Stratification for RRMM and Risk-Adapted Therapy: Sequencing of Therapies in RRMM
Abstract
:Simple Summary
Abstract
1. Introduction
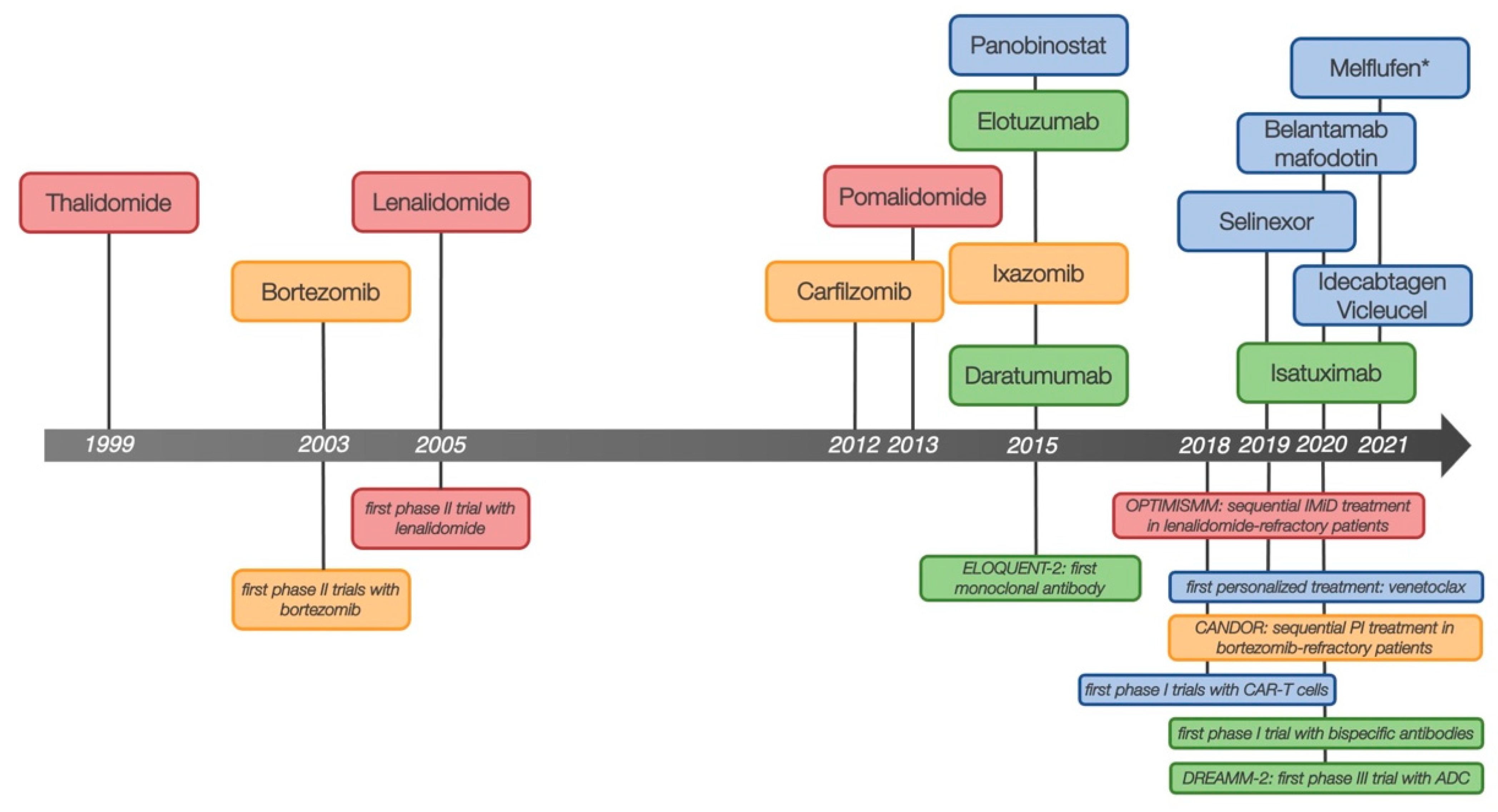
2. RRMM in a Changing Treatment Landscape
2.1. Lenalidomide-Refractory Disease
2.2. Bortezomib-Refractory Disease
2.3. Daratumumab-Refractory Disease
2.4. Multi-Drug Refractoriness and New Treatment Options
3. Patient-Related Factors with Impact on Decision-Making
3.1. High Risk Cytogenetics
3.2. Extramedullary Disease
3.3. Renal Impairment
3.4. Elderly and Frail Patients
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, D.S.; Dimopoulos, M.A.; Ludwig, H.; Facon, T.; Goldschmidt, H.; Jakubowiak, A.; San-Miguel, J.; Obreja, M.; Blaedel, J.; Stewart, A.K. Improvement in Overall Survival With Carfilzomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2018, 36, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Orlowski, R.Z.; Moreau, P.; Niesvizky, R.; Ludwig, H.; Oriol, A.; Chng, W.J.; Goldschmidt, H.; Yang, Z.; Kimball, A.S.; Dimopoulos, M. Carfilzomib-Dexamethasone Versus Bortezomib-Dexamethasone in Relapsed or Refractory Multiple Myeloma: Updated Overall Survival, Safety, and Subgroups. Clin. Lymphoma Myeloma Leuk. 2019, 19, 522–530.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimopoulos, M.A.; Lonial, S.; White, D.; Moreau, P.; Weisel, K.; San-Miguel, J.; Shpilberg, O.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; et al. Elotuzumab, lenalidomide, and dexamethasone in RRMM: Final overall survival results from the phase 3 randomized ELOQUENT-2 study. Blood Cancer J. 2020, 10, 91. [Google Scholar] [CrossRef]
- Richardson, P.G.; Kumar, S.K.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; et al. Final Overall Survival Analysis of the TOURMALINE-MM1 Phase III Trial of Ixazomib, Lenalidomide, and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma. J. Clin. Oncol. 2021, 39, 2430–2442. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.K.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Phase 3 Randomized Study of Daratumumab Plus Lenalidomide and Dexamethasone (D-Rd) Versus Lenalidomide and Dexamethasone (Rd) in Patients with Newly Diagnosed Multiple Myeloma (NDMM) Ineligible for Transplant (MAIA). Blood 2018, 132, LBA-2. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Bene, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef]
- Mateos, M.V.; Dimopoulos, M.A.; Cavo, M.; Suzuki, K.; Jakubowiak, A.; Knop, S.; Doyen, C.; Lucio, P.; Nagy, Z.; Kaplan, P.; et al. Daratumumab plus Bortezomib, Melphalan, and Prednisone for Untreated Myeloma. N. Engl. J. Med. 2018, 378, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.; Costa, L.J.; Anderson, L.D., Jr.; et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: The GRIFFIN trial. Blood 2020, 136, 936–945. [Google Scholar] [CrossRef]
- Gay, F.; Musto, P.; Rota Scalabrini, D.; Galli, M.; Belotti, A.; Zamagni, E.; Bertamini, L.; Zambello, R.; Quaresima, M.; De Sabbata, G.; et al. Survival Analysis of Newly Diagnosed Transplant-Eligible Multiple Myeloma Patients in the Randomized Forte Trial. Blood 2020, 136, 35–37. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hajek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up(dagger). Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Moreau, P.; Kumar, S.K.; San Miguel, J.; Davies, F.; Zamagni, E.; Bahlis, N.; Ludwig, H.; Mikhael, J.; Terpos, E.; Schjesvold, F.; et al. Treatment of relapsed and refractory multiple myeloma: Recommendations from the International Myeloma Working Group. Lancet Oncol. 2021, 22, e105–e118. [Google Scholar] [CrossRef]
- Mikhael, J.; Ismaila, N.; Cheung, M.C.; Costello, C.; Dhodapkar, M.V.; Kumar, S.; Lacy, M.; Lipe, B.; Little, R.F.; Nikonova, A.; et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J. Clin. Oncol. 2019, 37, 1228–1263. [Google Scholar] [CrossRef]
- Chari, A.; Romanus, D.; Palumbo, A.; Blazer, M.; Farrelly, E.; Raju, A.; Huang, H.; Richardson, P. Randomized Clinical Trial Representativeness and Outcomes in Real-World Patients: Comparison of 6 Hallmark Randomized Clinical Trials of Relapsed/Refractory Multiple Myeloma. Clin. Lymphoma Myeloma Leuk. 2020, 20, 8–17.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- San-Miguel, J.F.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Gunther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: A multicentre, randomised, double-blind phase 3 trial. Lancet Oncol. 2014, 15, 1195–1206. [Google Scholar] [CrossRef]
- San-Miguel, J.F.; Hungria, V.T.; Yoon, S.S.; Beksac, M.; Dimopoulos, M.A.; Elghandour, A.; Jedrzejczak, W.W.; Gunther, A.; Nakorn, T.N.; Siritanaratkul, N.; et al. Overall survival of patients with relapsed multiple myeloma treated with panobinostat or placebo plus bortezomib and dexamethasone (the PANORAMA 1 trial): A randomised, placebo-controlled, phase 3 trial. Lancet Haematol. 2016, 3, e506–e515. [Google Scholar] [CrossRef]
