A Polygenic Risk Score Predicts Incident Prostate Cancer Risk in Older Men but Does Not Select for Clinically Significant Disease
Abstract
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Genotyping
2.3. Endpoint
2.4. Calculation of Polygenic Risk Score
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Prostate Cancer Diagnoses
3.3. Association of PRS with Incident Prostate Cancer Risk
3.4. Prevalent Prostate Cancer
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pernar, C.H.; Ebot, E.M.; Wilson, K.M.; Mucci, L.A. The Epidemiology of Prostate Cancer. Cold Spring Harb. Perspect. Med. 2018, 8, a030361. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, O.; de Bono, J.S. Metastatic Prostate Cancer. N. Engl. J. Med. 2018, 378, 645–657. [Google Scholar] [CrossRef]
- Hayes, J.H.; Barry, M.J. Screening for Prostate Cancer with the Prostate-Specific Antigen Test: A Review of Current Evidence. JAMA 2014, 311, 1143–1149. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Bibbins-Domingo, K.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Ebell, M.; Epling, J.W., Jr.; et al. Screening for Prostate Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1901–1913. [Google Scholar] [PubMed]
- Clinical Practice Guidelines on PSA Testing PCFA. Available online: https://www.prostate.org.au/awareness/for-healthcare-professionals/clinical-practice-guidelines-on-psa-testing/ (accessed on 29 June 2021).
- American Cancer Society Recommendations for Prostate Cancer Early Detection. Available online: https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/acs-recommendations.html (accessed on 29 June 2021).
- Boyle, H.J.; Alibhai, S.; Decoster, L.; Efstathiou, E.; Fizazi, K.; Mottet, N.; Oudard, S.; Payne, H.; Prentice, M.; Puts, M.; et al. Updated Recommendations of the International Society of Geriatric Oncology on Prostate Cancer Management in Older Patients. Eur. J. Cancer 2019, 116, 116–136. [Google Scholar] [CrossRef]
- Louie, K.S.; Seigneurin, A.; Cathcart, P.; Sasieni, P. Do Prostate Cancer Risk Models Improve the Predictive Accuracy of PSA Screening? A Meta-Analysis. Ann. Oncol. 2015, 26, 848–864. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; Bellmunt, J.; Bolla, M.; Briers, E.; Cumberbatch, M.G.; De Santis, M.; Fossati, N.; Gross, T.; Henry, A.M.; Joniau, S.; et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2017, 71, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Stabile, A.; Giganti, F.; Rosenkrantz, A.B.; Taneja, S.S.; Villeirs, G.; Gill, I.S.; Allen, C.; Emberton, M.; Moore, C.M.; Kasivisvanathan, V. Multiparametric MRI for Prostate Cancer Diagnosis: Current Status and Future Directions. Nat. Rev. Urol. 2020, 17, 41–61. [Google Scholar] [CrossRef]
- Peisch, S.F.; Van Blarigan, E.L.; Chan, J.M.; Stampfer, M.J.; Kenfield, S.A. Prostate Cancer Progression and Mortality: A Review of Diet and Lifestyle Factors. World J. Urol. 2017, 35, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Brookman-May, S.D.; Campi, R.; Henríquez, J.D.S.; Klatte, T.; Langenhuijsen, J.F.; Brausi, M.; Linares-Espinós, E.; Volpe, A.; Marszalek, M.; Akdogan, B.; et al. Latest Evidence on the Impact of Smoking, Sports, and Sexual Activity as Modifiable Lifestyle Risk Factors for Prostate Cancer Incidence, Recurrence, and Progression: A Systematic Review of the Literature by the European Association of Urology Section of Oncological Urology (ESOU). Eur. Urol. Focus 2019, 5, 756–787. [Google Scholar] [PubMed] [Green Version]
