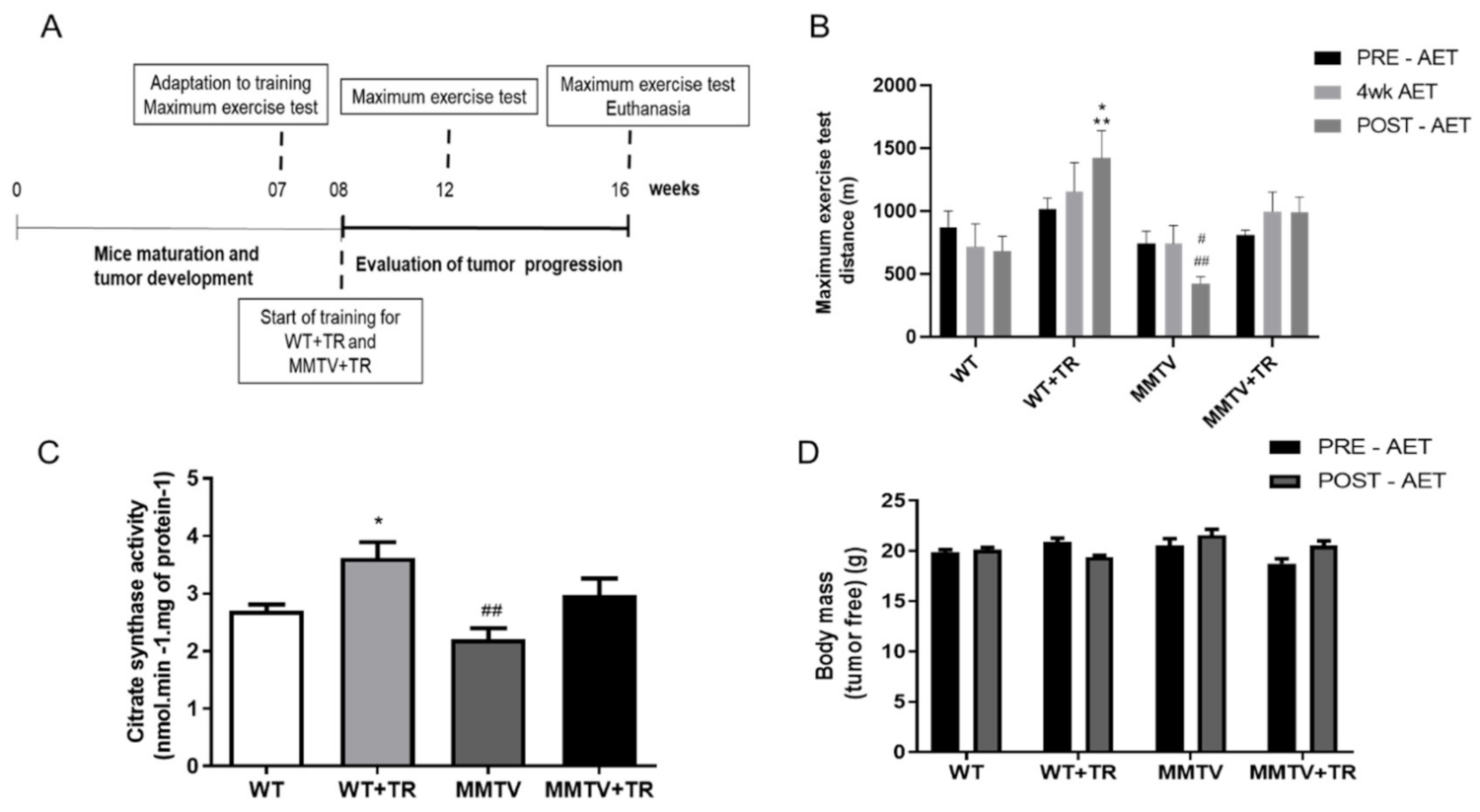
18]. Mice were trained 5 days a week for 60-min duration. The exercise intensity was set at 60% of the maximal speed achieved in the maximal exercise test. The CT26+TR groups and the WT+TR were 8 weeks old in the beginning of the training protocol and continued training until they were 14 weeks old. CT26 tumor cells were injected at 12 weeks old. Therefore, the CT26+TR mice remained training cancer-free for 4 weeks and 2 more weeks after tumor cell injection. The MMTV+TR and WT+TR groups were 8 weeks old at the beginning of the training protocol, and the exercise training lasted 8 weeks. Tumors spontaneously appeared in the MMTV and MMTV+TR mice between week 10 and week 13 of age.