- Stewart, A.K.; Rajkumar, S.V.; Dimopoulos, M.A.; Masszi, T.; Spicka, I.; Oriol, A.; Hajek, R.; Rosinol, L.; Siegel, D.S.; Mihaylov, G.G.; et al. Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N. Engl. J. Med. 2015, 372, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreau, P.; Masszi, T.; Grzasko, N.; Bahlis, N.J.; Hansson, M.; Pour, L.; Sandhu, I.; Ganly, P.; Baker, B.W.; Jackson, S.R.; et al. Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 374, 1621–1634. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, A.; Chanan-Khan, A.; Weisel, K.; Nooka, A.K.; Masszi, T.; Beksac, M.; Spicka, I.; Hungria, V.; Munder, M.; Mateos, M.V.; et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 754–766. [Google Scholar] [CrossRef]
- Mateos, M.V.; Sonneveld, P.; Hungria, V.; Nooka, A.K.; Estell, J.A.; Barreto, W.; Corradini, P.; Min, C.K.; Medvedova, E.; Weisel, K.; et al. Daratumumab, Bortezomib, and Dexamethasone Versus Bortezomib and Dexamethasone in Patients With Previously Treated Multiple Myeloma: Three-year Follow-up of CASTOR. Clin. Lymphoma Myeloma Leuk. 2020, 20, 509–518. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.A.; Oriol, A.; Nahi, H.; San-Miguel, J.; Bahlis, N.J.; Usmani, S.Z.; Rabin, N.; Orlowski, R.Z.; Komarnicki, M.; Suzuki, K.; et al. Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2016, 375, 1319–1331. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.A.; San-Miguel, J.; Belch, A.; White, D.; Benboubker, L.; Cook, G.; Leiba, M.; Morton, J.; Ho, P.J.; Kim, K.; et al. Daratumumab plus lenalidomide and dexamethasone versus lenalidomide and dexamethasone in relapsed or refractory multiple myeloma: Updated analysis of POLLUX. Haematologica 2018, 103, 2088–2096. [Google Scholar] [CrossRef] [Green Version]
- Dimopoulos, M.A.; Moreau, P.; Palumbo, A.; Joshua, D.; Pour, L.; Hajek, R.; Facon, T.; Ludwig, H.; Oriol, A.; Goldschmidt, H.; et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016, 17, 27–38. [Google Scholar] [CrossRef]
- Richardson, P.G.; Oriol, A.; Beksac, M.; Liberati, A.M.; Galli, M.; Schjesvold, F.; Lindsay, J.; Weisel, K.; White, D.; Facon, T.; et al. Pomalidomide, bortezomib, and dexamethasone for patients with relapsed or refractory multiple myeloma previously treated with lenalidomide (OPTIMISMM): A randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 781–794. [Google Scholar] [CrossRef]
- Grosicki, S.; Simonova, M.; Spicka, I.; Pour, L.; Kriachok, I.; Gavriatopoulou, M.; Pylypenko, H.; Auner, H.W.; Leleu, X.; Doronin, V.; et al. Once-per-week selinexor, bortezomib, and dexamethasone versus twice-per-week bortezomib and dexamethasone in patients with multiple myeloma (BOSTON): A randomised, open-label, phase 3 trial. Lancet 2020, 396, 1563–1573. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Quach, H.; Mateos, M.V.; Landgren, O.; Leleu, X.; Siegel, D.; Weisel, K.; Yang, H.; Klippel, Z.; Zahlten-Kumeli, A.; et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): Results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020, 396, 186–197. [Google Scholar] [CrossRef]
- Kumar, S.K.; Harrison, S.J.; Cavo, M.; de la Rubia, J.; Popat, R.; Gasparetto, C.; Hungria, V.; Salwender, H.; Suzuki, K.; Kim, I.; et al. Venetoclax or placebo in combination with bortezomib and dexamethasone in patients with relapsed or refractory multiple myeloma (BELLINI): A randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2020, 21, 1630–1642. [Google Scholar] [CrossRef]
- Moreau, P.; Dimopoulos, M.A.; Mikhael, J.; Yong, K.; Capra, M.; Facon, T.; Hajek, R.; Spicka, I.; Baker, R.; Kim, K.; et al. Isatuximab, carfilzomib, and dexamethasone in relapsed multiple myeloma (IKEMA): A multicentre, open-label, randomised phase 3 trial. Lancet 2021, 397, 2361–2371. [Google Scholar] [CrossRef]
- Chari, A.; Rodriguez-Otero, P.; McCarthy, H.; Suzuki, K.; Hungria, V.; Sureda Balari, A.; Perrot, A.; Hulin, C.; Magen, H.; Iida, S.; et al. Subcutaneous daratumumab plus standard treatment regimens in patients with multiple myeloma across lines of therapy (PLEIADES): An open-label Phase II study. Br. J. Haematol. 2021, 192, 869–878. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Terpos, E.; Boccadoro, M.; Delimpasi, S.; Beksac, M.; Katodritou, E.; Moreau, P.; Baldini, L.; Symeonidis, A.; Bila, J.; et al. Daratumumab plus pomalidomide and dexamethasone versus pomalidomide and dexamethasone alone in previously treated multiple myeloma (APOLLO): An open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 801–812. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef]
- Attal, M.; Richardson, P.G.; Rajkumar, S.V.; San-Miguel, J.; Beksac, M.; Spicka, I.; Leleu, X.; Schjesvold, F.; Moreau, P.; Dimopoulos, M.A.; et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): A randomised, multicentre, open-label, phase 3 study. Lancet 2019, 394, 2096–2107. [Google Scholar] [CrossRef]
