Real-World Data from a Refractory Triple-Negative Breast Cancer Cohort Selected Using a Clinical Data Warehouse Approach
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
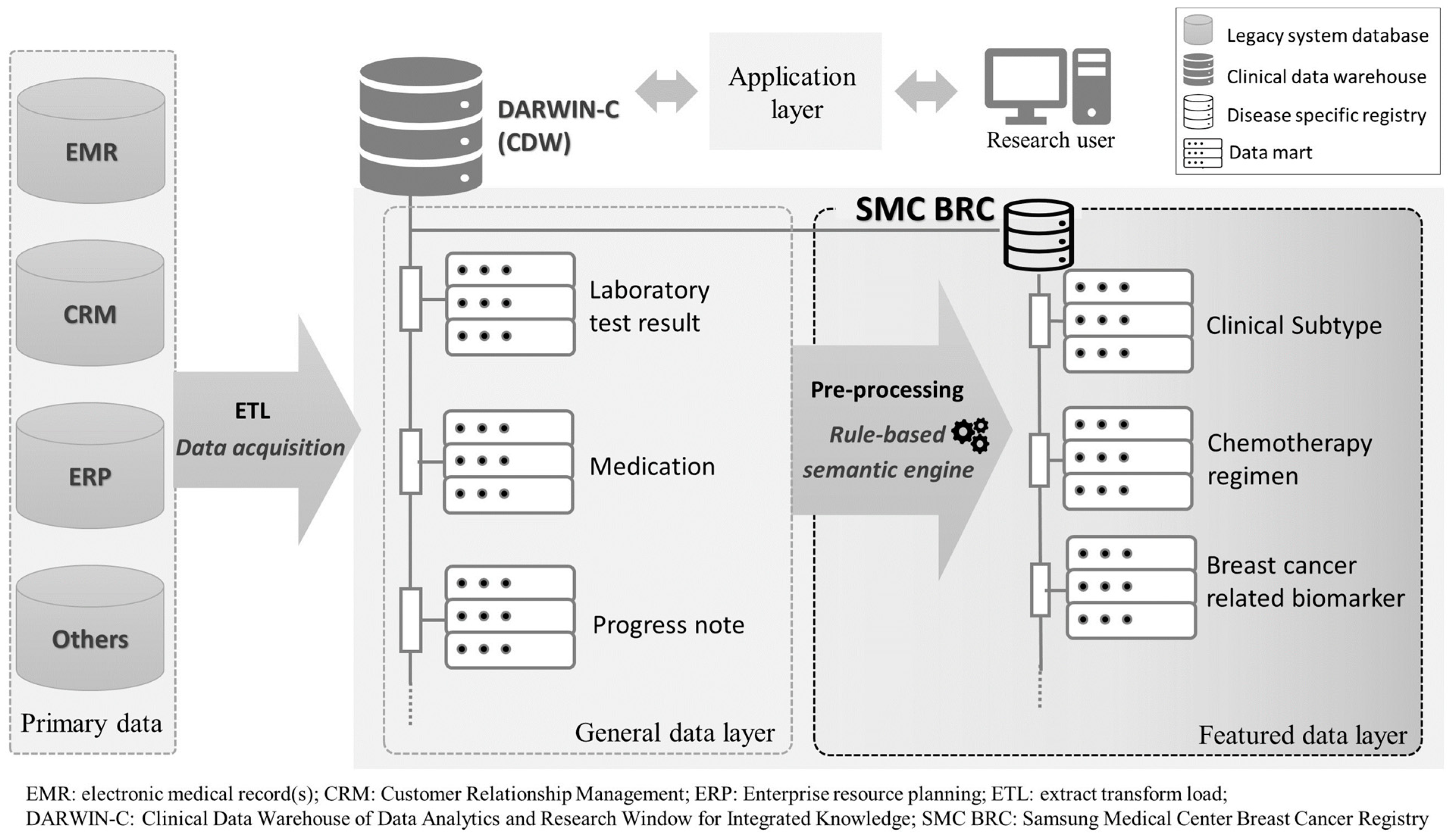
2.1. Data Source: The SMC Breast Cancer Registry
2.2. Study Population
2.3. Clinical and Pathological Definitions
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Chemotherapy
3.3. Survival and Prognosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harbeck, N.; Gnant, M. Breast cancer. Lancet 2017, 389, 1134–1150. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K.; et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients: A single-institution compilation compared with the National Cancer Institute’s Surveillance, Epidemiology, and End Results database. Cancer 2007, 110, 876–884. [Google Scholar] [CrossRef]
- Miles, D.W.; Dieras, V.; Cortes, J.; Duenne, A.A.; Yi, J.; O’Shaughnessy, J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: Pooled and subgroup analyses of data from 2447 patients. Ann. Oncol. 2013, 24, 2773–2780. [Google Scholar] [CrossRef]
- Yardley, D.A.; Coleman, R.; Conte, P.; Cortes, J.; Brufsky, A.; Shtivelband, M.; Young, R.; Bengala, C.; Ali, H.; Eakel, J.; et al. nab-Paclitaxel plus carboplatin or gemcitabine versus gemcitabine plus carboplatin as first-line treatment of patients with triple-negative metastatic breast cancer: Results from the tnAcity trial. Ann. Oncol. 2018, 29, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Dieras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Schmid, P.; Dent, R.; O’Shaughnessy, J. Pembrolizumab for Early Triple-Negative Breast Cancer. Reply. N. Engl. J. Med. 2020, 382, e108. [Google Scholar] [CrossRef] [PubMed]
- James, M.; Dixit, A.; Robinson, B.; Frampton, C.; Davey, V. Outcomes for Patients with Non-metastatic Triple-negative Breast Cancer in New Zealand. Clin. Oncol. 2019, 31, 17–24. [Google Scholar] [CrossRef]
- Polley, M.C.; Dickler, M.N.; Sinnwell, J.; Tenner, K.; de la Haba, J.; Loibl, S.; Goetz, M.P.; Bergh, J.; Roberston, J.; Couch, F.; et al. A clinical calculator to predict disease outcomes in women with hormone receptor-positive advanced breast cancer treated with first-line endocrine therapy. Breast Cancer Res. Treat. 2021, 189, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Tang, S.C.; Li, K.; Wu, J.; Li, X.; Ren, H.; Sun, X. Predicting the Survival of Triple-Negative Breast Cancer in Different Stages: A SEER Population Based Research Referring to Clinicopathological Factors. Cancer Investig. 