2.3. Evaluation of the Maximal Physical Capacity
Exercise capacity was assessed by maximal distance running in the maximal exercise test. The exercise test protocol was conducted, as described by Ferreira et al. [
18]. Briefly, mice were placed on a treadmill, and speed started at 3 m/min and was increased by 3 m/min every 3 min until exhaustion.
2.4. Grip Strength Meter
The grip test was used to evaluate limb traction force and muscular function in the CT26 model. The mice were positioned in the equipment (New Primer, São Paulo, Brazil), suspended by the tail, and stimulated to draw the grid coupled to a dynamometer. Three measurements were performed, and the highest value was selected. The grip test was performed at the end of the training protocol.
2.5. Ambulation Test
The ambulation behavioral test was used for the analysis of skeletal muscle function in the MMTV-PyMT model. This test estimates the contractile force and motor coordination. Mice hind legs were placed in contact with non-toxic black paint and then placed inside a rectangular wooden box of 1 m (homemade and lined with white paper) for walking. Subsequently, the stride length was measured.
2.6. Tissue Preparation
After euthanasia, the soleus, tibialis anterior (TA), gastrocnemius and plantaris muscles and tibia bone were harvested and weighed. Muscle mass was assessed by the measurement of the ratio of tissue weight in grams to tibia length in millimeters (muscle weight g/mm).
2.7. Skeletal Muscle Oxidative Enzyme Activity
To evaluate citrate synthase activity, the gastrocnemius muscle was homogenized in 1 g tissue: 7 µL of extraction buffer containing 50 mM Tris-base, pH 7.4 plus 1.0 mM EDTA at 4 °C. The samples were centrifuged at 3000×
g for 15 min at 4 °C, and the supernatant was used to perform the enzymatic kinetics. Protein was measured using the method of Bradford et al. [
19], using bovine serum albumin as a standard. The maximal activity of the enzyme was determined according to the method of Alp and Newsholme [
20] from the quantification of the complex formed between coenzyme A with 5,5′dithiobis 2-nitrobenzoic acid (DTNB) added to the assay buffer, thus forming a yellow complex. The assay buffer was prepared with 100 mM Tris-base, 0.4 mM DTNB, 1.24 mM Acetyl-CoA, 1% (
v/
v) Triton X-100 and the homogenate. The reaction was initiated by the addition of 18.9 mM oxaloacetate to the reaction mixture. The enzymatic kinetics was recording and performed at 25 °C for an interval of 10 min, at 412 nm using Victor (Victor3 1420 Multilabel Counter from PerkinElmer, Waltham, MA, USA). The result of the enzyme activity was expressed in nmol·min
−1·mg protein
−1.
2.8. Analysis of MicroRNA
Frozen TA skeletal muscle samples were used for RNA extraction. Fifteen milligrams of the tissues were homogenized in Trizol (700 μL), and RNA was isolated according to the manufacturer’s instructions (#15596026 Thermo Fisher Scientific, Waltham, MA, USA). Serum RNA was extracted through the miRNeasy Serum/Plasma Kit (#217184 Qiagen, Germantown, MD, USA) following the manufacturer’s instructions. MicroRNAs expression was measured using real-time PCR and the TaqMan MicroRNA Assay (Applied Biosystems, Waltham, CA, USA) as specified by the manufacturer’s instructions. A detailed method was already described by Fernandes et al. [
21]. Briefly, real-time PCR reaction included TaqMan Universal PCR master mix II, nuclease-free water, RT product and primers TaqMan MicroRNA Assay for microRNA-486 (Thermo Fisher #2228, Waltham, MA, USA), microRNA-206 (Thermo Fisher #0510), microRNA-133a (Thermo Fisher #2246), microRNA-133b (Thermo Fisher #2247) and microRNA-1 (Thermo Fisher #2064). We also performed U6 snRNA (Thermo Fisher #001973) expression using the same method. The reactions were incubated in a 96-well optical plate. The microRNAs expression was normalized by subtraction of values of U6 expression. Relative quantities of target gene expression in WT vs. other groups were compared after normalization to the values of the reference gene (ΔCT). Fold changes in microRNA expression were calculated using the differences in ΔCT values between the samples (ΔΔCT) and the equation 2
−ΔΔCT. Results were expressed as % of control groups (WT).
