A Multicenter Epidemiological Study on Second Malignancy in Non-Syndromic Pheochromocytoma/Paraganglioma Patients in Italy
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| NAME | SURNAME | TUMOR REGISTRY |
| Guido | Mazzoleni | South Tyrol Tumour Registry, Italy |
| Fabio | Vittadello | South Tyrol Tumour Registry, Italy |
| Rosario | Tumino | Cancer Registry, Provincial Health Authority (ASP) Ragusa, Italy |
| Ausilia | Sferrazza | Cancer Registry, Provincial Health Authority (ASP) Ragusa, Italy |
| Marco | Pompili | Cancer Registry Marche, Italy |
| Susanna | Vitarelli | Cancer Registry Marche, Italy |
| Francesco | Cuccaro | Cancer Registry of Puglia, Italy |
| Giuseppa | Candela | Cancer Registry Trapani-Agrigento ASP Trapani, Italy |
| Roberto | Rizzello | Trento Province Cancer Registry, Trento, Italy |
| Silvano | Piffer | Trento Province Cancer Registry, Trento, Italy |
| Maria | Michiara | Cancer Registry of Parma, Parma, Italy |
| Antonino | Musolino | Cancer Registry of Parma, Parma, Italy |
| Milena | Maule | Unit of Cancer Epidemiology, Turin, Italy |
| Carlotta | Sacerdote | Unit of Cancer Epidemiology, Turin, Italy |
| Alessandra | Ravaioli | Cancer Registry Romagna, Italy |
| Orietta | Giuliani | Cancer Registry Romagna, Italy |
| Maria Vittoria | Celesia | Cancer Registry Liguria, Italy |
| Enza | Marani | Cancer Registry Liguria, Italy |
| Diego | Serraino | Oncology referral Center Aviano, Italy |
| Luigino | Dal Maso | Oncology referral Center Aviano, Italy |
| Fernando | Palma | Cancer Registry Foggia, Section Cancer Registry Puglia, Italy |
| Mario | Fusco | Napoli 3 South Cancer Registry, Italy |
| Maria Francesca | Vitale | Napoli 3 South Cancer Registry, Italy |
| Giuliano | Carozzi | Modena Cancer Registry, Italy |
| Claudia | Cirilii | Modena Cancer Registry, Italy |
| Giovanna | Tagliabue | Lombardy Cancer Registry, Italy |
| Paolo | Contiero | Lombardy Cancer Registry, Italy |
| Santa | Valenti Clemente | Reggio Calabria Tumour Registry, Italy |
| Romina | Vincenzi | Reggio Calabria Tumour Registry, Italy |
| Rocco | Galasso | Regional Cancer Registry Basilicata, Italy |
| Fabrizio | Quarta | Lecce Tumour Registry, Italy |
| Anna | Melcarne | Lecce Tumour Registry, Italy |
| Rossella | Cavallo | Salerno Tumour Registry, Italy |
| Lucia | Mangone | Reggio-Emilia Tumour Registry, Italy |
| Isabella | Bisceglia | Reggio-Emilia Tumour Registry, Italy |
| Carlo Giacomo | Sciacchitano | CT-ME-EN Tumour Registry, Italy |
| Fiorella | Paderni | CT-ME-EN Tumour Registry, Italy |
| Annarita | Citarella | Benevento Tumour Registry, Italy |