- Richardson, P.; Ocio, E.; Oriol, A.; Larocca, A.; Rodriguez Otero, P.; Moreb, J.; Bladé, J.; Hassoun, H.; Cavo, M.; Alegre, A.; et al. OP-106 Horizon—Melflufen Therapy for RRMM Patients Refractory to Daratumumab and/or Pomalidomide; Updated Results and First Report on PFS. Blood 2018, 132, 600. [Google Scholar] [CrossRef]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Lonial, S.; Weiss, B.M.; Usmani, S.Z.; Singhal, S.; Chari, A.; Bahlis, N.J.; Belch, A.; Krishnan, A.; Vescio, R.A.; Mateos, M.V.; et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): An open-label, randomised, phase 2 trial. Lancet 2016, 387, 1551–1560. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Nahi, H.; Plesner, T.; Weiss, B.M.; Bahlis, N.J.; Belch, A.; Voorhees, P.M.; Laubach, J.P.; van de Donk, N.; Ahmadi, T.; et al. Daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma: Final results from the phase 2 GEN501 and SIRIUS trials. Lancet Haematol. 2020, 7, e447–e455. [Google Scholar] [CrossRef]
- Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Yee, A.J.; Huff, C.A.; Moreau, P.; Dingli, D.; Cole, C.; Lonial, S.; et al. Oral Selinexor-Dexamethasone for Triple-Class Refractory Multiple Myeloma. N. Engl. J. Med. 2019, 381, 727–738. [Google Scholar] [CrossRef] [PubMed]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Zhao, W.H.; Liu, J.; Wang, B.Y.; Chen, Y.X.; Cao, X.M.; Yang, Y.; Zhang, Y.L.; Wang, F.X.; Zhang, P.Y.; Lei, B.; et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018, 11, 141. [Google Scholar] [CrossRef]
- Gay, F.; Jackson, G.; Rosinol, L.; Holstein, S.A.; Moreau, P.; Spada, S.; Davies, F.; Lahuerta, J.J.; Leleu, X.; Bringhen, S.; et al. Maintenance Treatment and Survival in Patients With Myeloma: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2018, 4, 1389–1397. [Google Scholar] [CrossRef]
- McCarthy, P.L.; Holstein, S.A.; Petrucci, M.T.; Richardson, P.G.; Hulin, C.; Tosi, P.; Bringhen, S.; Musto, P.; Anderson, K.C.; Caillot, D.; et al. Lenalidomide Maintenance After Autologous Stem-Cell Transplantation in Newly Diagnosed Multiple Myeloma: A Meta-Analysis. J. Clin. Oncol. 2017, 35, 3279–3289. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Weisel, K.; Moreau, P.; Anderson, L.D., Jr.; White, D.; San-Miguel, J.; Sonneveld, P.; Engelhardt, M.; Jenner, M.; Corso, A.; et al. Pomalidomide, bortezomib, and dexamethasone for multiple myeloma previously treated with lenalidomide (OPTIMISMM): Outcomes by prior treatment at first relapse. Leukemia 2021, 35, 1722–1731. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Nooka, A.K.; Joseph, N.S.; Kaufman, J.L.; Heffner, L.T.; Gupta, V.A.; Gleason, C.; Boise, L.H.; Lonial, S. Clinical efficacy of daratumumab, pomalidomide, and dexamethasone in patients with relapsed or refractory myeloma: Utility of re-treatment with daratumumab among refractory patients. Cancer 2019, 125, 2991–3000. [Google Scholar] [CrossRef] [PubMed]
- Gavriatopoulou, M.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Fotiou, D.; Roussou, M.; Migkou, M.; Ziogas, D.C.; Kanellias, N.; Terpos, E.; Dimopoulos, M.A. The addition of IMiDs for patients with daratumumab-refractory multiple myeloma can overcome refractoriness to both agents. Blood 2018, 131, 464–467. [Google Scholar] [CrossRef]
- Becnel, M.R.; Horowitz, S.B.; Thomas, S.K.; Iyer, S.P.; Patel, K.K.; Manasanch, E.E.; Weber, D.M.; Kaufman, G.P.; Lee, H.C.; Orlowski, R.Z. Descriptive Analysis of Isatuximab Use Following Prior Daratumumab in Patients with Relapsed/Refractory Multiple Myeloma. Blood 2020, 136, 20–21. [Google Scholar] [CrossRef]
- Mikhael, J.; Belhadj-Merzoug, K.; Hulin, C.; Vincent, L.; Moreau, P.; Gasparetto, C.; Pour, L.; Spicka, I.; Vij, R.; Zonder, J.; et al. A phase 2 study of isatuximab monotherapy in patients with multiple myeloma who are refractory to daratumumab. Blood Cancer J. 2021, 11, 89. [Google Scholar] [CrossRef]
- Mateos, M.V.; Blade, J.; Bringhen, S.; Ocio, E.M.; Efebera, Y.; Pour, L.; Gay, F.; Sonneveld, P.; Gullbo, J.; Richardson, P.G. Melflufen: A Peptide-Drug Conjugate for the Treatment of Multiple Myeloma. J. Clin. Med. 2020, 9, 3120. [Google Scholar] [CrossRef]
- Richardson, P.G.; Oriol, A.; Larocca, A.; Blade, J.; Cavo, M.; Rodriguez-Otero, P.; Leleu, X.; Nadeem, O.; Hiemenz, J.W.; Hassoun, H.; et al. Melflufen and Dexamethasone in Heavily Pretreated Relapsed and Refractory Multiple Myeloma. J. Clin. Oncol. 2021, 39, 757–767. [Google Scholar] [CrossRef]
- Ocio, E.M.; Efebera, Y.A.; Hájek, R.; Granell, M.; Maisnar, V.; Straub, J.; Eveillard, J.-R.; Karlin, L.; Ribrag, V.; Mateos, M.-V.; et al. ANCHOR (OP-104): Melflufen Plus Dexamethasone (dex) and Daratumumab (dara) or Bortezomib (BTZ) in Relapsed/Refractory Multiple Myeloma (RRMM) Refractory to an IMiD and/or a Proteasome Inhibitor (PI)—Updated Efficacy and Safety. Blood 2020, 136, 9–10. [Google Scholar] [CrossRef]
- Carpenter, R.O.; Evbuomwan, M.O.; Pittaluga, S.; Rose, J.J.; Raffeld, M.; Yang, S.; Gress, R.E.; Hakim, F.T.; Kochenderfer, J.N. B-cell maturation antigen is a promising target for adoptive T-cell therapy of multiple myeloma. Clin. Cancer Res. 2013, 19, 2048–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia 2020, 34, 985–1005. [Google Scholar] [CrossRef] [PubMed]
