Interstitial Photodynamic Therapy for Glioblastomas: A Standardized Procedure for Clinical Use
Abstract
:Simple Summary
Abstract
1. Introduction
2. Method
2.1. Planning Procedure
2.1.1. Brain Imaging
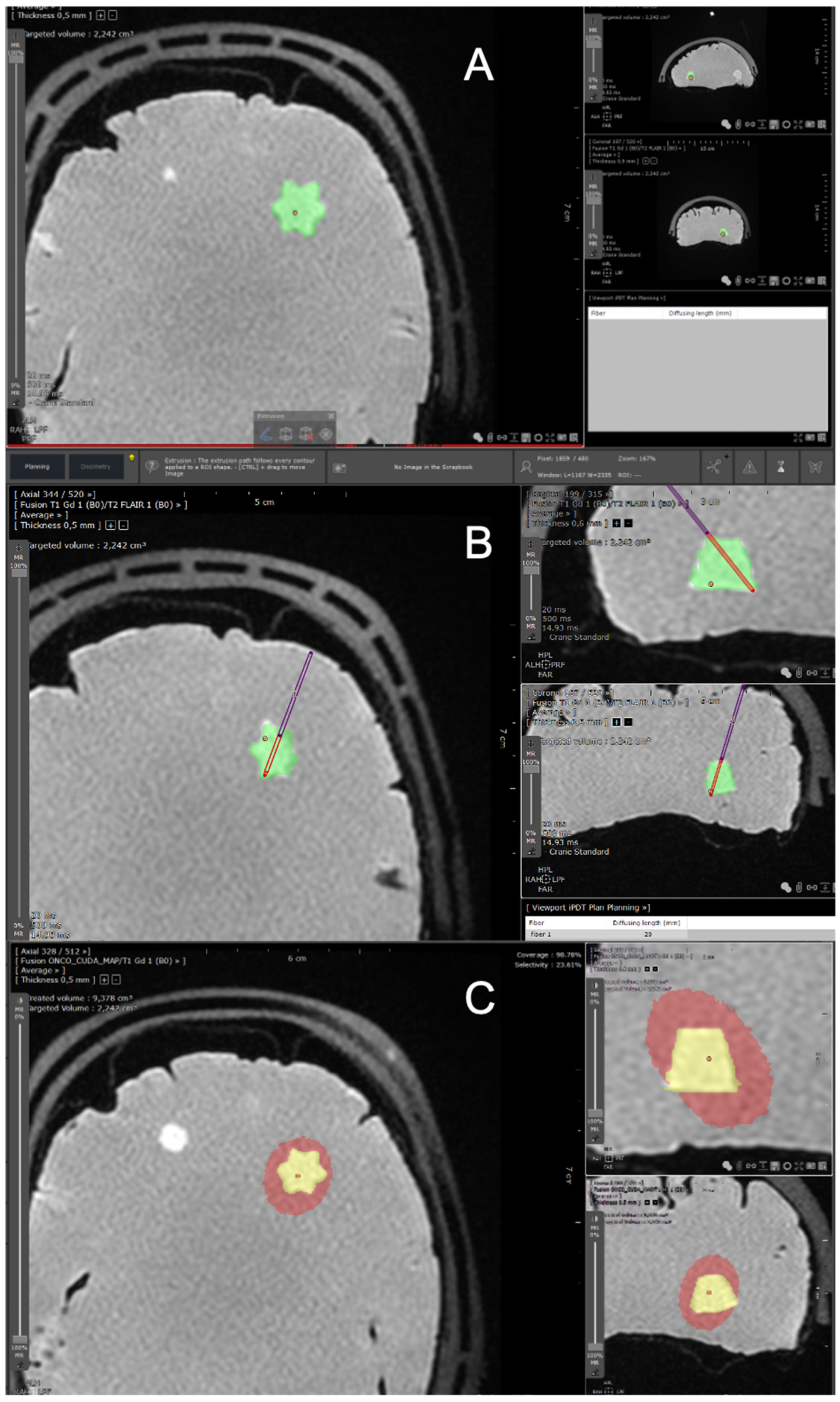
2.1.2. Segmentation Process
2.1.3. Host Software for the TPS
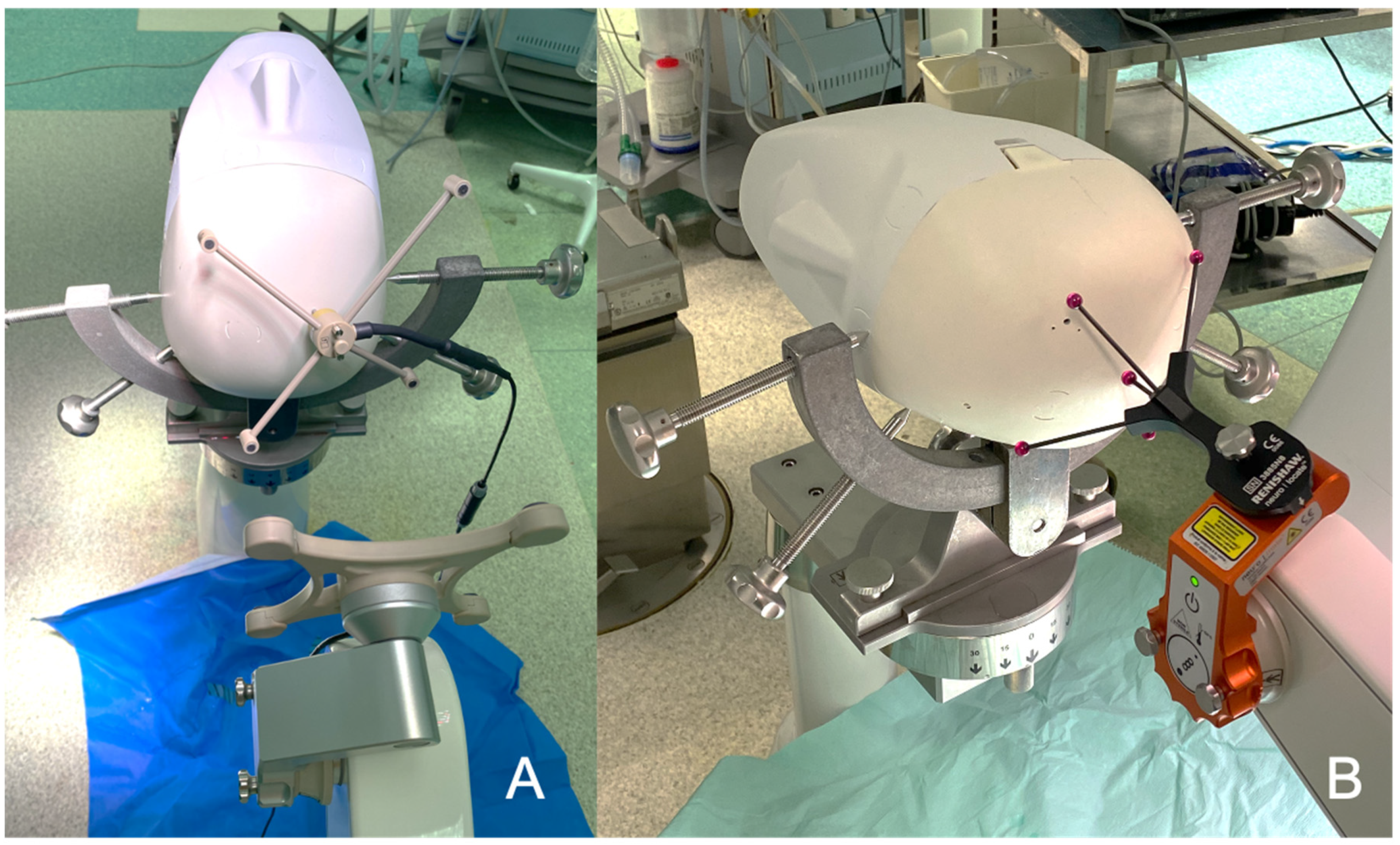
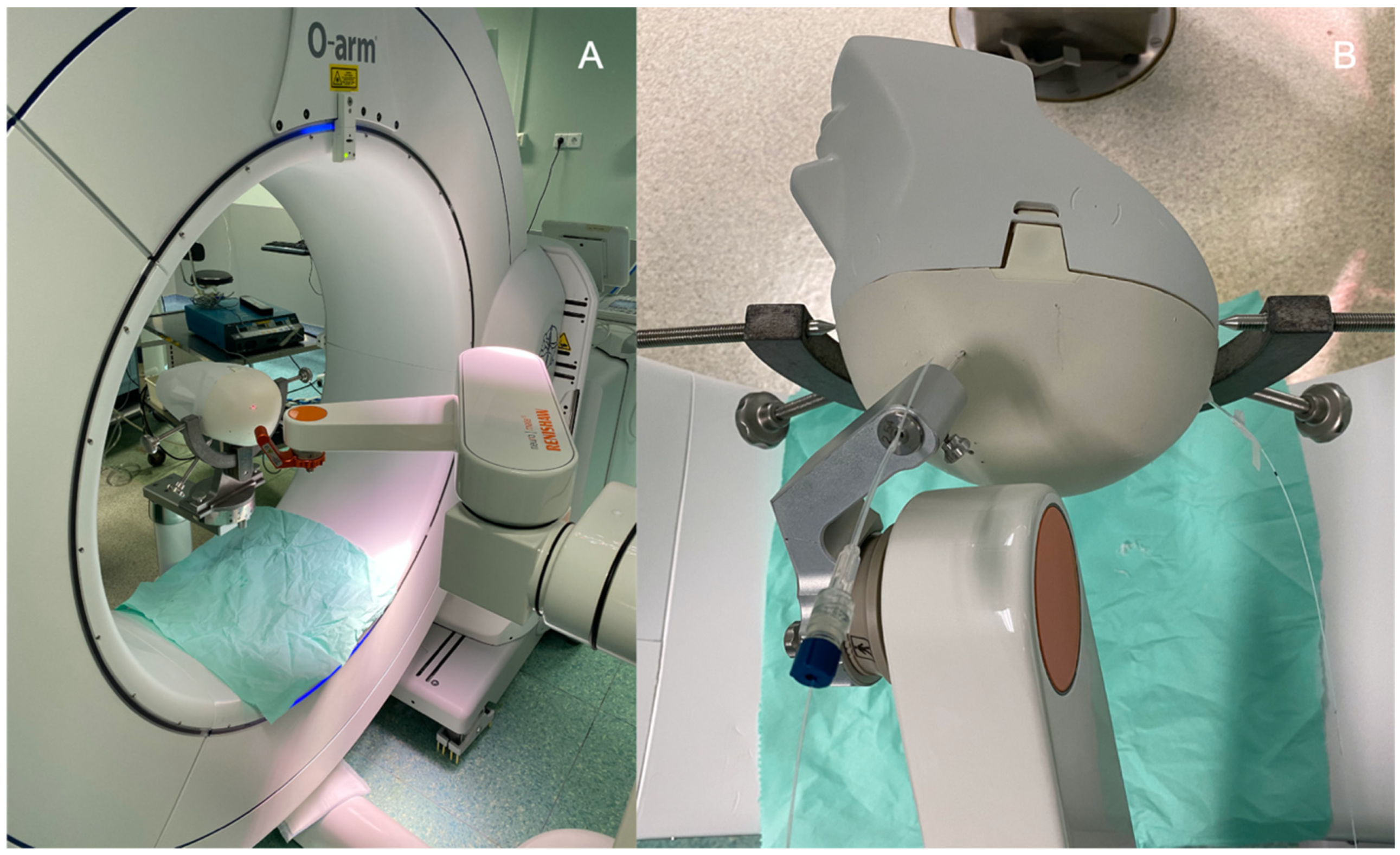
2.1.4. Optical Fibers Positioning
2.1.5. Laser Devices
2.1.6. Monte Carlo Simulations
Specifications of the “Mcxyz” Program for Interstitial 5-ALA PDT
- (a)
- Specification of the wavelength of interest
- (b)
- Specification of the light source power
- (c)
- Specification of the 3D Cartesian grid of voxels
- (d)
- Specification of the optical properties of the 3D grid of voxels
- (e)
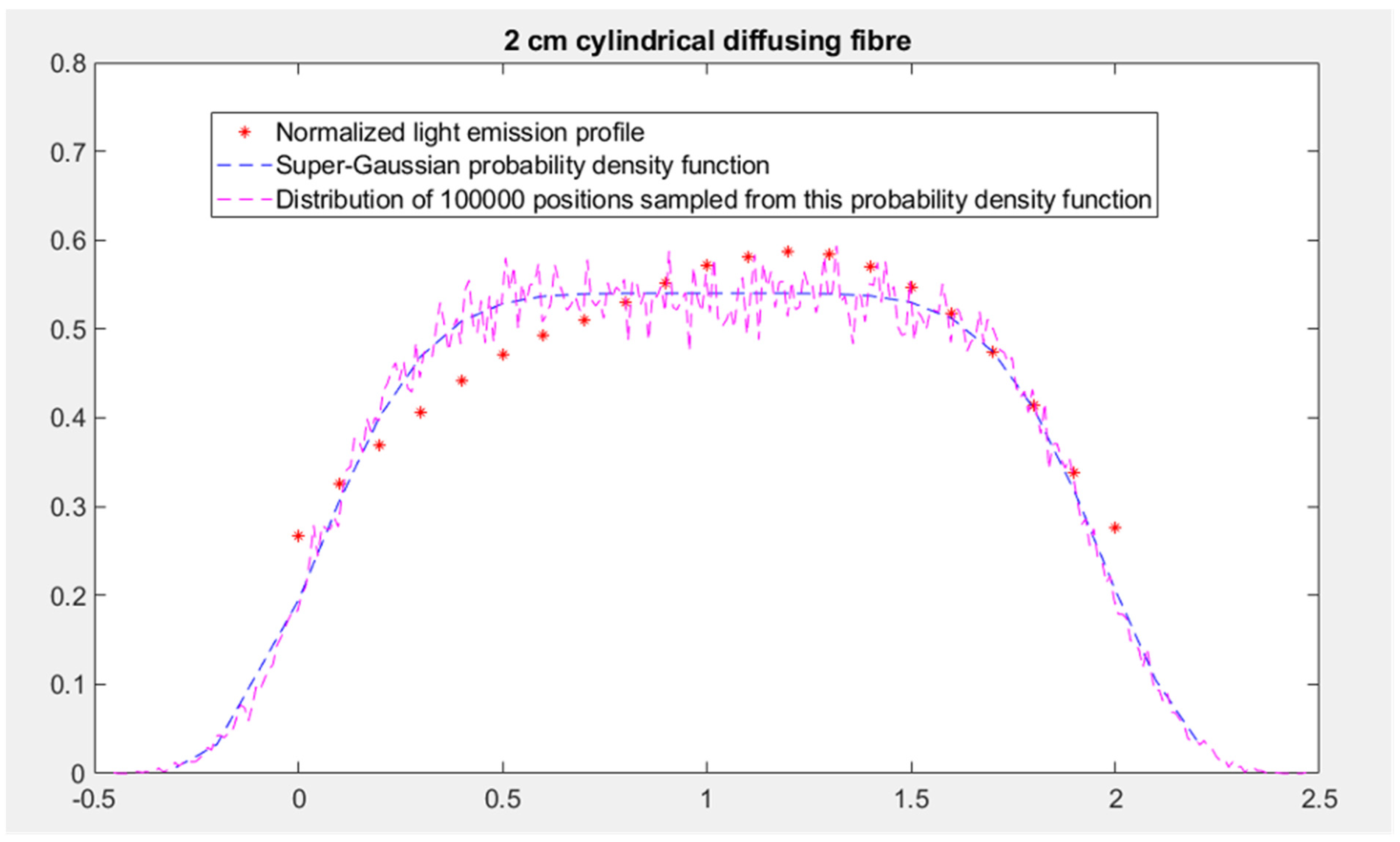
- Specification of the sampling method for the initial position and propagation direction of the photon packets
- (f)
- Specification of the number of photon packets and use of the parallelization
Definition of the Effective Treated Volume
2.2. Surgical Procedure
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omuro, A.; DeAngelis, L.M. Glioblastoma and Other Malignant Gliomas: A Clinical Review. JAMA 2013, 310, 1842–1850. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- McGirt, M.J.; Chaichana, K.L.; Gathinji, M.; Attenello, F.J.; Than, K.; Olivi, A.; Weingart, J.D.; Brem, H.; Quiñones-Hinojosa, A. redo Independent Association of Extent of Resection with Survival in Patients with Malignant Brain Astrocytoma: Clinical Article. J. Neurosurg. 2009, 110, 156–162. [Google Scholar] [CrossRef] [Green Version]