| Antonella | Sutera Sardo | Catanzaro Tumour Registry, Italy |
| Massimo | Rugge | Veneto Tumour Registry, Italy |
Appendix B
- Istituto Oncologico Veneto IRCCS, Padova;
- Dipartimento di Scienze Biomediche Sperimentali e Cliniche, AOU Careggi, Firenze;
- Centro Specialistico Ipertensioni Secondarie, Dipartimento di Medicina Interna e Specialità Mediche, Università di Roma “Sapienza”, Policlinico Umberto I, Roma;
- Endocrinologia, Diabetologia e Metabolismo, Dipartimento di Scienze Mediche, Università di Torino, Città della Salute e della Scienza, Torino e Endocrinologia Oncologica, Dipartimento di Scienze Mediche, Università di Torino, Città della Salute e della Scienza, Torino;
- Medicina Interna ed Endocrinologia, Dipartimento di Scienze Cliniche e Biologiche, Università di Torino, AOU San Luigi, Orbassano Torino;
- Unità di Endocrinologia e Malattie Metaboliche Fondazione IRCCS Cà Granda Ospedale Maggiore Policlinico, Milano;
- Dipartimento di medicina Clinica e Chirurgia, Divisione di Endocrinologia Università Federico II Napoli;
- Endocrinologia AO S. Croce e Carle, Cuneo;
- Unità di Endocrinologia Istituto Nazionale Tumori Regina Elena, Roma;
- Medicina Interna e Ipertensione, Dipartimento di Scienze Mediche, Università di Torino, Città della Salute e della Scienza, Torino;
- Endocrinologia, Dipartimento di Medicina Clinica e Molecolare, Università Roma “Sapienza”, Ospedale Sant’Andrea, Roma;
- Endocrinologia, AO Ordine Mauriziano, Torino
References
- Lenders, J.W.; Eisenhofer, G.; Mannelli, M.; Pacak, K. Phaeochromocytoma. Lancet 2005, 366, 665–675. [Google Scholar] [CrossRef]
- Andrews, K.A.; Ascher, D.B.; Pires, D.E.V.; Barnes, D.R.; Vialard, L.; Casey, R.T.; Bradshaw, N.; Adlard, J.; Aylwin, S.; Brennan, P.; et al. Tumour risks and genotype–phenotype correlations associated with germline variants in succinate dehydrogenase subunit genes SDHB, SDHC and SDHD. J. Med. Genet. 2018, 55, 384–394. [Google Scholar] [CrossRef]
- Dahia, P.L.M. Pheochromocytoma and paraganglioma pathogenesis: Learning from genetic heterogeneity. Nat. Rev. Cancer 2014, 14, 108–119. [Google Scholar] [CrossRef]
- Currás-Freixes, M.; Piñeiro-Yañez, E.; Montero-Conde, C.; Apellániz-Ruiz, M.; Calsina, B.; Mancikova, V.; Remacha, L.; Richter, S.; Ercolino, T.; Rogowski-Lehmann, N.; et al. PheoSeq: A Targeted Next-Generation Sequencing Assay for Pheochromocytoma and Paraganglioma Diagnostics. J. Mol. Diagn. 2017, 19, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Renella, R.; Carnevale, J.; Schneider, K.A.; Hornick, J.L.; Rana, H.Q.; Janeway, K.A. Exploring the association of succinate dehydrogenase complex mutations with lymphoid malignancies. Fam. Cancer 2014, 13, 507–511. [Google Scholar] [CrossRef]