- Gagelmann, N.; Ayuk, F.; Atanackovic, D.; Kröger, N. B cell maturation antigen-specific chimeric antigen receptor T cells for relapsed or refractory multiple myeloma: A meta-analysis. Eur. J. Haematol. 2020, 104, 318–327. [Google Scholar] [CrossRef]
- Chari, A.; Suvannasankha, A.; Fay, J.W.; Arnulf, B.; Kaufman, J.L.; Ifthikharuddin, J.J.; Weiss, B.M.; Krishnan, A.; Lentzsch, S.; Comenzo, R.; et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. Blood 2017, 130, 974–981. [Google Scholar] [CrossRef]
- Sonneveld, P.; Avet-Loiseau, H.; Lonial, S.; Usmani, S.; Siegel, D.; Anderson, K.C.; Chng, W.J.; Moreau, P.; Attal, M.; Kyle, R.A.; et al. Treatment of multiple myeloma with high-risk cytogenetics: A consensus of the International Myeloma Working Group. Blood 2016, 127, 2955–2962. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.K.; Bergsagel, P.L.; Greipp, P.R.; Dispenzieri, A.; Gertz, M.A.; Hayman, S.R.; Kumar, S.; Lacy, M.Q.; Lust, J.A.; Russell, S.J.; et al. A practical guide to defining high-risk myeloma for clinical trials, patient counseling and choice of therapy. Leukemia 2007, 21, 529–534. [Google Scholar] [CrossRef] [Green Version]
- Greipp, P.R.; San Miguel, J.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Blade, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Avet-Loiseau, H.; Leleu, X.; Roussel, M.; Moreau, P.; Guerin-Charbonnel, C.; Caillot, D.; Marit, G.; Benboubker, L.; Voillat, L.; Mathiot, C.; et al. Bortezomib plus dexamethasone induction improves outcome of patients with t(4;14) myeloma but not outcome of patients with del(17p). J. Clin. Oncol. 2010, 28, 4630–4634. [Google Scholar] [CrossRef]
- Cavo, M.; Tacchetti, P.; Patriarca, F.; Petrucci, M.T.; Pantani, L.; Galli, M.; Di Raimondo, F.; Crippa, C.; Zamagni, E.; Palumbo, A.; et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet 2010, 376, 2075–2085. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Lonial, S.; Betts, K.A.; Chen, C.; Zichlin, M.L.; Brun, A.; Signorovitch, J.E.; Makenbaeva, D.; Mekan, S.; Sy, O.; et al. Elotuzumab plus lenalidomide and dexamethasone in relapsed/refractory multiple myeloma: Extended 4-year follow-up and analysis of relative progression-free survival from the randomized ELOQUENT-2 trial. Cancer 2018, 124, 4032–4043. [Google Scholar] [CrossRef] [PubMed]
- Jakubowiak, A.; Offidani, M.; Pegourie, B.; De La Rubia, J.; Garderet, L.; Laribi, K.; Bosi, A.; Marasca, R.; Laubach, J.; Mohrbacher, A.; et al. Randomized phase 2 study: Elotuzumab plus bortezomib/dexamethasone vs bortezomib/dexamethasone for relapsed/refractory MM. Blood 2016, 127, 2833–2840. [Google Scholar] [CrossRef] [Green Version]
- Weisel, K.; Spencer, A.; Lentzsch, S.; Avet-Loiseau, H.; Mark, T.M.; Spicka, I.; Masszi, T.; Lauri, B.; Levin, M.D.; Bosi, A.; et al. Daratumumab, bortezomib, and dexamethasone in relapsed or refractory multiple myeloma: Subgroup analysis of CASTOR based on cytogenetic risk. J. Hematol. Oncol. 2020, 13, 115. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, J.L.; Dimopoulos, M.A.; White, D.; Benboubker, L.; Cook, G.; Leiba, M.; Morton, J.; Joy Ho, P.; Kim, K.; Takezako, N.; et al. Daratumumab, lenalidomide, and dexamethasone in relapsed/refractory myeloma: A cytogenetic subgroup analysis of POLLUX. Blood Cancer J. 2020, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- Chng, W.J.; Goldschmidt, H.; Dimopoulos, M.A.; Moreau, P.; Joshua, D.; Palumbo, A.; Facon, T.; Ludwig, H.; Pour, L.; Niesvizky, R.; et al. Carfilzomib-dexamethasone vs bortezomib-dexamethasone in relapsed or refractory multiple myeloma by cytogenetic risk in the phase 3 study ENDEAVOR. Leukemia 2017, 31, 1368–1374. [Google Scholar] [CrossRef]
- Harrison, S.J.; Perrot, A.; Alegre, A.; Simpson, D.; Wang, M.C.; Spencer, A.; Delimpasi, S.; Hulin, C.; Sunami, K.; Facon, T.; et al. Subgroup analysis of ICARIA-MM study in relapsed/refractory multiple myeloma patients with high-risk cytogenetics. Br. J. Haematol. 2021, 194, 120–131. [Google Scholar] [CrossRef]
- Nooka, A.K.; Yee, A.J.; Huff, C.A.; Vogl, D.T.; Gavriatopoulou, M.; Chari, A.; Moreau, P.; Dingli, D.; Cole, C.E.; Lonial, S.; et al. Influence of Cytogenetics in Patients with Relapsed Refractory Multiple Myeloma Treated with Oral Selinexor and Dexamethasone: A Post-Hoc Analysis of the STORM Study. Blood 2019, 134, 1872. [Google Scholar] [CrossRef]
- Bhutani, M.; Foureau, D.M.; Atrash, S.; Voorhees, P.M.; Usmani, S.Z. Extramedullary multiple myeloma. Leukemia 2020, 34, 1–20. [Google Scholar] [CrossRef]
- Rosinol, L.; Beksac, M.; Zamagni, E.; Van de Donk, N.; Anderson, K.C.; Badros, A.; Caers, J.; Cavo, M.; Dimopoulos, M.A.; Dispenzieri, A.; et al. Expert review on soft-tissue plasmacytomas in multiple myeloma: Definition, disease assessment and treatment considerations. Br. J. Haematol. 2021, 194, 496–507. [Google Scholar] [CrossRef]