2020, 38, 549–558. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, S.; Zhang, X.B.; Wang, P.; Hou, G.F.; Zhang, J. Clinicopathological and prognostic characteristics of triple-negative breast cancer (TNBC) in Chinese patients: A retrospective study. Asian Pac. J. Cancer Prev. 2013, 14, 3779–3784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef] [Green Version]
- Skinner, K.E.; Haiderali, A.; Huang, M.; Schwartzberg, L.S. Real-world effectiveness outcomes in patients diagnosed with metastatic triple-negative breast cancer. Future Oncol. 2021, 17, 931–941. [Google Scholar] [CrossRef]
- Mantel, N.; Haenszel, W. Statistical aspects of the analysis of data from retrospective studies of disease. J. Natl. Cancer Inst. 1959, 22, 719–748. [Google Scholar]
- Khozin, S.; Abernethy, A.P.; Nussbaum, N.C.; Zhi, J.; Curtis, M.D.; Tucker, M.; Lee, S.E.; Light, D.E.; Gossai, A.; Sorg, R.A.; et al. Characteristics of Real-World Metastatic Non-Small Cell Lung Cancer Patients Treated with Nivolumab and Pembrolizumab During the Year Following Approval. Oncologist 2018, 23, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, A.C.; Hammond, M.E.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J. Clin. Oncol. 2007, 25, 118–145. [Google Scholar] [CrossRef] [Green Version]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litiere, S.; de Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [Green Version]
- Gagalova, K.K.; Elizalde, M.A.L.; Portales-Casamar, E.; Görges, M. What You Need to Know Before Implementing a Clinical Research Data Warehouse: Comparative Review of Integrated Data Repositories in Health Care Institutions. JMIR Form. Res. 2020, 4, e17687. [Google Scholar] [CrossRef]
- Abernethy, A.P.; Gippetti, J.; Parulkar, R.; Revol, C. Use of electronic health record data for quality reporting. J. Oncol. Pract. 2017, 13, 530–534. [Google Scholar] [CrossRef]
- Booth, C.M.; Karim, S.; Mackillop, W.J. Real-world data: Towards achieving the achievable in cancer care. Nat. Rev. Clin. Oncol. 2019, 16, 312–325. [Google Scholar] [CrossRef]
- Garrison, L.P., Jr.; Neumann, P.J.; Erickson, P.; Marshall, D.; Mullins, C.D. Using real-world data for coverage and payment decisions: The ISPOR Real-World Data Task Force report. Value Health 2007, 10, 326–335. [Google Scholar] [CrossRef] [Green Version]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, W.D.; Smith, I.E.; Reis-Filho, J.S. Triple-negative breast cancer. N. Engl. J. Med. 2010, 363, 1938–1948. [Google Scholar] [CrossRef] [Green Version]