- Vanharanta, S.; Buchta, M.; McWhinney, S.R.; Virta, S.K.; Peçzkowska, M.; Morrison, C.D.; Lehtonen, R.J.; Januszewicz, A.; Järvinen, H.; Juhola, M.; et al. Early-Onset Renal Cell Carcinoma as a Novel Extraparaganglial Component of SDHB-Associated Heritable Paraganglioma. Am. J. Hum. Genet. 2004, 74, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Miettinen, M.; Lasota, J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs)—A review. Int. J. Biochem. Cell Biol. 2014, 53, 514–519. [Google Scholar] [CrossRef]
- Ugarte-Camara, M.; Fernandez-Prado, R.; Lorda, I.; Rossello, G.; Gonzalez-Enguita, C.; Cannata-Ortiz, P.; Ortiz, A. Positive/retained SDHB immunostaining in renal cell carcinomas associated to germline SDHB-deficiency: Case report. Diagn. Pathol. 2019, 14, 42. [Google Scholar] [CrossRef]
- Gill, A.J. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2018, 72, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Killian, J.K.; Miettinen, M.; Walker, R.L.; Wang, Y.; Zhu, Y.J.; Waterfall, J.J.; Noyes, N.; Retnakumar, P.; Yang, Z.; Smith, W.I.; et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci. Transl. Med. 2014, 6, 268ra177. [Google Scholar] [CrossRef]
- Roszko, K.L.; Blouch, E.; Blake, M.; Powers, J.; Tischler, A.; Hodin, R.; Sadow, P.; Lawson, E.A. Case Report of a Prolactinoma in a Patient With a Novel MAX Mutation and Bilateral Pheochromocytomas. J. Endocr. Soc. 2017, 1, 1401–1407. [Google Scholar] [CrossRef]
- Hernandez, K.G.; Ezzat, S.; Morel, C.F.; Swallow, C.; Otremba, M.; Dickson, B.; Asa, S.; Mete, O. Familial pheochromocytoma and renal cell carcinoma syndrome: TMEM127 as a novel candidate gene for the association. Virchows Arch. 2015, 466, 727–732. [Google Scholar] [CrossRef]
- Muller, M.; Ferlicot, S.; Guillaud-Bataille, M.; Le Teuff, G.; Genestie, C.; Deveaux, S.; Slama, A.; Poulalhon, N.; Escudier, B.; Albiges, L.; et al. Reassessing the clinical spectrum associated with hereditary leiomyomatosis and renal cell carcinoma syndrome in French FH mutation carriers. Clin. Genet. 2017, 92, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Xekouki, P.; Brennand, A.; Whitelaw, B.; Pacak, K.; Stratakis, C.A. The 3PAs: An Update on the Association of Pheochromocytomas, Paragangliomas, and Pituitary Tumors. Horm. Metab. Res. 2019, 51, 419–436. [Google Scholar] [CrossRef]
- O’Toole, S.M.; Dénes, J.; Robledo, M.; A Stratakis, C.; Korbonits, M. 15 YEARS OF PARAGANGLIOMA: The association of pituitary adenomas and phaeochromocytomas or paragangliomas. Endocr. -Related Cancer 2015, 22, T105–T122. [Google Scholar] [CrossRef]