- Patel, A.R.; Klein, E.A. Risk Factors for Prostate Cancer. Nat. Clin. Pract. Urol. 2009, 6, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Foerster, B.; Pozo, C.; Abufaraj, M.; Mari, A.; Kimura, S.; D’Andrea, D.; John, H.; Shariat, S.F. Association of Smoking Status With Recurrence, Metastasis, and Mortality Among Patients With Localized Prostate Cancer Undergoing Prostatectomy or Radiotherapy: A Systematic Review and Meta-Analysis. JAMA Oncol. 2018, 4, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Allott, E.H.; Masko, E.M.; Freedland, S.J. Obesity and Prostate Cancer: Weighing the Evidence. Eur. Urol. 2013, 63, 800–809. [Google Scholar] [CrossRef] [Green Version]
- Cuzick, J.; Thorat, M.A.; Andriole, G.; Brawley, O.W.; Brown, P.H.; Culig, Z.; Eeles, R.A.; Ford, L.G.; Hamdy, F.C.; Holmberg, L.; et al. Prevention and Early Detection of Prostate Cancer. Lancet Oncol. 2014, 15, e484–e492. [Google Scholar] [CrossRef] [Green Version]
- Albright, F.; Stephenson, R.A.; Agarwal, N.; Teerlink, C.C.; Lowrance, W.T.; Farnham, J.M.; Albright, L.A.C. Prostate Cancer Risk Prediction Based on Complete Prostate Cancer Family History. Prostate 2015, 75, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, D.K.; Schmidt, K.T.; Chau, C.H.; Figg, W.D. Germline Genetics of Prostate Cancer: Prevalence of Risk Variants and Clinical Implications for Disease Management. Cancers 2021, 13, 2154. [Google Scholar] [CrossRef]
- Schumacher, F.R.; Al Olama, A.A.; Berndt, S.I.; Benlloch, S.; Ahmed, M.; Saunders, E.J.; Dadaev, T.; Leongamornlert, D.; Anokian, E.; Cieza-Borrella, C.; et al. Association Analyses of More than 140,000 Men Identify 63 New Prostate Cancer Susceptibility Loci. Nat. Genet. 2018, 50, 928–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Unger, R.H.; et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef] [Green Version]
- Mancuso, N.; Rohland, N.; Rand, K.A.; Tandon, A.; Allen, A.; Quinque, D.; Mallick, S.; Li, H.; Stram, A.; Sheng, X.; et al. The Contribution of Rare Variation to Prostate Cancer Heritability. Nat. Genet. 2016, 48, 30–35. [Google Scholar] [CrossRef]
- Leongamornlert, D.; Mahmud, N.; Tymrakiewicz, M.; Saunders, E.; Dadaev, T.; Castro, E.; Goh, C.; Govindasami, K.; Guy, M.; O’Brien, L.; et al. Germline BRCA1 Mutations Increase Prostate Cancer Risk. Br. J. Cancer 2012, 106, 1697–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, R.; Aly, M.; Clements, M.; Zheng, L.; Adolfsson, J.; Xu, J.; Grönberg, H.; Wiklund, F. A Population-Based Assessment of Germline HOXB13 G84E Mutation and Prostate Cancer Risk. Eur. Urol. 2014, 65, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen-Dumont, T.; MacInnis, R.J.; Steen, J.A.; Theys, D.; Tsimiklis, H.; Hammet, F.; Mahmoodi, M.; Pope, B.J.; Park, D.J.; Mahmood, K.; et al. Rare Germline Genetic Variants and Risk of Aggressive Prostate Cancer. Int. J. Cancer 2020, 147, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Mars, N.; Koskela, J.T.; Ripatti, P.; Kiiskinen, T.T.J.; Havulinna, A.S.; Lindbohm, J.V.; Ahola-Olli, A.; Kurki, M.; Karjalainen, J.; Palta, P.; et al. Polygenic and Clinical Risk Scores and Their Impact on Age at Onset and Prediction of Cardiometabolic Diseases and Common Cancers. Nat. Med. 2020, 26, 549–557. [Google Scholar] [CrossRef]
- Vilhjálmsson, B.J.; Yang, J.; Finucane, H.K.; Gusev, A.; Lindström, S.; Ripke, S.; Genovese, G.; Loh, P.-R.; Bhatia, G.; Do, R.; et al. Modeling Linkage Disequilibrium Increases Accuracy of Polygenic Risk Scores. Am. J. Hum. Genet. 2015, 97, 576–592. [Google Scholar] [CrossRef] [Green Version]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2016, 40, 244–252. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.J.; Wolfe, R.; Woods, R.L.; Tonkin, A.M.; Donnan, G.A.; Nelson, M.R.; Reid, C.M.; Lockery, J.E.; Kirpach, B.; Storey, E.; et al. Effect of Aspirin on Cardiovascular Events and Bleeding in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1509–1518. [Google Scholar] [CrossRef]