- Mittendorf, E.A.; Zhang, H.; Barrios, C.H.; Saji, S.; Jung, K.H.; Hegg, R.; Koehler, A.; Sohn, J.; Iwata, H.; Telli, M.L.; et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): A randomised, double-blind, phase 3 trial. Lancet 2020, 396, 1090–1100. [Google Scholar] [CrossRef]
- Miles, D.; Gligorov, J.; Andre, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- Steward, L.; Conant, L.; Gao, F.; Margenthaler, J.A. Predictive factors and patterns of recurrence in patients with triple negative breast cancer. Ann. Surg. Oncol. 2014, 21, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Radosa, J.C.; Eaton, A.; Stempel, M.; Khander, A.; Liedtke, C.; Solomayer, E.F.; Karsten, M.; Pilewskie, M.; Morrow, M.; King, T.A. Evaluation of Local and Distant Recurrence Patterns in Patients with Triple-Negative Breast Cancer According to Age. Ann. Surg. Oncol. 2017, 24, 698–704. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Xue, X.; Hu, C.; Xu, H.; Kou, D.; Li, R.; Li, M. Comparison of Clinicopathological Features and Prognosis in Triple-Negative and Non-Triple Negative Breast Cancer. J. Cancer 2016, 7, 167–173. [Google Scholar] [CrossRef] [Green Version]


| Group | De Novo mTNBC | Recurrence of mTNBC after Curative Surgery | ||
|---|---|---|---|---|
| Nonrefractory mTNBC | Refractory mTNBC | |||
| Refractory mTNBC after Surgery | Unresectable mTNBC d/t Progression during NAC | |||
| Patient, no. | 69 | 131 | 207 | 44 |
| 251 | ||||
| Age | ||||
| At diagnosis, median, yr. (range) | 49 (28–89) | 51 (29–80) | 45 (24–90) | 46 (28–76) |
| 46 (24–90) | ||||
| <40 | 15 (22) | 20 (15) | 67 (32) | 16 (36) |
| 83 (33) | ||||
| <60 | 53 (77) | 111 (85) | 192 (93) | 41(93) |
| 233 (93) | ||||
| ≥60 | 16 (23) | 20 (15) | 15(7) | 3 (7) |
| 18 (7) | ||||
| Menopausal status at diagnosis | ||||
| Premenopause | 29 (42) | 52 (40) | 130 (63) | 27 (61) |
| 157 (63) | ||||
| Postmenopause | 34 (49) | 64 (49) | 64 (31) | 14 (32) |
| 78 (31) | ||||
| Unknown | 6 (9) | 15 (11) | 13 (6) | 3 (7) |
| 16 (6) | ||||
| Histologic subtypes | ||||
| Ductal | 62 (90) | 113 (86) | 180 (87) | 43 (98) |
| 223 (89) | ||||
| Lobular | 1 (1) | 3 (2) | 2 (1) | 0 |
| 2 (1) | ||||
| Metaplastic | 1 (1) | 9 (7) | 17 (8) | 0 |
| 17 (7) | ||||
| Other | 5 (7) | 6 (5) | 8 (4) | 1 (2) |