- Canu, L.; Puglisi, S.; Berchialla, P.; De Filpo, G.; Brignardello, F.; Schiavi, F.; Ferrara, A.M.; Zovato, S.; Luconi, M.; Pia, A.; et al. Depositated in Figshare 5 June 2021. [CrossRef]
- AIOM-AIRTUM. I Numeri del Cancro in Italia. Available online: https://www.aiom.it/wp-content/uploads/2019/09/2019_Numeri_Cancro-operatori-web.pdf (accessed on 1 February 2021).
- Lam, A.K.-Y. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef]
- Tischler, A.; de Krijger, R. Phaeocromocytoma. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; pp. 183–189. [Google Scholar]
- Kimura, N.; Cappella, C. Extraadrenal paraganglioma. In WHO Classification of Tumours of Endocrine Organs, 4th ed.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; pp. 190–195. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Khorram-Manesh, A.; Jansson, S.; Wangberg, B.; Nilsson, O.; Tisell, L.-E.; Ahlman, H. Mortality Associated with Pheochromocytoma: Increased Risk for Additional Tumors. Ann. N. Y. Acad. Sci. 2006, 1073, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Papathomas, T.G.; Oudijk, L.; Persu, A.; Gill, A.J.; Van Nederveen, F.; Tischler, A.; Tissier, F.; Volante, M.; Matias-Guiu, X.; Smid, M.; et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: A multicenter interobserver variation analysis using virtual microscopy: A Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod. Pathol. 2015, 28, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, C.; Abbe, P.; Hemesath, T.J.; Bille, K.; Fisher, D.E.; Ortonne, J.-P.; Ballotti, R. Microphthalmia Gene Product as a Signal Transducer in cAMP-Induced Differentiation of Melanocytes. J. Cell Biol. 1998, 142, 827–835. [Google Scholar] [CrossRef]
- Bertolotto, C.; Lesueur, F.; Giuliano, S.; Strub, T.; De Lichy, M.; Bille, K.; Dessen, P.; D’Hayer, B.; Mohamdi, H.; Remenieras, A.; et al. A SUMOylation-defective MITF germline mutation predisposes to melanoma and renal carcinoma. Nature 2011, 480, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Woods, S.L.; Boyle, G.M.; Aoude, L.G.; MacGregor, S.; Zismann, V.; Gartside, M.; Cust, A.E.; Haq, R.; Harland, M.; et al. A novel recurrent mutation in MITF predisposes to familial and sporadic melanoma. Nature 2011, 480, 99–103. [Google Scholar] [CrossRef]
- Castro-Vega, L.J.; Kiando, S.R.; Burnichon, N.; Buffet, A.; Amar, L.; Simian, C.; Berdelou, A.; Galan, P.; Schlumberger, M.; Bouatia-Naji, N.; et al. The MITF, p.E318K Variant, as a Risk Factor for Pheochromocytoma and Paraganglioma. J. Clin. Endocrinol. Metab. 2016, 101, 4764–4768. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Yang, H.; Pass, H.; Krausz, T.; Testa, J.R.; Gaudino, G. BAP1 and cancer. Nat. Rev. Cancer 2013, 13, 153–159. [Google Scholar] [CrossRef]