- Leroy, H.; Vermandel, M.; Lejeune, J.; Mordon, S.; Reyns, N. Fluorescence Guided Resection and Glioblastoma in 2015: A Review. Laser Surg. Med. 2015, 47, 441–451. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.-J.; ALA-Glioma Study Group. Fluorescence-Guided Surgery with 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase III Trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Slotty, P.J.; Siantidis, B.; Beez, T.; Steiger, H.J.; Sabel, M. The Impact of Improved Treatment Strategies on Overall Survival in Glioblastoma Patients. Acta Neurochir. 2013, 155, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Petrecca, K.; Guiot, M.-C.; Panet-Raymond, V.; Souhami, L. Failure Pattern Following Complete Resection plus Radiotherapy and Temozolomide Is at the Resection Margin in Patients with Glioblastoma. J. Neuro-Oncol. 2013, 111, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, V.; Hinkka, S.; Färkkilä, M.; Jääskeläinen, J. Debulking or Biopsy of Malignant Glioma in Elderly People—A Randomised Study. Acta Neurochir. 2003, 145, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.W.; Dougherty, T.J. HOW DOES PHOTODYNAMIC THERAPY WORK? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.; Vignion-Dewalle, A.S.; Thecua, E.; Lecomte, F.; Maire, C.; Deleporte, P.; Béhal, H.; Kerob, D.; Duhamel, A.; Mordon, S.; et al. Photodynamic Therapy for Actinic Keratosis of the Forehead and Scalp: A Randomized, Controlled, Phase II Clinical Study Evaluating the Noninferiority of a New Protocol Involving Irradiation with a Light-Emitting, Fabric-Based Device (the Flexitheralight Protocol) Compared with the Conventional Protocol Involving Irradiation with the Aktilite CL 128 Lamp. Br. J. Dermatol. 