- McNeil, J.J.; Woods, R.L.; Nelson, M.R.; Reid, C.M.; Kirpach, B.; Wolfe, R.; Storey, E.; Shah, R.C.; Lockery, J.E.; Tonkin, A.M.; et al. Effect of Aspirin on Disability-Free Survival in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1499–1508. [Google Scholar] [CrossRef]
- McNeil, J.J.; Nelson, M.R.; Woods, R.L.; Lockery, J.E.; Wolfe, R.; Reid, C.M.; Kirpach, B.; Shah, R.C.; Ives, D.G.; Storey, E.; et al. Effect of Aspirin on All-Cause Mortality in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1519–1528. [Google Scholar] [CrossRef]
- ASPREE Investigator Group. Study Design of ASPirin in Reducing Events in the Elderly (ASPREE): A Randomized, Controlled Trial. Contemp. Clin. Trials 2013, 36, 555–564. [Google Scholar] [CrossRef] [Green Version]
- Nelson, M.R.; Reid, C.M.; Ames, D.; Beilin, L.J.; Donnan, G.A.; Gibbs, P.; Johnston, C.I.; Krum, H.; Storey, E.; Tonkin, A.; et al. Feasibility of Conducting a Primary Prevention Trial of Low-dose Aspirin for Major Adverse Cardiovascular Events in Older People in Australia: Results from the ASPirin in Reducing Events in the Elderly (ASPREE) Pilot Study. Med. J. Aust. 2008, 189, 105–109. [Google Scholar] [CrossRef]
- Lockery, J.E.; Collyer, T.A.; Abhayaratna, W.P.; Fitzgerald, S.M.; McNeil, J.J.; Nelson, M.R.; Orchard, S.G.; Reid, C.; Stocks, N.P.; Trevaks, R.E.; et al. Recruiting General Practice Patients for Large Clinical Trials: Lessons from the Aspirin in Reducing Events in the Elderly (ASPREE) Study. Med. J. Aust. 2019, 210, 168–173. [Google Scholar] [CrossRef] [Green Version]
- McNeil, J.J.; Woods, R.L.; Nelson, M.R.; Murray, A.M.; Reid, C.M.; Kirpach, B.; Storey, E.; Shah, R.C.; Wolfe, R.S.; Tonkin, A.M.; et al. Baseline Characteristics of Participants in the ASPREE (ASPirin in Reducing Events in the Elderly) Study. J. Gerontol. Ser. A 2017, 72, 1586–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riaz, M.; Huq, A.; Ryan, J.; Orchard, S.G.; Tiller, J.; Lockery, J.; Woods, R.L.; Wolfe, R.; Renton, A.E.; Goate, A.M.; et al. Effect of APOE and a Polygenic Risk Score on Incident Dementia and Cognitive Decline in a Healthy Older Population. Aging Cell 2021, 20, e13384. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, A.; Yan, M.; Riaz, M.; Polekhina, G.; Orchard, S.G.; Tiller, J.; Wolfe, R.; Joshi, A.; Cao, Y.; McInerney-Leo, A.M.; et al. Genomic Risk Score for Melanoma in a Prospective Study of Older Individuals. J. Natl. Cancer Inst. 2021, 113, 1379–1385. [Google Scholar] [CrossRef] [PubMed]
- Consortium, T. 1000 G.P.; The 1000 Genomes Project Consortium A Global Reference for Human Genetic Variation. Nature 2015, 526, 68–74. [Google Scholar]
- Taliun, D.; Harris, D.N.; Kessler, M.D.; Carlson, J.; Szpiech, Z.A.; Torres, R.; Taliun, S.A.G.; Corvelo, A.; Gogarten, S.M.; Kang, H.M.; et al. Sequencing of 53,831 Diverse Genomes from the NHLBI TOPMed Program. Nature 2021, 590, 290–299. [Google Scholar] [CrossRef]
- McNeil, J.J.; Gibbs, P.; Orchard, S.G.; Lockery, J.E.; Bernstein, W.B.; Cao, Y.; Ford, L.; Haydon, A.; Kirpach, B.; Macrae, F.; et al. Effect of Aspirin on Cancer Incidence and Mortality in Older Adults. J. Natl. Cancer Inst. 2021, 113, 258–265. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-Generation PLINK: Rising to the Challenge of Larger and Richer Datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Lambert, S.A.; Gil, L.; Jupp, S.; Ritchie, S.C.; Xu, Y.; Buniello, A.; McMahon, A.; Abraham, G.; Chapman, M.; Parkinson, H.; et al. The Polygenic Score Catalog as an Open Database for Reproducibility and Systematic Evaluation. Nat. Genet. 2021, 53, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M.; Lumley, T. Package “survival”. R Top Doc 2015, 128, 28–33. [Google Scholar]
- Team, R.C. Others R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 17 November 2021).