- Maffeis, V.; Cappellesso, R.; Nicolè, L.; Guzzardo, V.; Menin, C.; Elefanti, L.; Schiavi, F.; Guido, M.; Fassina, A. Loss of BAP1 in Pheochromocytomas and Paragangliomas Seems Unrelated to Genetic Mutations. Endocr. Pathol. 2019, 30, 276–284. [Google Scholar] [CrossRef]
- Bugalho, M.J.; Silva, A.L.; Domingues, R. Coexistence of paraganglioma/pheochromocytoma and papillary thyroid carcinoma: A four-case series analysis. Fam. Cancer 2015, 14, 603–607. [Google Scholar] [CrossRef]
- Miettinen, M.; Sarlomo-Rikala, M.; Cue, P.M.; Czapiewski, P.; Langfort, R.; Waloszczyk, P.; Wazny, K.; Biernat, W.; Lasota, J.; Wang, Z. Mapping of Succinate Dehydrogenase Losses in 2258 Epithelial Neoplasms. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 31–36. [Google Scholar] [CrossRef]
- Plouin, P.F.; Amar, L.; Dekkers, O.M.; Fassnacht, M.; Gimenez-Roqueplo, A.P.; Lenders, J.W.M.; Lussey-Lepoutre, C.; Steichen, O. European Society of Endocrinology Clinical Practice Guideline for long-term follow-up of patients operated on for a phaeochromocytoma or a paraganglioma. Eur. J. Endocrinol. 2016, 174, G1–G10. [Google Scholar] [CrossRef]
- Mannelli, M.; Canu, L.; Ercolino, T.; Rapizzi, E.; Martinelli, S.; Parenti, G.; De Filpo, G.; Nesi, G. DIAGNOSIS of ENDOCRINE DISEASE: SDHx mutations: Beyond pheochromocytomas and paragangliomas. Eur. J. Endocrinol. 2018, 178, R11–R17. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K. PCR-Based Detection Methods for Single-Nucleotide Polymorphism or Mutation. Adv. Clin. Chem. 2017, 80, 45–72. [Google Scholar] [CrossRef]
- Mitra, A.; Singh, S.V.; Garg, V.K.; Sharma, M.; Chaturvedi, R.; Rath, S.K. Protective association exhibited by the single nucleotide polymorphism (SNP) rs1052133 in the gene human 8-oxoguanine DNA glycosylase (hOGG1) with the risk of squamous cell carcinomas of the head & neck (SCCHN) among north Indians. Indian J. Med. Res. 2011, 133, 605–612. [Google Scholar]
- Hoffman, J.; Fejerman, L.; Hu, D.; Huntsman, S.; Li, M.; John, E.M.; Torres-Mejía, G.; Kushi, L.H.; Ding, Y.C.; Weitzel, J.; et al. Identification of novel common breast cancer risk variants at the 6q25 locus among Latinas. Breast Cancer Res. 2019, 21, 3. [Google Scholar] [CrossRef]