2019, 180, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Mordon, S.; Vignion-Dewalle, A.S.; Abi-Rached, H.; Thecua, E.; Lecomte, F.; Vicentini, C.; Deleporte, P.; Béhal, H.; Kerob, D.; Hommel, T.; et al. The Conventional Protocol vs. a Protocol Including Illumination with a Fabric-based Biophotonic Device (the Phosistos Protocol) in Photodynamic Therapy for Actinic Keratosis: A Randomized, Controlled, Noninferiority Clinical Study. Br. J. Dermatol. 2020, 182, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Wang, K.K. Photodynamic Therapy for Gastrointestinal Cancer. Photochem. Photobiol. 2020, 96, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Meulemans, J.; Delaere, P.; Poorten, V.V. Photodynamic Therapy in Head and Neck Cancer: Indications, Outcomes, and Future Prospects. Curr. Opin. Otolaryngol. 2019, 27, 136–141. [Google Scholar] [CrossRef]
- Dupont, C.; Vermandel, M.; Leroy, H.; Quidet, M.; Lecomte, F.; Delhem, N.; Mordon, S.; Reyns, N. INtraoperative PhotoDYnamic Therapy for GliOblastomas (INDYGO): Study Protocol for a Phase I Clinical Trial. Neurosurgery 2018, 84, E414–E419. [Google Scholar] [CrossRef] [Green Version]
- Vermandel, M.; Dupont, C.; Lecomte, F.; Leroy, H.-A.; Tuleasca, C.; Mordon, S.; Hadjipanayis, C.G.; Reyns, N. Standardized Intraoperative 5-ALA Photodynamic Therapy for Newly Diagnosed Glioblastoma Patients: A Preliminary Analysis of the INDYGO Clinical Trial. J. Neuro-Oncol. 2021, 152, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Dupont, C.; Vignion, A.; Mordon, S.; Reyns, N.; Vermandel, M. Photodynamic Therapy for Glioblastoma: A Preliminary Approach for Practical Application of Light Propagation Models. Laser Surg. Med. 2018, 50, 523–534. [Google Scholar] [CrossRef] [Green Version]