- Varettoni, M.; Corso, A.; Pica, G.; Mangiacavalli, S.; Pascutto, C.; Lazzarino, M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: A longitudinal study on 1003 consecutive patients. Ann. Oncol. 2010, 21, 325–330. [Google Scholar] [CrossRef]
- Deng, S.; Xu, Y.; An, G.; Sui, W.; Zou, D.; Zhao, Y.; Qi, J.; Li, F.; Hao, M.; Qiu, L. Features of extramedullary disease of multiple myeloma: High frequency of p53 deletion and poor survival: A retrospective single-center study of 834 cases. Clin. Lymphoma Myeloma. Leuk. 2015, 15, 286–291. [Google Scholar] [CrossRef]
- Pour, L.; Sevcikova, S.; Greslikova, H.; Kupska, R.; Majkova, P.; Zahradova, L.; Sandecka, V.; Adam, Z.; Krejci, M.; Kuglik, P.; et al. Soft-tissue extramedullary multiple myeloma prognosis is significantly worse in comparison to bone-related extramedullary relapse. Haematologica 2014, 99, 360–364. [Google Scholar] [CrossRef] [Green Version]
- Yee, A.J.; Huff, C.A.; Chari, A.; Vogl, D.T.; Gavriatopoulou, M.; Nooka, A.K.; Moreau, P.; Dingli, D.; Cole, C.E.; Lonial, S.; et al. Response to Therapy and the Effectiveness of Treatment with Selinexor and Dexamethasone in Patients with Penta-Exposed Triple-Class Refractory Myeloma Who Had Plasmacytomas. Blood 2019, 134, 3140. [Google Scholar] [CrossRef]
- Beksac, M.; Richardson, P.G.; Unal, A.; Corradini, P.; DeLimpasi, S.; Gulbas, Z.; Kerridge, I.; Mikala, G.; Neylon, A.; Symeonidis, A.; et al. Isatuximab Plus Pomalidomide and Dexamethasone in Patients with Relapsed/Refractory Multiple Myeloma and Soft-Tissue Plasmacytomas: Icaria-Mm Subgroup Analysis. In Proceedings of the European Hematology Association Congress, Frankfurt, Germany, 11–14 June 2020. [Google Scholar] [CrossRef]
- Zhou, X.; Fluchter, P.; Nickel, K.; Meckel, K.; Messerschmidt, J.; Bockle, D.; Knorz, S.; Steinhardt, M.J.; Krummenast, F.; Danhof, S.; et al. Carfilzomib Based Treatment Strategies in the Management of Relapsed/Refractory Multiple Myeloma with Extramedullary Disease. Cancers 2020, 12, 1035. [Google Scholar] [CrossRef] [Green Version]
- Short, K.D.; Rajkumar, S.V.; Larson, D.; Buadi, F.; Hayman, S.; Dispenzieri, A.; Gertz, M.; Kumar, S.; Mikhael, J.; Roy, V.; et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia 2011, 25, 906–908. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, J.; Zhao, W.-H.; Chen, Y.-X.; Cao, X.-M.; Yang, Y.; Zhang, Y.-L.; Wang, F.-X.; Zhang, P.-Y.; Lei, B.; et al. Chimeric Antigen Receptor T Cell Therapy in the Relapsed or Refractory Multiple Myeloma with Extramedullary Disease--a Single Institution Observation in China. Blood 2020, 136, 6. [Google Scholar] [CrossRef]
- Usmani, S.Z.; Weiss, B.M.; Plesner, T.; Bahlis, N.J.; Belch, A.; Lonial, S.; Lokhorst, H.M.; Voorhees, P.M.; Richardson, P.G.; Chari, A.; et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016, 128, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Sonneveld, P.; Leung, N.; Merlini, G.; Ludwig, H.; Kastritis, E.; Goldschmidt, H.; Joshua, D.; Orlowski, R.Z.; Powles, R.; et al. International Myeloma Working Group Recommendations for the Diagnosis and Management of Myeloma-Related Renal Impairment. J. Clin. Oncol. 2016, 34, 1544–1557. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Luo, X.; Zu, Y.; Issa, H.A.; Li, L.; Ye, H.; Yang, T.; Hu, J.; Wei, L. Severe renal impairment as an adverse prognostic factor for survival in newly diagnosed multiple myeloma patients. J. Clin. Lab. Anal. 2020, 34, e23416. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, M.A.; Leleu, X.; Moreau, P.; Richardson, P.G.; Liberati, A.M.; Harrison, S.J.; Miles Prince, H.; Ocio, E.M.; Assadourian, S.; Campana, F.; et al. Isatuximab plus pomalidomide and dexamethasone in relapsed/refractory multiple myeloma patients with renal impairment: ICARIA-MM subgroup analysis. Leukemia 2021, 35, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Schjesvold, F.; Weisel, K.; Moreau, P.; Anderson, L.D., Jr.; White, D.; Rodriguez-Otero, P.; Sonneveld, P.; Engelhardt, M.; Jenner, M.; et al. Pomalidomide, bortezomib, and dexamethasone at first relapse in lenalidomide-pretreated myeloma: A subanalysis of OPTIMISMM by clinical characteristics. Eur. J. Haematol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.; Martin, T., III; Moreau, P.; Baker, R.; Pour, L.; Min, C.-K.; Leleu, X.; Mohty, M.; Reinoso Segura, M.; Turgut, M.; et al. Isatuximab Plus Carfilzomib and Dexamethasone Versus Carfilzomib and Dexamethasone in Relapsed Multiple Myeloma Patients with Renal Impairment: Ikema Subgroup Analysis. Blood 2020, 136, 46–47. [Google Scholar] [CrossRef]
- Dimopoulos, M.; Siegel, D.; White, D.J.; Boccia, R.; Iskander, K.S.; Yang, Z.; Kimball, A.S.; Mezzi, K.; Ludwig, H.; Niesvizky, R. Carfilzomib vs. bortezomib in patients with multiple myeloma and renal failure: A subgroup analysis of ENDEAVOR. Blood 2019, 133, 147–155. [Google Scholar] [CrossRef] [Green Version]
- SEER Cancer Stat Facts: Myeloma. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 26 October 2021).