- Zhu, J.; Jia, W.; Wu, C.; Fu, W.; Xia, H.; Liu, G.; He, J. Base Excision Repair Gene Polymorphisms and Wilms Tumor Susceptibility. EBioMedicine 2018, 33, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.P.H.; De Herder, W. Energy and metabolic alterations in predisposition to pheochromocytomas and paragangliomas: The so-called Warburg (and more) effect, 15 years on. Endocr.-Relat. Cancer 2015, 22, E5–E7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mravec, B.; Dubravicky, J.; Tibensky, M.; Horvathova, L. Effect of the nervous system on cancer: Analysis of clinical studies. Bratisl. Lek. List. 2019, 120, 119–123. [Google Scholar] [CrossRef]
- Mravec, B.; Horvathova, L.; Hunakova, L. Neurobiology of Cancer: The Role of β-Adrenergic Receptor Signaling in Various Tumor Environments. Int. J. Mol. Sci. 2020, 21, 7958. [Google Scholar] [CrossRef] [PubMed]
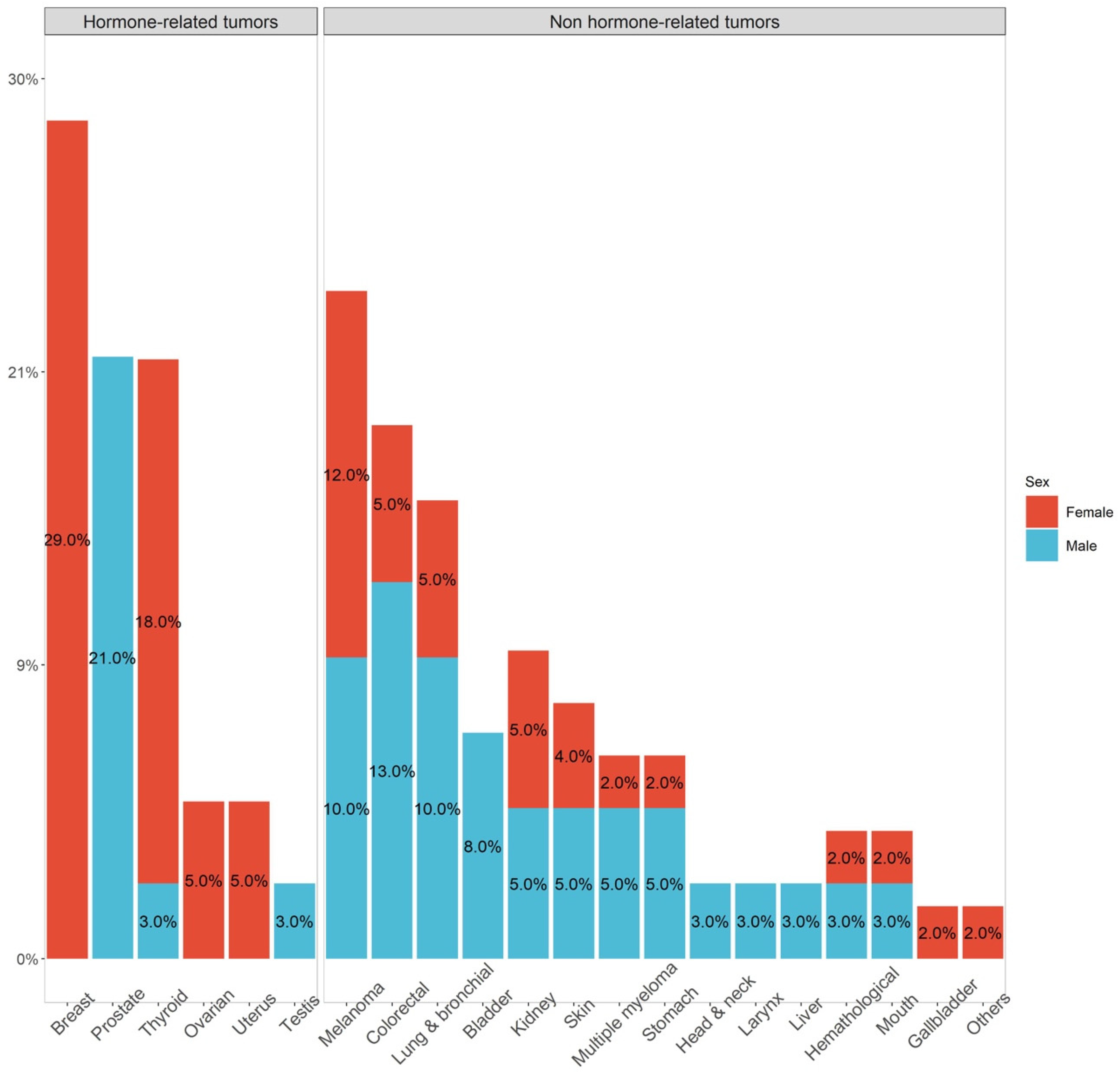
| Characteristics | n. of Evaluated Patients | N |
|---|---|---|
| Sex Female Male | 741 | 415/741 (56.0%) 326/741 (44.0%) |
| Age (years) at PPGL diagnosis, median | 741 | 49 [IQR: 36–60] |
| Metastatic PPGL | 612 | 54 (8.8%) |
| Functioning PPGL | 572 | 379 (66.3%) |
| PPGL localization | 741 | |
| Abdominal PGL | 172 (23.1%) | |
| Mediastinal PGL | 2 (0.3%) | |
| HNPGL | 3 (0.4%) | |
| PCC | 37 (5.0%) | |
| Abdominal PGL + PCC | 58 (7.8%) | |
| Mediastinal PGL + HNPGL | 56 (7.6%) | |
| Abdominal PGL + HNPGL | 5 (0.7%) | |
| PCC + HNPGL | 408 (55.1%) | |
| Family history of tumor | 727 | 264 (36.3%) |
| Risk factors | ||
| Smoke | 672 | 159 (23.7%) |
| Alcohol | 678 | 32 (4.7%) |
| Exposure to toxic substances | 625 | 29 (4.6%) |
| Genetic analysis | 515 | |
| Wild type | 349 (67.8%) | |
| SDHD | 86 (16.7%) | |
| SDHB | 45 (8.7%) | |
| MAX | 12 (2.3%) | |
| TMEM127 | 11 (2.1%) | |
| SDHC | 7 (1.4%) | |
| SDHA | 4 (0.8%) | |
| SDHAF2 | 1 (0.2%) | |
| Cluster 1 | 515 | 141 (26.6%) |
| Cluster 2 | 515 | 23 (4.5%) |
| Second malignant tumor | 741 | 95 (12.8%) |
| Death | 26 (3.5%) | |
| Death for PCC/PGL | 11 (1.5%) | |
| Follow up months, median | 48 [IQR: 12–108] |
| Characteristics | Patients with Mutation (n. 166) | Patients without Mutation (n. 349) | p Value |
|---|---|---|---|
| Sex | 0.537 | ||
| Female | 92/166 (55.4%) | 205/349 (58.7%) | |
| Male | 74/166 (44.6%) | 144/349 (41.3%) | |
| Age (years) at PPGL diagnosis, median | 37 (IQR: 28–46.5) | 52 (IQR: 41–61) | <0.001 |
| Age (years) at second malignancy | 57 (IQR: 47–65.5) | 56.5 (IQR: 37.8–64) | 0.527 |
| Metastatic PPGL | 21 (14.5%) | 21 (6.8%) | 0.014 |
| Functioning PPGL | 39 (30.5%) | 202 (70.1%) | <0.001 |
| HNPGL | 92 (55.4%) | 84 (24.1%) | <0.001 |
| Family history of tumor | 62 (38.5%) | 174 (50.0%) | 0.020 |
| Risk factor: smoke | 30 (20.5%) | 90 (28.1%) | 0.105 |
| Risk factor: alcohol | 4 (2.7%) | 12 (3.7%) | 0.781 |