- Muragaki, Y.; Akimoto, J.; Maruyama, T.; Iseki, H.; Ikuta, S.; Nitta, M.; Maebayashi, K.; Saito, T.; Okada, Y.; Kaneko, S.; et al. Phase II Clinical Study on Intraoperative Photodynamic Therapy with Talaporfin Sodium and Semiconductor Laser in Patients with Malignant Brain Tumors: Clinical Article. J. Neurosurg. 2013, 119, 845–852. [Google Scholar] [CrossRef]
- Weller, M.; Cloughesy, T.; Perry, J.R.; Wick, W. Standards of Care for Treatment of Recurrent Glioblastoma—Are We There Yet? Neuro-Oncology 2012, 15, 4–27. [Google Scholar] [CrossRef] [Green Version]
- Shafirstein, G.; Bellnier, D.; Oakley, E.; Hamilton, S.; Potasek, M.; Beeson, K.; Parilov, E. Interstitial Photodynamic Therapy—A Focused Review. Cancers 2017, 9, 12. [Google Scholar] [CrossRef] [Green Version]
- Johansson, A.; Faber, F.; Kniebühler, G.; Stepp, H.; Sroka, R.; Egensperger, R.; Beyer, W.; Kreth, F.-W. Protoporphyrin IX Fluorescence and Photobleaching during Interstitial Photodynamic Therapy of Malignant Gliomas for Early Treatment Prognosis. Laser Surg. Med. 2013, 45, 225–234. [Google Scholar] [CrossRef]
- Leroy, H.; Delmaire, C.; Rhun, E.; Drumez, E.; Lejeune, J.; Reyns, N. High-Field Intraoperative MRI and Glioma Surgery: Results after the First 100 Consecutive Patients. Acta Neurochir. 2019, 161, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.; Vermandel, M.; Vignion-Dewalle, A.; Leroux, B.; Maurage, C.; Duhamel, A.; Mordon, S.; Reyns, N. Interstitial Photodynamic Therapy and Glioblastoma: Light Fractionation in a Preclinical Model: INTERSTITIAL PHOTODYNAMIC THERAPY AND GLIOBLASTOMA. Laser Surg. Med. 2016, 49, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Leroy, H.