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Lacaze, P.; Bakshi, A.; Riaz, M.; Orchard, S.G.; Tiller, J.; Neumann, J.T.; Carr, P.R.; Joshi, A.D.; Cao, Y.; Warner, E.T.; et al. Genomic Risk Prediction for Breast Cancer in Older Women. Cancers 2021, 13, 3533. [Google Scholar] [CrossRef]
- Neumann, J.T.; Riaz, M.; Bakshi, A.; Polekhina, G.; Thao, L.T.P.; Nelson, M.R.; Woods, R.L.; Abraham, G.; Inouye, M.; Reid, C.M.; et al. Predictive Performance of a Polygenic Risk Score for Incident Ischemic Stroke in a Healthy Older Population. Stroke 2021, 52, 2882–2891. [Google Scholar] [CrossRef]
- Neumann, J.T.; Riaz, M.; Bakshi, A.; Polekhina, G.; Thao, L.T.P.; Nelson, M.R.; Woods, R.L.; Abraham, G.; Inouye, M.; Reid, C.M.; et al. A Polygenic Risk Score for Coronary Heart Disease Performs Well in Individuals Aged 70 Years and Older. medRxiv 2021. [Google Scholar] [CrossRef]
- Plym, A.; Penney, K.L.; Kalia, S.; Kraft, P.; Conti, D.V.; Haiman, C.; Mucci, L.A.; Kibel, A.S. Evaluation of a Multiethnic Polygenic Risk Score Model for Prostate Cancer. J. Natl. Cancer Inst. 2021, djab058. [Google Scholar] [CrossRef]
- Black, M.H.; Li, S.; LaDuca, H.; Lo, M.-T.; Chen, J.; Hoiness, R.; Gutierrez, S.; Tippin-Davis, B.; Lu, H.-M.; Gielzak, M.; et al. Validation of a Prostate Cancer Polygenic Risk Score. Prostate 2020, 80, 1314–1321. [Google Scholar] [CrossRef]
- Pashayan, N.; Duffy, S.W.; Neal, D.E.; Hamdy, F.C.; Donovan, J.L.; Martin, R.M.; Harrington, P.; Benlloch, S.; Amin Al Olama, A.; Shah, M.; et al. Implications of Polygenic Risk-Stratified Screening for Prostate Cancer on Overdiagnosis. Genet. Med. 2015, 17, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Sipeky, C.; Talala, K.M.; Tammela, T.L.J.; Taari, K.; Auvinen, A.; Schleutker, J. Prostate Cancer Risk Prediction Using a Polygenic Risk Score. Sci. Rep. 2020, 10, 17075. [Google Scholar] [CrossRef]