| Risk factor: exposure to toxic substances | 0 (0.0%) | 15 (4.8%) | 0.025 |
| Second malignant tumor | 0.113 | ||
| Before | 4 (22.2%) | 34 (49.3%) | |
| After | 10 (55.6%) | 23 (33.3%) | |
| Simultaneously | 4 (22.2%) | 12 (17.4%) |
| Characteristics | Patients with Second Malignant Tumors (n 95) | Patients without Second Malignant Tumors (n 646) | p Value |
|---|---|---|---|
| Sex Female Male | 56/95 (58.9%) 39/95 (41.1%) | 359/646 (55.6%) 287/646 (44.4%) | 0.61 |
| Age (years) at PPGL diagnosis, median | 58 (IQR: 50–65.8) | 47 (IQR: 35–58) | <0.001 |
| Metastatic PPGL | 5/76 (6.6%) | 49/536 (9.1%) | 0.60 |
| Functioning forms | 51/72 (70.8%) | 328/500 (65.6%) | 0.46 |
| PPGL localization | 0.43 | ||
| Abdominal PGL | 23/95 (24.3%) | 149/646 (23.1%) | |
| Mediastinal PGL | 0/95 (0.0%) | 2/646 (0.3%) | |
| HNPGL | 0/95 (0.0%) | 3/646 (0.4%) | |
| PCC | 2/95 (2.1%) | 35/646 (5.4%) | |
| Abdominal PGL + PCC | 4/95 (4.2%) | 54/646 (8.4%) | |
| Mediastinal PGL + HNPGL | 6/95 (6.3%) | 50/646 (7.7%) | |
| Abdominal PGL + HNPGL | 0/95 (0.0%) | 5/646 (0.8%) | |
| PCC + HNPGL | 60/95 (63.1%) | 348/646 (53.9%) | |
| Positive family history of cancer | 36/89 (40.4%) | 228/638 (35.7%) | 0.45 |
| Risk factors | |||
| Smoke | 21/83 (25.3%) | 138/589 (23.4%) | 0.81 |
| Alcohol | 4/86 (4.7%) | 28/592 (4.7%) | 1.00 |
| Exposure to toxic substances | 3/77 (3.9%) | 26/548 (4.7%) | 0.97 |
| Germ-line mutation | 10/56 (17.9%) | 156/459 (34.0%) | 0.01 |
| Genetic test | |||
| Wild type | 46/56 (82.1%) | 303/459 (66.0%) | 0.18 |
| SDHA | 0/56 (0.0%) | 4/459 (0.9%) | 1.00 |
| SDHB | 1/56 (1.7%) | 44/459 (9.6%) | 0.08 |
| SDHC | 0/56 (0.0%) | 7/459 (1.5%) | 0.73 |
| SDHD | 5/56 (8.3%) | 81/459 (17.2%) | 0.12 |
| SDHAF2 | 0/56 (0.0%) | 1/459 (0.2%) | 1.00 |
| MAX | 2/56 (3.6%) | 10/459 (2.2%) | 0.86 |
| TMEM127 | 2/56 (3.5%) | 9/459 (2.0%) | 0.79 |
| Cluster 1 | 6/54 (11.1%) | 137/459 (29.8%) | 0.006 |
| Cluster 2 | 4/54 (7.4%) | 19/459 (4.1%) | 0.45 |
| Years between PPGL and second malignant tumor, median | 6 (IQR: 2–14) | ||
| Death | 7/95 (7.4%) | 19/646 (2.9%) | 0.06 |
| Death for PPGL | 3/95 (3.2%) | 8/646 (1.2%) | 0.32 |
| Follow up months, median | 36 (IQR: 12–108) | 48 (IQR: 15–108) | 0.47 |
| HR | 95% CI | p Value | ||
|---|---|---|---|---|
| Males vs. females | 0.79 | 0.45 | 1.39 | 0.42 |
| Age category | ||||
| 50–59 years vs. <50 years | 2.27 | 1.13 | 4.53 | 0.021 |
| >60 years vs. <50 years | 2.22 | 1.05 | 4.69 | 0.036 |
| Metastatic PPGLs (yes vs. no) | 0.20 | 0.03 | 1.49 | 0.12 |
| Functioning PPGLs (no vs. yes) | 0.80 | 0.38 | 1.66 | 0.54 |