- Jones, A.; Bowcock, S.; Rachet, B. Survival trends in elderly myeloma patients. Eur. J. Haematol. 2021, 106, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.; Larocca, A.; Facon, T.; Zweegman, S.; Engelhardt, M. Defining the vulnerable patient with myeloma-a frailty position paper of the European Myeloma Network. Leukemia 2020, 34, 2285–2294. [Google Scholar] [CrossRef]
- Engelhardt, M.; Domm, A.S.; Dold, S.M.; Ihorst, G.; Reinhardt, H.; Zober, A.; Hieke, S.; Baayen, C.; Muller, S.J.; Einsele, H.; et al. A concise revised Myeloma Comorbidity Index as a valid prognostic instrument in a large cohort of 801 multiple myeloma patients. Haematologica 2017, 102, 910–921. [Google Scholar] [CrossRef]
- Schjesvold, F.H.; Richardson, P.G.; Facon, T.; Alegre, A.; Spencer, A.; Jurczyszyn, A.; Sunami, K.; Frenzel, L.; Min, C.K.; Guillonneau, S.; et al. Isatuximab plus pomalidomide and dexamethasone in elderly patients with relapsed/refractory multiple myeloma: ICARIA-MM subgroup analysis. Haematologica 2021, 106, 1182–1187. [Google Scholar] [CrossRef]
- Facon, T.; Moreau, P.; Martin, T.G.; Spicka, I.; Oriol, A.; Koh, Y.; Lim, A.; Mikala, G.; Rosiñol, L.; Yağci, M.; et al. MM-092: Isatuximab Plus Carfilzomib and Dexamethasone Versus Carfilzomib and Dexamethasone in Elderly Patients with Relapsed Multiple Myeloma: IKEMA Subgroup Analysis. Clin. Lymphoma Myeloma Leuk. 2021, 21, S420. [Google Scholar] [CrossRef]
| Trial Name | Combination | Phase | n | Prev. Lines of Therapy (Range) | Combination Approved by FDA/EMA | Response | Progression-Free Survival (Months) | Hazard Ratio (95% CI) | Survival Benefit | MRD Rate |
|---|---|---|---|---|---|---|---|---|---|---|
| PANORAMA-1 2014 [14] | PanoVd vs. Vd | 3 | 387 vs. 381 | 1–3, 1: 51% vs. 52% | FDA EMA | ORR: 60.7% vs. 54.6% | 11.99 vs. 8.08 | 0.63 (0.52–0.76), p < 0.0001 | no [15] OS 40.3 vs. 35.8 months, HR 0.94 (0.78–1.14), p = 0.54 | n.r. |
| ASPIRE 2015 [16] | KRd vs. Rd | 3 | 396 vs. 396 | 1–3, median 2 (1–3) | FDA EMA | ORR 87.1% vs. 66.7% ≥VGPR 69.9% vs. 40.4% | 26.3 vs. 17.6 | 0.69 (0.57–0.83), p = 0.0001 | Yes [1] Median follow-up: 67.1 months OS 48.3 vs. 40.4 months, HR 0.794 (0.667–0.945), p = 0.0045 | n.r. |
| ELOQUENT-2 2015 [17] | EloRd vs. Rd | 3 | 321 vs. 325 | 1–3, median 2 (1–4) | FDA EMA | ORR 79% vs. 66% ≥VGPR 33% vs. 28% | 19.4 vs. 14.9 | 0.70 (0.57–0.85), p < 0.001 | yes [3] Minimum follow-up: 70.1 months 48.3 vs. 39.6 months, HR 0.82 (0.68–1.00), p = 0.0408 | n.r. |
| TOURMALINE-MM1 2016 [18] | IxaRd vs. Rd | 3 | 360 vs. 362 | 1–3, 1: 62% vs. 60% | FDA EMA | ORR 78% vs. 72% ≥VGPR 48% vs. 39% | 20.6 vs. 14.7 | 0.74 (0.59–0.94), p = 0.01 | no [4] Median follow-up: 85 months mOS 53.6 vs. 51.6 months, HR 0.939 (0.784 to 1.125), p = 0.495 | n.r. |
| CASTOR 2016 [19] | DaraVd vs. Vd | 3 | 251 vs. 247 | ≥1, median 2 (1–10) | FDA EMA | ORR: 82.9% vs. 63.2%, p < 0.001 ≥VGPR: 59.2% vs. 29.1%, p < 0.001 | NR vs. 7.2 | 0.39 (0.28–0.53), p < 0.001 | MRD 10-5 assessed at CR: 14% vs. 2%, p < 0.000001 [20] | |
| POLLUX 2016 [21] | DaraRd vs. Rd | 3 | 286 vs. 283 | ≥1, median 1 (1–11) | FDA EMA | ORR 92.9% vs. 76.4%, p < 0.001 ≥VGPR: 75.8% vs. 44.2% | NR vs. 18.4 | 0.37 (0.27–0.52), p < 0.001 | MRD 10-5 assessed at CR: 26.2% vs. 6.4%, p < 0.000001 [22] | |
| ENDEAVOR 2016 [23] | Kd vs. Vd | 3 | 464 vs. 465 | 1–3, median 2 (IQR 1–2) | FDA EMA | ORR 77% vs. 63% ≥VGPR 54% vs. 29% | 18.7 vs. 9.4 | 0.53 (0.44–0.65), p < 0.0001 | yes [2] Median follow-up 44.3 vs. 43.7 months OS 47.8 vs. 38.8 months, HR 0.76 (0.63–0.92), p = 0.0017 | n.r. |
| OPTIMISMM 2019 [24] | PVd vs. Vd | 3 | 281 vs. 278 | 1–3, median 2 (IQR 1–2) | EMA | ORR 82.2% vs. 50.0% ≥VGPR 52.7% vs. 18.3% | 11.2 vs. 7.1 | 0.61 (0.49–0.77), p < 0.0001 | n.r. | |
| BOSTON 2020 [25] | SVd vs. Vd | 3 | 195 vs. 207 | 1–3, median 2 (IQR 1–2) | FDA | ORR 76.4% vs. 62.3% ≥VGPR 44.6% vs. 32.4% | 13.93 vs. 9.46 | 0.70 (0.53–0.93), p = 0.0075 | MRD 10-5 assessed at CR or better: 5% vs. 4% | |