2.9. Immunohistochemistry
TA skeletal muscle was harvested and embedded in Tissue-Tek® O.C.T, frozen in isopentane and then in liquid nitrogen. Immunohistochemistry was conducted to analyze the cross-section area; sections (10-micrometer thick) were obtained from TA muscle using a Leica-CM 1850 cryostat (Leica Microsystem, Welzlar, Germany). The muscle sections were fixed with 4% formalin (Sigma-Aldrich, HT501128, São Paulo, Brazil) for 10 min at room temperature, permeabilized in 0.2% Triton X-100 (Biorad, 01-0407, Hercules, CA, USA) and 1% bovine serum albumin (BSA; VWR Amresco Chemicals, E588, Radnor, PA, USA) diluted in phosphate buffer saline (PBS; Sigma-Aldrich, P4417, Saint Louis, MO, USA) for 10 min. Blocking was performed with 10% goat serum (Sigma-Aldrich, G9023, São Paulo, Brazil) in PBS for 45 min. Glass slides were incubated with a solution containing the primary antibody anti-laminin (1:100 dilutions, Thermo fisher, PA1-16730, USA) for delimiting muscle fibers, with 1.5% goat serum in PBS for 1 h 30 min at room temperature. After proper washing, the sections were incubated with a solution with a secondary fluorescent antibody (1:500 dilutions; Alexa Fluor 488 goat anti-rabbit, Life Technologies, A11008, Waltham, MA, USA). The images were captured with a 200× magnification and a 20× objective. The images were recorded on a computer connected to a fluorescent microscope and connected to a photographic system (magnification, 200×) (Leica Qwin, Leica Microsystems, Wetzlar, Germany). The quantification of the cross-sectional area for each fiber was evaluated using the program Image J software (Image J Corporation based in NIH image, Bethesda, MD, USA). The results were expressed in μm2.
2.10. Immunoblotting
The protein levels PTEN, PI3K, AKT, mTOR, FOXO3a and FOXO3a phosphorylated (pFOXO3a) in the TA muscle were analyzed using Western blotting. Samples were loaded to SDS-PAGE on polyacrylamide gels (6–15%). After electrophoresis, proteins were electro-transferred to a nitrocellulose membrane (BioRad Biosciences, Hercules, CA, USA). Equal loading of samples (30μg) and transfer efficiency were monitored with the use of 0.5% Ponceau S staining of the blot membrane. The blot membranes were incubated with polyclonal antibodies anti-AKT (#9272, Cell Signaling Tech, Danvers, MA, USA), anti-phospho-AKT-SER473 ([pAKT-SER473] #9271s, Cell Signaling Tech, Danvers, MA, USA), anti-PI3K (#ab32569, Abcam, Cambridge, UK), anti-PTEN (#9559, Cell Signaling Tech, Danvers, MA, USA), anti-mTOR (#2972, Cell Signaling Tech, Danvers, MA, USA), FOXO3a (#ab12162, Abcam, Cambridge, UK), FOXO3ap (#ab15478, Abcam, Cambridge, UK) and anti-GAPDH (ab37168, Abcam, Cambridge, UK). The bands were analyzed using Image J software (Image J Corporation based on NIH image, Bethesda, MD, USA). Skeletal muscle GAPDH expression levels were used to normalize the results, which are expressed as a percentage of control expression.