| 9 (4) | ||||
| Ki-67 | ||||
| 1+ | 6 (9) | 29 (22) | 19 (9) | 6 (14) |
| 25 (10) | ||||
| 2+ | 12 (17) | 25 (19) | 38 (18) | 7 (16) |
| 45 (18) | ||||
| ≥3 | 46 (67) | 62 (47) | 146 (71) | 31 (70) |
| 177 (71) | ||||
| Unknown | 5 (7) | 15 (11) | 3 (1) | 0 |
| 3 (1) | ||||
| BRCA mutation | ||||
| BRCA1 | 3/13 (23) | 2/15 (13) | 9/53 (17) | 1/5 (20) |
| 10/58 (17) | ||||
| BRCA2 | 1/13 (8) | 0/15 | 3/53 (6) | 0/5 |
| 3/58 (5) | ||||
| Distant metastasis site at diagnosis of MBC | ||||
| Bone | 29 (42) | 36 (27) | 51 (25) | 11 (25) |
| 62 (25) | ||||
| Brain | 0 | 8 (6) | 31 (15) | 4 (9) |
| 35 (14) | ||||
| Liver | 16 (23) | 20(15) | 34 (16) | 6 (14) |
| 40 (16) | ||||
| Lung | 24 (35) | 59 (45) | 73 (35) | 18 (41) |
| 91 (36) | ||||
| First-line palliative chemotherapy | ||||
| AC | 35 (51) | 5 (4) | 7 (3) | 4 (9) |
| Taxane only | 9 (13) | 38 (29) | 43 (21) | 6 (14) |
| 49 (20) | ||||
| Taxane + G | 0 | 15 (11) | 8 (4) | 1 (2) |
| Taxane + platinum | 10 (15) | 34 (26) | 51 (25) | 13 (30) |
| 64 (25) | ||||
| GP (C) | 3 (4) | 6 (5) | 60 (29) | 11 (25) |
| 71 (28) | ||||
| NX | 0 | 0 | 2 (1) | 1 (2) |
| Capecitabine | 8 (12) | 25 (19) | 18 (9) | 7 (16) |
| Other | 4 (6) | 8 (6) | 18 (9) | 1 (2) |
| Second-line palliative chemotherapy | ||||
| Patients, no. | 57/69 (83) | 105/131 (80) | 167/207 (81) | 35/44 (80) |
| 202 (80) | ||||
| AC | 16 (28) | 10 (10) | 8 (5) | 1 (3) |
| Taxane only | 12 (21) | 7 (7) | 24 (14) | 3 (9) |
| Taxane + G | 0 | 1 (1) | 0 | 0 |
| Taxane + platinum | 11 (19) | 12 (11) | 19 (11) | 2 (6) |
| GP (C) | 5 (9) | 27 (26) | 43 (26) | 8 (23) |
| 51 (25) | ||||
| NX | 1 (2) | 0 | 4 (2) | 1 (3) |
| Capecitabine | 10 (18) | 39 (37) | 32 (19) | 13 (37) |
| 45 (22) | ||||
| Other | 2 (4) | 9 (9) | 37 (22) | 7 (20) |
| No., (%) | Nonrefractory mTNBC | Refractory mTNBC |
|---|---|---|
| Patient, no. | 131 | 207 |
| Neoadjuvant chemotherapy | ||
| Yes | 31 (24) | 130 (63) |
| pCR | 2/31 (6) | 4/130 (3) |
| Grade, histologic | ||
| Well | 5 (4) | 4 (2) |
| Moderate | 40 (31) | 38 (18) |
| Poorly differentiated | 76 (58) | 156 (75) |
| Unknown | 10 (8) | 9 (4) |
| pT stage | ||
| No residual tumor | 2 (2) | 4 (2) |
| 1 | 46 (35) | 55 (27) |
| 2 | 75 (57) | 101 (49) |
| 3 | 8 (6) | 44 (21) |
| 4 | 0 | 3 (1) |
| pN stage | ||
| 0 | 58 (44) | 83 (40) |
| 1 | 41 (31) | 65 (31) |
| 2 | 21 (16) | 30 (14) |
| 3 | 11 (8) | 29 (14) |
| Group | Recurrence of mTNBC after Curative Surgery | |||