- Sun, X.; Xue, A.; Qi, T.; Chen, D.; Shi, D.; Wu, Y.; Zheng, Z.; Zeng, J.; Yang, J. Tumor Mutational Burden Is Polygenic and Genetically Associated with Complex Traits and Diseases. Cancer Res. 2021, 81, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Sha, D.; Jin, Z.; Budczies, J.; Kluck, K.; Stenzinger, A.; Sinicrope, F.A. Tumor Mutational Burden as a Predictive Biomarker in Solid Tumors. Cancer Discov. 2020, 10, 1808–1825. [Google Scholar] [CrossRef]
- Hieronymus, H.; Schultz, N.; Gopalan, A.; Carver, B.S.; Chang, M.T.; Xiao, Y.; Heguy, A.; Huberman, K.; Bernstein, M.; Assel, M.; et al. Copy Number Alteration Burden Predicts Prostate Cancer Relapse. Proc. Natl. Acad. Sci. USA 2014, 111, 11139–11144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hieronymus, H.; Murali, R.; Tin, A.; Yadav, K.; Abida, W.; Moller, H.; Berney, D.; Scher, H.; Carver, B.; Scardino, P.; et al. Tumor Copy Number Alteration Burden Is a Pan-Cancer Prognostic Factor Associated with Recurrence and Death. Elife 2018, 7, e37294. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.A.; Fraser, M.; Livingstone, J.; Espiritu, S.M.G.; Thorne, H.; Huang, V.; Lo, W.; Shiah, Y.-J.; Yamaguchi, T.N.; Sliwinski, A.; et al. Germline BRCA2 Mutations Drive Prostate Cancers with Distinct Evolutionary Trajectories. Nat. Commun. 2017, 8, 13671. [Google Scholar] [CrossRef]

| Characteristics | ASPREE |
|---|---|
| Participants | 5701 |
| Age at randomisation (mean (SD)) | 74.9 (4.2) |
| Age Group (%) | |
| 70–74 | 3566 (62.6) |
| 75–79 | 1384 (24.3) |
| 80–84 | 585 (10.3) |
| 85+ | 166 (2.9) |
| Current or former smoker (%) | 3203 (56.2) |
| Diabetes (%) | 637 (11.2) |
| Randomized to Aspirin (%) | 2837 (49.8) |
| Body-mass-index (kg/m2) − mean (SD) | 27.9 (3.8) |
| Current alcohol consumption (%) | 4880 (85.6) |
| Family history of prostate cancer (%) | 499 (8.8) |
| Polygenic Risk Score − mean (SD) | −0.46 (0.15) |
| Prevalent Prostate Cancer (%) (self-reported at enrolment) | |
| None | 4725 (88.5) |
| <49 years | 4 (0.0) |
| ≥50 years | 654 (11.5) |
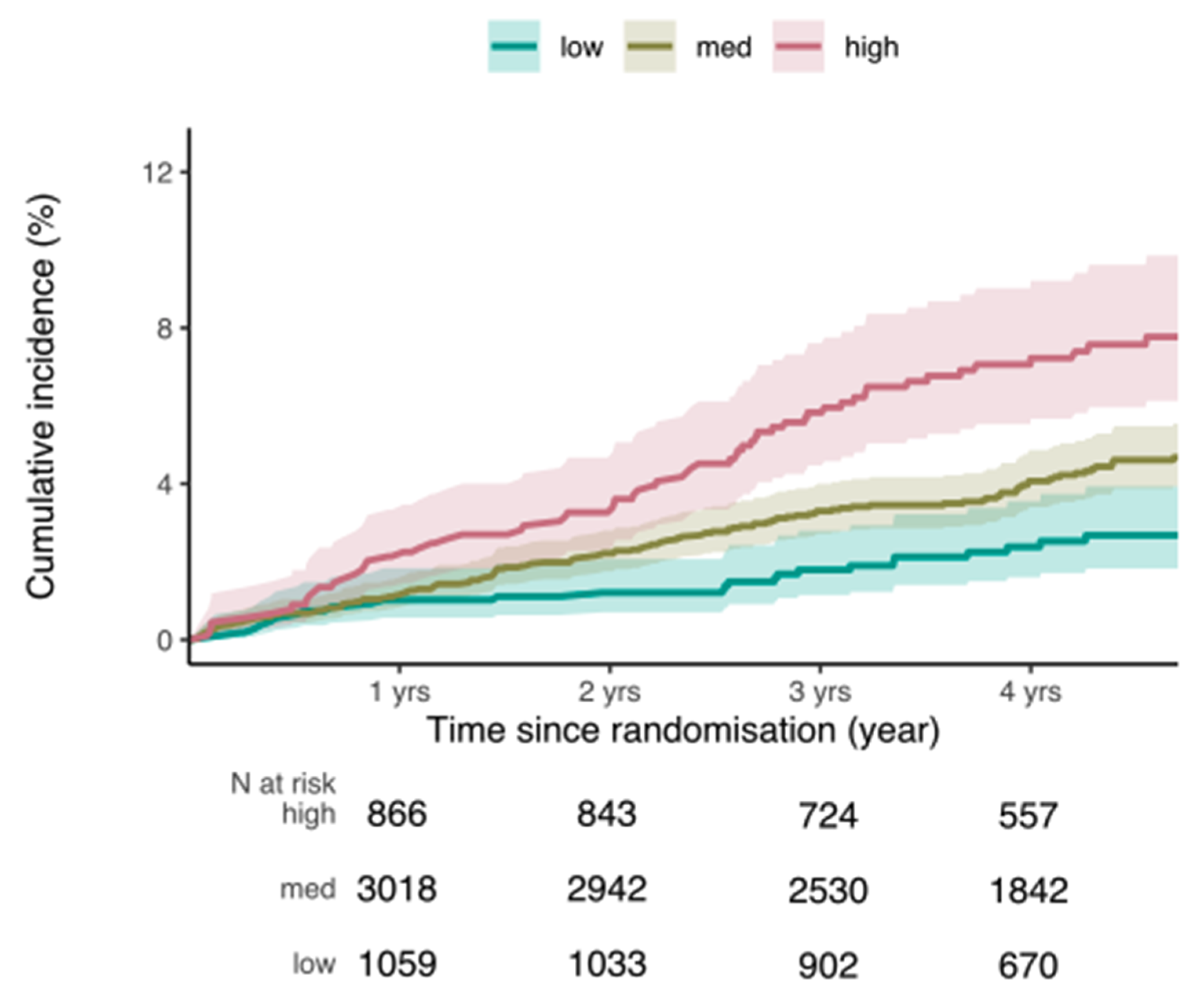
| Incident prostate cancer. (218 clinically confirmed cases during the ASPREE trial, excluding all prevalent cases) | ||||||