2.11. Statistical Analysis
The results are presented as mean ± SEM. Statistical analysis was performed using two-way ANOVA. To indicate how closely two variables, change in relationship to each other, Pearson’s correlation coefficient was used. Probability values of p ≤ 0.05 were accepted as statistically significant. The Tukey post hoc test (STATISTICA software; StatSoft, Tulsa, OK, USA) was used for individual comparisons between means when a significant change was observed with ANOVA.
4. Discussion
We showed that cancer induced a downregulation of microRNA-486 expression in the TA skeletal muscle and serum of CT26 and MMTV mice. These effects were not prevented by AET. Furthermore, cancer induced a downregulation of microRNA-206 expression in the serum of CT26 mice. Additionally, microRNA-206 was upregulated in the TA skeletal muscle, serum and tumor of the MMTV model, and these effects were prevented by AET. In addition, circulating myomiRs have been suggested as a prognosis cancer biomarker, thus the downregulation of miRNA-486 and -206 in colon cancer, as well as the downregulation miRNA-486 and upregulation of miRNA-206 in breast cancer might be good serum biomarkers to these two kinds of cancer. In addition, the serum miRNA-206 expression of CT26 and MMTV can be a good biomarker of the effects of AET preventing the effects of cancer, as well as of miRNA-206 in TA. According to our knowledge, this is the first study showing the characterization of the myomiRs’ expression in CT26 and MMTV-PyMT animal models submitted to an AET protocol.
The main findings observed in the CT26 model were microRNA-486 expression downregulation in TA skeletal muscle in CT26 and CT26+TR groups, increased protein PTEN levels decreasing the PI3K/Akt/mTOR pathway and AET not being effective in restoring the crucial protein synthesis pathway in skeletal muscle.
We are the first to show the possible role of microRNA-486 in CT26 mice skeletal muscle. Our results suggest that the decreased expression of microRNA-486 leads to a loss in skeletal muscle mass, function and aerobic capacity since it causes impairment in the protein synthesis pathway by targeting the PTEN pathway. These data corroborate a study by Small et al. [
22] that inversely showed in vitro and in vivo that PTEN protein levels, the target of microRNA-486, was reduced by microRNA-486 overexpression, which enhances PI3K/AKT signaling [
22]. Additionally, decreasing miRNA-486 expression in normal myoblasts results in the impossibility of cell migration and fusion, while overexpression induces muscle regeneration [
23]. Consistent with these results, downregulation of microRNA-486 expression was evident in the skeletal muscle of patients with Duchene muscular dystrophy [
23].
The decreased expression of microRNA-486 in cancer groups of the MMTV-PyMT model were not followed by changes in PTEN protein expression. However, PI3K and total AKT (not pAKT/AKT) protein expression were downregulated in MMTV and MMTV+TR compared with the WT group. Chen et al. [
24] also investigated the effects of microRNA-486 in the MMTV-PyMT model; the authors showed a trend to decrease microRNA-486 expression in skeletal muscle and elevated PTEN expression and lower pAKT, without changes in muscle phenotype [
24]. Therefore, our findings also show that the muscle and body mass (
Figure 2D and
Figure 4A) are preserved in the MMTV group. However, two points should be noticed. Firstly, a low performance at the maximal exercise test can be the first sign of the onset of muscle dysfunction in non-cachectic mice. Secondly, AET prevented this response (
Figure 2B). The similarity in microRNA-486 expression in the skeletal muscle of CT26 and MMTV mice cancer groups suggests that downregulation of microRNA-486 may be an early muscle biomarker of impairing skeletal muscle function in MMTV cancer mice.