-A.; Vermandel, M.; Leroux, B.; Duhamel, A.; Lejeune, J.-P.; Mordon, S.; Reyns, N. MRI Assessment of Treatment Delivery for Interstitial Photodynamic Therapy of High-Grade Glioma in a Preclinical Model. Laser Surg. Med. 2017, 50, 460–468. [Google Scholar] [CrossRef]
- Vermandel, M.; Quidet, M.; Vignion-Dewalle, A.; Leroy, H.; Leroux, B.; Mordon, S.; Reyns, N. Comparison of Different Treatment Schemes in 5-ALA Interstitial Photodynamic Therapy for High-Grade Glioma in a Preclinical Model: An MRI Study. Photodiagnosis Photodyn. Ther. 2018, 25, 166–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porz, N.; Bauer, S.; Pica, A.; Schucht, P.; Beck, J.; Verma, R.K.; Slotboom, J.; Reyes, M.; Wiest, R. Multi-Modal Glioblastoma Segmentation: Man versus Machine. PLoS ONE 2014, 9, e96873. [Google Scholar] [CrossRef] [Green Version]
- Beck, T.J.; Kreth, F.W.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Stepp, H.; Stummer, W.; Baumgartner, R. Interstitial Photodynamic Therapy of Nonresectable Malignant Glioma Recurrences Using 5-Aminolevulinic Acid Induced Protoporphyrin IX. Laser Surg. Med. 2007, 39, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Coupling 3D Monte Carlo Light Transport in Optically Heterogeneous Tissues to Photoacoustic Signal Generation. Photoacoustics 2014, 2, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Valdés, P.A.; Leblond, F.; Kim, A.; Harris, B.T.; Wilson, B.C.; Fan, X.; Tosteson, T.D.; Hartov, A.; Ji, S.; Erkmen, K.; et al. Quantitative Fluorescence in Intracranial Tumor: Implications for ALA-Induced PpIX as an Intraoperative Biomarker: Clinical Article. J. Neurosurg. 