|---|---|---|---|---|
| De Novo mTNBC | Nonrefractory mTNBC | Refractory mTNBC | ||
| Median, Months | Refractory mTNBC after Surgery | Nonresectable TNBC d/t Progression during NAC | ||
| DFS (range) | - | 30.1 (18.3–217.9) | 7.6 (7.4–17.9) | |
| PFS (95% CI) | 3.8 (3.3–4.3) | 6.2 (5.2–7.2) | 4.5 (3.7–5.3) | 3.7 (1.6–5.8) |
| 4.2 (1.6–5.8) | ||||
| 2nd PFS (range) | 3.7 | 5.3 | 3.0 | 2.1 |
| 2.6 (0–25.0) | ||||
| OS (95% CI) | 17.3 (15.9–18.7) | 24.8 (21.4–28.2) | 14.4 (12.5–16.5) | 12.9 (9.7–16.1) |
| 14.3 (12.5–16.1) | ||||
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| Age < 40 | 0.997 (0.793–1.252) | 0.977 | NA | NA |
| Non-IDC histology | 0.677 (0.403–1.139) | 0.141 | NA | NA |
| Ki-67 4+ (76–100%) | 0.978 (0.795–1.201) | 0.829 | NA | NA |
| Bone metastasis | 1.299 (1.045–1.615) | 0.018 | 1.184 (0.649–1.77) | 0.135 |
| Brain metastasis | 1.162 (0.820–1.646) | 0.398 | NA | NA |
| Liver metastasis | 1.947 (1.506–2.518) | <0.001 | 2.009 (1.552–2.600) | <0.001 |
| Lung metastasis | 0.918 (0.746–1.129) | 0.416 | NA | |
| Leptomeningeal seeding | 1.744 (1.230–2.473) | 0.002 | 1.862 (1.311–2.645) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Kim, H.J.; Kim, H.; Kim, H.R.; Jo, H.; Hong, J.; Kim, R.; Kim, J.-Y.; Ahn, J.S.; Im, Y.-H.; et al. Real-World Data from a Refractory Triple-Negative Breast Cancer Cohort Selected Using a Clinical Data Warehouse Approach. Cancers 2021, 13, 5835. https://doi.org/10.3390/cancers13225835
Kim H, Kim HJ, Kim H, Kim HR, Jo H, Hong J, Kim R, Kim J-Y, Ahn JS, Im Y-H, et al. Real-World Data from a Refractory Triple-Negative Breast Cancer Cohort Selected Using a Clinical Data Warehouse Approach. Cancers. 2021; 13(22):5835. https://doi.org/10.3390/cancers13225835
Chicago/Turabian StyleKim, Hana, Hyo Jung Kim, Hongsik Kim, Hye Ryeon Kim, Hyunji Jo, Joohyun Hong, Ryul Kim, Ji-Yeon Kim, Jin Seok Ahn, Young-Hyuck Im, and et al. 2021. "Real-World Data from a Refractory Triple-Negative Breast Cancer Cohort Selected Using a Clinical Data Warehouse Approach" Cancers 13, no. 22: 5835. https://doi.org/10.3390/cancers13225835
APA StyleKim, H., Kim, H. J., Kim, H., Kim, H. R., Jo, H., Hong, J., Kim, R., Kim, J.-Y., Ahn, J. S., Im, Y.-H., Lee, S. K., Kim, H., Shin, S.-Y., & Park, Y. H. (2021). Real-World Data from a Refractory Triple-Negative Breast Cancer Cohort Selected Using a Clinical Data Warehouse Approach. Cancers, 13(22), 5835. https://doi.org/10.3390/cancers13225835