The microRNA-206 expression was upregulated in the MMTV group and downregulated as an effect of AET in WT+TR and MMTV+TR (
Figure 6B). Skeletal muscle has remarkable conditions to adapt and adjust to different stimulus such as AET, activating a variety of signaling pathways and improving metabolic and structural function. In this context, it is well known that AET activates insulin-like growth factor-1 (IGF1), signaling an important anabolic role modulating skeletal muscle mass and metabolism [
25]. MicroRNA-206 directly regulates IGF1 gene expression [
25,
26]. IGF1 is a molecular target validated using a luciferase assay to the microRNA-206 [
26,
27]. Therefore, microRNA-206 upregulation in the MMTV group (
Figure 6B) could be inhibiting the IGF1 signaling pathway corroborating to impair the performance in the maximal exercise test in sedentary breast cancer mice, even the body and skeletal mass, fiber number, cross-sectional area and function were still preserved (
Figure 2D and
Figure 4). Corroborating these results, we observed a negative correlation between microRNA-206 expression and muscle oxidative metabolism and physical capacity (
Figure 8A–C). These findings suggest an association between microRNA-206 expression and physical performance and lead speculations regarding microRNA-206 expression being a rate-limiting step in this process or at least having some effect on skeletal muscle metabolism and physical performance in maximal exercise test (
Figure 2B). Importantly, changes of microRNA-206 expression have been associated with skeletal muscle disorders such as Duchenne muscular dystrophy [
28].
Therefore, our results suggest that the downregulation of microRNA-486 and the upregulation of microRNA-206 are contributing to the impairment in exercise capacity and muscle functioning in the MMTV group that is prevented by AET with the downregulating of microRNA-206 expression in the MMTV+TR group. This result is probably due to the fact that microRNA-206 is a skeletal-muscle-specific microRNA inducing more robust effects than a non-muscle specific microRNA.
MyomiRs, in turn, are a class of microRNAs with enriched expression in skeletal and cardiac muscles that act by controlling myogenesis, homeostasis, muscle metabolism and regeneration [
6]. In terms of function, myomiRs target genes related with skeletal muscle differentiation and growth [
6,
29,
30]. To this end, we also analyzed the microRNA-1, -133a and -133b; however, their expressions were not modified to any groups (
Figure S1). Furthermore, to investigate whether the myomiRs would promote a possible skeletal muscle differentiation, we analyzed the expression of the predict genes to fiber myogenesis, histone deacetylase 4 (HDAC4) and paired box transcript factors 7 (Pax 7) and 3 (Pax 3), the expressions of were not modified as well (
Figure S2). Thus, other myogenic pathways [
31] that were not investigated here could be involved in preserving the skeletal muscle mass in non-cachectic MMTV-PyMT breast cancer mice.
CT26 and MMTV sedentary mice showed low aerobic capacity, which was preserved by the AET (
Figure 1B and
Figure 2B). These results are not related to the changes in energy consumption for both models (data not shown). Additionally, the low aerobic capacity is a strong predictor of mortality in cancer patients [
32]. Neil-Sztramko et al. [
33] showed that in patients with breast cancer, the aerobic capacity and strength of the upper limbs are severely decreased before, during and after chemotherapy treatment, which is independent of age. The aerobic capacity impairment may be associated with the development of other comorbidities [
33]. Thus, AET could be an important coadjutant non-pharmacological treatment mainly for breast cancer patients. In the CT26 model, the low aerobic capacity could be associated with the muscle wasting and of the fiber cross-sectional areao, oxidative metabolism and muscle function that was not preserved by AET, although the evidence in the literature describing the mechanisms by which the AET is effective to mitigate skeletal muscle wasting, as reviewed by Alves et al. [
34], preserves the individual’s quality of life with chronic diseases.