2011, 115, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Myrzakhmetov, B.; Arnoux, P.; Mordon, S.; Acherar, S.; Tsoy, I.; Frochot, C. Photophysical Properties of Protoporphyrin IX, Pyropheophorbide-a, and Photofrin® in Different Conditions. Pharmaceuticals 2021, 14, 138. [Google Scholar] [CrossRef]
- Johansson, A.; Palte, G.; Schnell, O.; Tonn, J.; Herms, J.; Stepp, H. 5-Aminolevulinic Acid-induced Protoporphyrin IX Levels in Tissue of Human Malignant Brain Tumors. Photochem. Photobiol. 2010, 86, 1373–1378. [Google Scholar] [CrossRef]
- Honda, N.; Ishii, K.; Kajimoto, Y.; Kuroiwa, T.; Awazu, K. Determination of Optical Properties of Human Brain Tumor Tissues from 350 to 1000 Nm to Investigate the Cause of False Negatives in Fluorescence-Guided Resection with 5-Aminolevulinic Acid. J. Biomed. Opt. 2018, 23, 075006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gebhart, S.C.; Lin, W.C.; Mahadevan-Jansen, A. In Vitro Determination of Normal and Neoplastic Human Brain Tissue Optical Properties Using Inverse Adding-Doubling. Phys. Med. Biol. 2006, 51, 2011–2027. [Google Scholar] [CrossRef] [PubMed]
- Yaroslavsky, A.N.; Schulze, P.C.; Yaroslavsky, I.V.; Schober, R.; Ulrich, F.; Schwarzmaier, H.-J. Optical Properties of Selected Native and Coagulated Human Brain Tissues in Vitro in the Visible and near Infrared Spectral Range. Phys. Med. Biol. 2002, 47, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.V. Tissue Optics and Photonics: Biological Tissue Structures. J. Biomed. Photonics Eng. 2015, 1, 3–21. [Google Scholar] [CrossRef]