| CANDOR 2020 [26] | DaraKd vs. Kd | 3 | 312 vs. 154 | 1–3, median 2 (IQR 1–2) | EMA FDA | ORR 84% vs. 75%, p = 0.008 ≥VGPR 69% vs. 49% | NR vs. 15.8 | 0.63 (0.46–0.85), p = 0.0027 | MRD 10-5 at 12 months: 18% vs. 4%, p < 0.0001 | |
| BELLINI 2020 [27] | VenVd vs. Vd | 3 | 194 vs. 97 | 1–3, 1: 47% vs. 45% | ORR t(11;14): 90% vs. 47% p = 0.0038 BCL2 high: 85% vs. 75%, p = 0.367 ≥VGPR t(11;14) 70% vs. 27%, p = 0.016 BCL2 high: 71% vs. 28%, p = 0.00013 | t(11;14): NR vs. 9.5 BCL2 high: 22.4 vs. 9.9 | t(11;14): 0.11 (0.02–0.56), p = 0.0040; BLC2 high 0.24 (0.12–0.48), p < 0.0001 | MRD 10-5 assessed at CR or sCR: 13% vs. 1%, p = 0.00066 t(11;14): 25% vs. 0%, p = 0.056 BCL 2 high: 18% vs. 0%, p = 0.025 | ||
| IKEMA 2021 [28] | IsaKd vs. Kd | 3 | 179 vs. 123 | 1–3, median 2 (IQR 1–3) | FDA EMA | ORR 86.6% vs. 82.9% ≥VGPR 72.6% vs. 56.1% | NR vs. 19.15 | 0.531 (0.318–0.889), p = 0.0007 | MRD 10-5 assessed at VGPR or better: 30% vs. 13%, p = 0.0004 | |
| PLEIADES 2020 [29] | RRMM Arm: s.c.D-Rd | 2 | 65 | ≥1, median 1 (1–5) | ORR 93.8% ≥VGPR 78.5% | MRD 10-5: 15.4% | ||||
| APOLLO 2021 [30] | DaraPd vs. Pd | 3 | 151 vs. 153 | ≥1, median 2 (1–5) | FDA EMA | ORR 69% vs. 46% ≥VGPR 51% vs. 20% | 12.4 vs. 6.9 | 0.63 (0.47–0.85), p = 0.00018 | MRD 10-5 assessed at CR or sCR: 9% vs. 2%, p = 0.010 | |
| ELOQUENT-3 2018 [31] | EloPd vs. Pd | 2 | 60 vs. 57 | ≥2, median 3 (2–8) | FDA EMA | ORR 53% vs. 26% ≥VGPR 33% vs. 18% | 10.3 vs. 4.7 | 0.54 (0.34–0.86), p = 0.008 | n.r. | |
| ICARIA 2019 [32] | Isa-Pd vs. Pd | 3 | 154 vs. 153 | ≥2, median 3 (IQR 2–4) | FDA EMA | ORR 60% vs. 35% ≥VGPR 32% vs. 9% | 11.53 vs. 6.47 | 0.596 (0.436–0.0814), p = 0.001 | MRD 10-5 assessed at CR or if clinically indicated: 5% vs. 0% | |
| HORIZON OP-106 2020 [33] | Single arm melflufen | 2 | 157 | ≥2, median 5 (2–12) | FDA | total cohort ORR 29%, ≥VGPR: 12% triple class refractory 26%, ≥VGPR: 11% | Total cohort: 4.22 Triple class refractory: 3.9 | n.r. | ||
| DREAMM-2 2020 [34] | Belantamab-mafodotin 2.5 mg/kg vs. 3.4 mg/kg | 2 | 97 vs. 99 | ≥3, median 6 vs. 7 (3–21), more than 4: 84% vs. 83% | FDA EMA | ORR 31% vs. 34% ≥VGPR 19% v.s 20% | 2.9 vs. 4.9 | n.r. | ||
| SIRIUS 2016 [35] | Dara-d | 2 | 106 | ≥3, median 5 (2–14) | FDA EMA | ORR 29.2% ≥VGPR 12.3% | 3.7 | n.r. | ||
| Usmani et al., 2020 [36] | Dara-d pooled analysis from GEN501 and SIRIUS trials | 2 | 148 | median 5 (4–7) | FDA EMA | ORR: 30.4% ≥VGPR 14% | 20.5 | n.r. | ||
| STORM 2019 [37] | Single Arm Selinexor | 2 | 122 | median 7 (3–18) | FDA EMA | ≥PR 26%, ≥MR 39% ≥VGPR 6.6% | 3.7 | n.r. | ||
| KarMMa 2021 [38] | Ide-cel CAR T cells | 2 | 128 | ≥3, median 6 (3–16) | FDA EMA | ORR: total cohort 73%, ≥VGPR: 65% 300 × 106: 69%, ≥VGPR: 42% 450 × 106: 81%, ≥VGPR: 53% | Total cohort: 8.8 300 × 106: 5.8 450 × 106: 12.1 | MRD 10-5 at CR or better: total cohort: 26% 300 × 106: 24% 450 × 106 28% | ||
| Cartitude-1 2021 [39] | Cilta-cel CAR T cells | 2 | 97 | Media 6 (IQR 4–8) | ORR 97% ≥VGPR 93% | Not reached, 12 months PFS rate 77% | MRD 10-5 in 57 evaluable patients: 93% | |||
| Legend-2 [40] | Cilta-cel CAR T cells | 1 | 57 | Median 3 (1–9) | ORR 88% ≥VGPR 74% | 15 | MRD 10-4: 63% |
| Clinical Trial including Bortezomib-Refractory Patients | |||||
|---|---|---|---|---|---|
| Trial | Combination | Phase | n | Bortezomib-Refractory | Response |
| SIRIUS 2016 [35] | Dara–d | 2 | 106 | 90% | ORR 27.4% |
| ICARIA 2019 [32] | IsaPd vs. Pd | 3 | 154 vs. 153 | PI refractory: 76% | HR 0.58 (0.41–0.82) |
| CANDOR 2020 [26] | DaraKd vs. Kd | 3 | 312 vs. 154 | 29% * | Refractory to bortezomib or ixazomib HR 0.84 (0.52–1.36) |
| HORIZON OP-106 2020 [50] | Single arm Melflufen | 2 | 157 | 64% | n.r. |
| DREAMM-2 2020 [34] | Belantamab–mafodotin 2.5 mg/kg vs. 3–4 mg/kg | 2 | 97 vs. 99 | 76% | ORR 29.7% vs. 31.1% |
| APOLLO 2021 [30] | Dara-Pd vs. Pd | 3 | 151 vs. 153 | PI refractory 48% | HR 0.73 (0.49–1.08) |