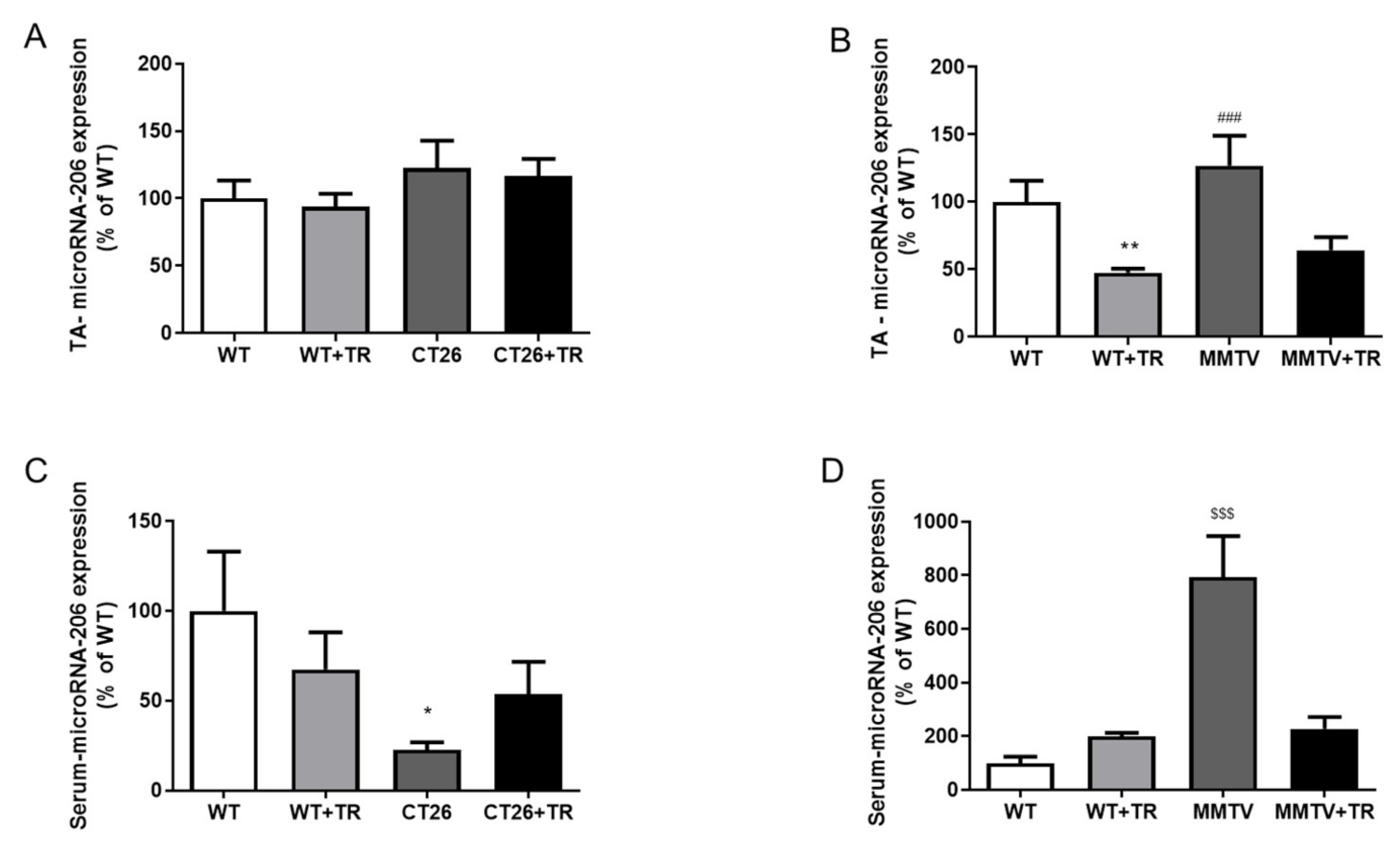
Circulating microRNAs promote cell to cell and tissue to tissue communication in an autocrine, paracrine and endocrine manner. The serum microRNA-486 expression was downregulated in the CT26, CT26+TR, MMTV and MMTV+TR groups (
Figure 5C,D) compared with their respective healthy WT groups, and a similar expression was observed in the TA skeletal muscle (
Figure 5A,B). These results suggest a link between the effects promoted by the tumor on skeletal muscle and the circulating of microRNA-486 levels. These data reinforce microRNAs as circulating non-invasive biomarkers for cancer [
35,
36], and the microRNA-486 can be used to monitor the muscle dysfunction and cachexia evolution in colon cancer.