- Lietke, S.; Schmutzer, M.; Schwartz, C.; Weller, J.; Siller, S.; Aumiller, M.; Heckl, C.; Forbrig, R.; Niyazi, M.; Egensperger, R.; et al. Interstitial Photodynamic Therapy Using 5-ALA for Malignant Glioma Recurrences. Cancers 2021, 13, 1767. [Google Scholar] [CrossRef]
- Muller, P.J.; Wilson, B.C. Photodynamic Therapy for Malignant Newly Diagnosed Supratentorial Gliomas. J. Clin. Laser Med. Surg. 1996, 14, 263–270. [Google Scholar] [CrossRef]
- Schwartz, C.; Rühm, A.; Tonn, J.-C.; Kreth, S.; Kreth, F.-W. SURG-25INTERSTITIAL PHOTODYNAMIC THERAPY OF DE-NOVO GLIOBLASTOMA MULTIFORME WHO IV. Neuro-Oncology 2015, 17, v219–v220. [Google Scholar] [CrossRef]
- Stummer, W.; Beck, T.; Beyer, W.; Mehrkens, J.H.; Obermeier, A.; Etminan, N.; Stepp, H.; Tonn, J.-C.; Baumgartner, R.; Herms, J.; et al. Long-Sustaining Response in a Patient with Non-Resectable, Distant Recurrence of Glioblastoma Multiforme Treated by Interstitial Photodynamic Therapy Using 5-ALA: Case Report. J. Neuro-Oncol. 2007, 87, 103–109. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Powers, S.K.; Witmer, P.; Brown, T. Optimal Light Dose for Interstitial Photodynamic Therapy in Treatment for Malignant Brain Tumors. Laser Surg. Med. 2000, 27, 224–234. [Google Scholar] [CrossRef]
- Leroy, H.-A.; Guérin, L.; Lecomte, F.; Baert, G.; Vignion, A.-S.; Mordon, S.; Reyns, N. Is Interstitial Photodynamic Therapy for Brain Tumors Ready for Clinical Practice? A Systematic Review. Photodiagnosis Photodyn. Ther. 2021, 36, 102492. [Google Scholar] [CrossRef]
- Curnow, A.; Haller, J.C.; Bown, S.G. Oxygen Monitoring during 5-Aminolaevulinic Acid Induced Photodynamic Therapy in Normal Rat Colon Comparison of Continuous and Fractionated Light Regimes. J. Photochem. Photobiol. B Biol. 2000, 58, 149–155. [Google Scholar] [CrossRef] [Green Version]