| Variable | PRS as Continuous Variable (per Standard Deviation) | PRS as Categorical Variable (low, medium, high) | ||||
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| PRS (per std dev) | 1.52 | (1.33; 1.74) | <0.0001 | |||
| Low PRS 0–20% (n = 23) | Reference | |||||
| Medium PRS >20–80% (n = 128) | 1.74 | (1.14; 2.66) | 0.01 | |||
| High PRS >80% (n = 57) | 2.99 | (1.90; 4.72) | <0.0001 | |||
| Incident prostate cancer in participants with age at randomisation between 70 and 74 years (126 clinically confirmed cases during the ASPREE trial, excluding all prevalent cases) | ||||||
| Variable | PRS as Continuous Variable (per Standard Deviation) | PRS as Categorical Variable (low, medium, high) | ||||
| Hazard Ratio | 95% CI | p-Value | Hazard Ratio | 95% CI | p-Value | |
| PRS (per std dev) | 1.65 | (1.39; 1.97) | <0.0001 | |||
| Low PRS 0–20% (n = 23) | Reference | |||||
| Medium PRS >20–80% (n = 128) | 1.78 | (1.00; 3.15) | 0.05 | |||
| High PRS >80% (n = 57) | 3.54 | (1.93; 6.49) | <0.0001 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakshi, A.; Riaz, M.; Orchard, S.G.; Carr, P.R.; Joshi, A.D.; Cao, Y.; Rebello, R.; Nguyen-Dumont, T.; Southey, M.C.; Millar, J.L.; et al. A Polygenic Risk Score Predicts Incident Prostate Cancer Risk in Older Men but Does Not Select for Clinically Significant Disease. Cancers 2021, 13, 5815. https://doi.org/10.3390/cancers13225815
Bakshi A, Riaz M, Orchard SG, Carr PR, Joshi AD, Cao Y, Rebello R, Nguyen-Dumont T, Southey MC, Millar JL, et al. A Polygenic Risk Score Predicts Incident Prostate Cancer Risk in Older Men but Does Not Select for Clinically Significant Disease. Cancers. 2021; 13(22):5815. https://doi.org/10.3390/cancers13225815
Chicago/Turabian StyleBakshi, Andrew, Moeen Riaz, Suzanne G. Orchard, Prudence R. Carr, Amit D. Joshi, Yin Cao, Richard Rebello, Tú Nguyen-Dumont, Melissa C. Southey, Jeremy L. Millar, and et al. 2021. "A Polygenic Risk Score Predicts Incident Prostate Cancer Risk in Older Men but Does Not Select for Clinically Significant Disease" Cancers 13, no. 22: 5815. https://doi.org/10.3390/cancers13225815