In the CT26 cancer colon mice, the serum microRNA-206 expression in the CT26 group was downregulated compared to all the other groups (
Figure 6C). Liu et al. [
37] confirmed microRNA-206 as an independent prognostic indicator for colorectal cancer. Patients with low microRNA-206 expression had a poor prognosis and worse survival [
37]. The authors demonstrated that serum microRNA-206 was a good diagnostic marker for discriminating colorectal patients from healthy controls. MicroRNA-206 levels were increased in the blood samples of colorectal patients who received surgical treatment [
37]. Similarly, our study showed that AET prevented the low circulating microRNA-206 expression in WT+TR and CT26+TR compared to the CT26 group (
Figure 6C), suggesting the AET as a potential coadjutant non-pharmacological therapy to colon cancer. Additionally, circulating microRNA-206 expression has highlighted its potential as a diagnosis and prognostic biomarker to monitor the effects of AET for colorectal cancer patients.
In the MMTV group, the TA and serum microRNA-206 expression was significantly increased compared with the other groups (
Figure 6B,D). These results suggest that the microRNA-206 downregulation in TA skeletal muscle promoted by AET could be important to preserve maximal exercise capacity (
Figure 2B) and metabolism (
Figure 2C), since a negative correlation between TA microRNA-206 expression and citrate synthase activity was observed (
Figure 8A). An interesting result was observed in the MMTV group; the circulating microRNA-206 expression was eight times higher than skeletal muscle (
Figure 6B,D) and the serum and TA microRNA-206 were both negatively correlated with the maximal exercise test (
Figure 8B,C). Therefore, the increase in TA microRNA-206 expression was correlated with the decreased muscle oxidative capacity impairing the maximal aerobic capacity, which is also corroborated by the increased circulating microRNA-206 expression.
For a long time, microRNA-206 has been reported as having a tumor suppressor role in breast cancer [
11]. Once the serum microRNA-206 expression was very high in the MMTV group and was decreased by AET, based on the hypothesis that microRNA-206 could be a tumor suppressor [
11], we also evaluate the microRNA-206 expression in the tumor samples (
Figure 7). The tumor microRNA-206 expression was downregulated by AET in MMTV+TR compared to the MMTV group (
Figure 7A,B). However, the tumor volume was not modified as an effect of the AET (
Figure 7C). Since the microRNA-206 expression is exclusive of skeletal muscle, these results together suggest a link among the skeletal muscle, circulation and tumor that can be modulated by the AET. Thus, breast cancer mice increased the microRNA-206 expression in these three tissues, and AET prevented this effect. These data reinforce microRNAs as non-invasive circulating biomarkers, and microRNA-206 expression is suggested as an independent prognostic indicator for breast cancer and as a tool to monitor the beneficial effect of AET.
It is noteworthy that the results related to microRNA-206 expression in breast cancer and the effects of AET are unclearly established, sometimes they are contradictory, mainly regarding the effects of AET. MicroRNA-206 expression is linked to many malignancies and plays an important role in several cancers’ tissues [
38,
39]. In breast cancer cell lines and breast cancer tissues, microRNA-206 expression was upregulated when compared to adjacent normal tissues. The upregulation of microRNA-206 expression promotes breast cancer cell invasion, migration, proliferation and colony formation in vitro, and the in vivo inhibition of tumor formation by anti-microRNA-206 in mice confirmed the suppression of proliferation [
40]. Our study shows that AET significantly decreases the tumor microR-206 expression compared with sedentary mice (
Figure 7A,B); however, the tumor volume was not modified (
Figure 7C). Conversely, it was found that a lowered microRNA-206 expression in the patient’s breast cancer tissues was related to larger tumor size and more advanced clinical stage [
41]. Recently, Calzia et al. [
42] showed myoblasts stimulated by gravity compared with a lack of gravity, mimicking a lack of physical activity, and released microvesicles containing microRNA-206, inhibiting breast cancer cell growth [
42]. Therefore, exercise training regulates several physiological processes; however, little is known regarding the mechanisms by which microRNAs regulate the tumor microenvironment, via endocrine and paracrine systems, modulated by exercise training from the distant tissues, for example, skeletal muscle. Nevertheless, this finding encourages further investigation in future studies.