| Variable | Healthy Parenchyma | Tumor | |||||
|---|---|---|---|---|---|---|---|
| CSF | Gray Matter | White Matter | Necrotic | Non-Enhancing | Enhancing | Oedema | |
| Absorption coefficient (/mm) | 0.004 | 0.13 | 0.08 | 0.17 | 0.08 | 0.17 | 0.08 |
| Scattering coefficient (/mm) | 0.009 | 9 | 40.5 | 24.1 | 69.7 | 24.1 | 40.5 |
| Anisotropy factor | 0.89 | 0.92 | 0.85 | 0.9 | 0.9 | 0.9 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leroy, H.-A.; Baert, G.; Guerin, L.; Delhem, N.; Mordon, S.; Reyns, N.; Vignion-Dewalle, A.-S. Interstitial Photodynamic Therapy for Glioblastomas: A Standardized Procedure for Clinical Use. Cancers 2021, 13, 5754. https://doi.org/10.3390/cancers13225754
Leroy H-A, Baert G, Guerin L, Delhem N, Mordon S, Reyns N, Vignion-Dewalle A-S. Interstitial Photodynamic Therapy for Glioblastomas: A Standardized Procedure for Clinical Use. Cancers. 2021; 13(22):5754. https://doi.org/10.3390/cancers13225754
Chicago/Turabian StyleLeroy, Henri-Arthur, Gregory Baert, Laura Guerin, Nadira Delhem, Serge Mordon, Nicolas Reyns, and Anne-Sophie Vignion-Dewalle. 2021. "Interstitial Photodynamic Therapy for Glioblastomas: A Standardized Procedure for Clinical Use" Cancers 13, no. 22: 5754. https://doi.org/10.3390/cancers13225754
APA StyleLeroy, H.-A., Baert, G., Guerin, L., Delhem, N., Mordon, S., Reyns, N., & Vignion-Dewalle, A.-S. (2021). Interstitial Photodynamic Therapy for Glioblastomas: A Standardized Procedure for Clinical Use. Cancers, 13(22), 5754. https://doi.org/10.3390/cancers13225754