| Parasympathetic vs. sympathetic lesions | 0.89 | 0.50 | 1.60 | 0.71 |
| Family history of cancer (yes vs. no) | 1.80 | 1.03 | 3.14 | 0.04 |
| Germ-line mutation vs. wild type | 0.27 | 0.11 | 0.63 | 0.003 |
| Cluster 1 (positive vs. negative) | 0.31 | 0.13 | 0.73 | 0.008 |
| Cluster 2 (positive vs. negative) | 0.82 | 0.24 | 2.74 | 0.75 |
| Risk factors (yes vs. no) | ||||
| Smoke | 1.18 | 0.58 | 2.40 | 0.64 |
| Alcohol | 2.46 | 0.75 | 8.06 | 0.14 |
| Exposure to toxic substances | 0.57 | 0.01 | 4.11 | 0.67 |
| A (n. 741) | B (n. 515) | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |||
| Age category | ||||||||
| 50–59 years vs. <50 years | 2.50 | 1.15 | 5.44 | 0.021 | 1.71 | 0.72 | 4.07 | 0.23 |
| >60 years vs. <40 years | 3.46 | 1.67 | 7.15 | <0.001 | 1.46 | 0.54 | 3.94 | 0.46 |
| Males vs. females | 1.23 | 0.63 | 2.41 | 0.54 | 1.18 | 0.53 | 2.60 | 0.69 |
| Smoker vs. non-smoker | 2.10 | 0.82 | 5.39 | 0.12 | 1.64 | 0.57 | 4.72 | 0.36 |
| Genetic test (positive vs. negative) | 0.25 | 0.10 | 0.63 | 0.003 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Canu, L.; Puglisi, S.; Berchialla, P.; De Filpo, G.; Brignardello, F.; Schiavi, F.; Ferrara, A.M.; Zovato, S.; Luconi, M.; Pia, A.; et al. A Multicenter Epidemiological Study on Second Malignancy in Non-Syndromic Pheochromocytoma/Paraganglioma Patients in Italy. Cancers 2021, 13, 5831. https://doi.org/10.3390/cancers13225831
Canu L, Puglisi S, Berchialla P, De Filpo G, Brignardello F, Schiavi F, Ferrara AM, Zovato S, Luconi M, Pia A, et al. A Multicenter Epidemiological Study on Second Malignancy in Non-Syndromic Pheochromocytoma/Paraganglioma Patients in Italy. Cancers. 2021; 13(22):5831. https://doi.org/10.3390/cancers13225831
Chicago/Turabian StyleCanu, Letizia, Soraya Puglisi, Paola Berchialla, Giuseppina De Filpo, Francesca Brignardello, Francesca Schiavi, Alfonso Massimiliano Ferrara, Stefania Zovato, Michaela Luconi, Anna Pia, and et al. 2021. "A Multicenter Epidemiological Study on Second Malignancy in Non-Syndromic Pheochromocytoma/Paraganglioma Patients in Italy" Cancers 13, no. 22: 5831. https://doi.org/10.3390/cancers13225831
APA StyleCanu, L., Puglisi, S., Berchialla, P., De Filpo, G., Brignardello, F., Schiavi, F., Ferrara, A. M., Zovato, S., Luconi, M., Pia, A., Appetecchia, M., Arvat, E., Letizia, C., Maccario, M., Parasiliti-Caprino, M., Altieri, B., Faggiano, A., Modica, R., Morelli, V., ... Reimondo, G. (2021). A Multicenter Epidemiological Study on Second Malignancy in Non-Syndromic Pheochromocytoma/Paraganglioma Patients in Italy. Cancers, 13(22), 5831. https://doi.org/10.3390/cancers13225831