| Clinical Trials including Lenalidomide-Refractory Patients | |||||
| Trial | Combination | Phase | n | Lenalidomide Refractory | Response |
| SIRIUS 2016 [35] | Dara–d | 2 | 106 | 88% | ORR 28% |
| ENDEAVOR 2016 [23] | Kd vs. Vd | 3 | 464 vs. 465 | 25.3% | HR 0.80 (0.57–1.11) |
| ELOQUENT-3 2018 [31] | EloPd vs. Pd | 2 | 60 vs. 57 | 87% | Double refractory: HR 0.56 (0.33–0.97) |
| ICARIA 2019 [32] | IsaPd vs. Pd | 3 | 154 vs. 153 | 93% | HR 0.59 (95% CI 0.43–0.82) |
| OPTIMISMM 2019 [24] | PVd vs. Vd | 3 | 281 vs. 278 | 70% | HR 0.65 (95% CI 0.50–0.84) |
| CANDOR 2020 [26] | DaraKd vs. Kd | 3 | 312 vs. 154 | 33% | HR 0.47 (95% CI 0.29–0.78) |
| BELLINI 2020 [27] | VenVd vs. Vd | 3 | 194 vs. 97 | 22% | n.r. |
| DREAMM-2 2020 [34] | Belantamab–mafodotin 2.5 mg/kg vs. 3.4 mg/kg | 2 | 97 vs. 99 | 89% | ORR 29.9% vs. 35.2% |
| APOLLO 2021 [30] | Dara-Pd vs. Pd | 3 | 151 vs. 153 | 80% | HR 0.66 (0.49–0.90) |
| IKEMA 2021 [28] | IsaKd vs. Kd | 3 | 179 vs. 123 | 32.8% | HR 0.58 (0.35–0.96) |
| Clinical Trials including Double and Triple Class Refractory Patients | |||||
| Trial | Combination | Phase | n | Refractoriness to Classes | Response |
| SIRIUS 2016 [35] | Dara–d | 2 | 106 | Double (PI + IMiD): 95% Triple (PI + IMiD +alkylating agent): 75% | ORR 29.7% ORR 22.8% |
| EQUULEUS (MMY1001) 2017 [55] | DaraPd–arm | 1b | 103 | Double (PI + IMiD): 71% | ORR 57.5% |
| ELOQUENT-3 2018 [31] | EloPd vs. Pd | 2 | 60 vs. 57 | Double (PI + Lenalidomide) 70% | HR 0.56 (0.33–0.97) |
| Usmani et al., 2020 [36] | Dara–d pooled analysis from GEN501 and SIRIUS trials | 2 | 148 | Double (PI + IMiD) 87% Triple: 68% | n.r. |
| STORM 2019 [37] | Single arm Selinexor | 2 | 122 | Triple (≥1 Imid, ≥1 PI, Daratumumab): 100% | ORR 39%, PR or better: 26% |
| ICARIA 2020 [32] | IsaPd vs. Pd | 3 | 154 vs. 153 | Double (PI + Lenalidomide): 71% | HR 0.58 (0.40–0.58) |
| HORIZON OP-106 2020 [50] | Single arm Melflufen | 2 | 157 | Triple (PI + IMiD + CD38): 76% | CBR: 39% ORR: 26% ≥VGPR: 11% |
| DREAMM-2 2020 [34] | Belantamab–mafodotin 2.5 mg/kg vs. 3.4 mg/kg | 2 | 97 vs. 99 | Triple (PI + IMiD + CD38 **): 100% | ORR: 30.9% vs. 34.9% |
| APOLLO 2021 [30] | DaraPd vs. Pd | 3 | 151 vs. 153 | Double Len + PI: 42% | HR 0.74 (0.49–1.12) |
| IKEMA 2021 [28] | IsaKd vs. Kd | 3 | 179 vs. 123 | Double (PI + IMiD) 21% | n.r. |
| Trial Name | Combination | Phase | n | Cytogenetics Available | Standard Risk vs. High Risk * | Response in Standard Risk Cytogenetics | Response in High Risk Cytogenetics |
|---|---|---|---|---|---|---|---|
| PANORAMA-1 2014 [14] | Pano-Vd vs. Vd | 3 | 387 vs. 381 | 26% | 82% vs. 18% | PFS n.r. HR: 0.88 (0.60–1.29) ORR n.r. | PFS: n.r. HR: 0.47 (0.18–1.25) ORR: n.r. |
| ASPIRE 2015 [16] | KRd vs. Rd | 3 | 396 vs. 396 | 53% | 76% vs. 24% | PFS: n.r. HR: 0.66 (0.48–0.90) ORR: n.r. | PFS: n.r. HR: 0.70 (0.43–1.16) ORR: n.r. |
| ELOQUENT-2 2015 [62] | EloRd vs. Rd | 3 | 321 vs. 325 | n.r. | approx. 70% vs. 20% † | PFS 19.7 vs. 16.6 months HR 0.77 (0.62–0.95), p = 0.0159 ORR: n.r. | PFS: 15.2 vs. 7.4 months HR 0.64 (0.43–0.98), p = 0.0331 ORR n.r. |
| Jakubowiak 2016 [63] | EloVd vs. Vd | 2 | 77 vs. 75 | 45% | 40% vs. 3% | HR: 0.62 (0.35–1.12) | PFS: n.r. HR: n.r. ORR: n.r. |
| TOURMALINE-MM1 2016 [18] | IxaRd vs. Rd | 3 | 360 vs. 362 | 84% | 75% vs. 25% | PFS: 20.6 vs. 15.6 months HR: 0.64 (n.r.) ORR: n.r. | PFS: 21.4 vs. 9.7 months HR: 0.54 (n.r.) ORR: n.r. |
| CASTOR 2016 [64] | DaraVd vs. Vd | 3 | 251 vs. 247 | 71% | 79% vs. 21% | PFS: 16.6 vs. 6.6 months HR: 0.26 (0.19–0.37), p < 0.0001 ORR: 84% vs. 62%, p < 0.0001, ≥VGPR: 62% vs. 28%, p < 0.0001 MRD 10−5: 11% vs. 3%, p = 0.0091 | PFS: 12.6 vs.6.2 months HR: 0.41 (0.21–0.83), p = 0.0106 ORR: 85% vs. 56%, p = 0.0512, ≥VGPR: 59% vs. 32%, p = 0.1259 MRD 10−5: 15% vs. 0%, p = 0.0271 |
| POLLUX 2016 [65] | DaraRd vs. Rd | 3 | 286 vs. 283 | 77% | 84% vs. 16% | PFS: NR vs. 18.6 months HR: 0.43 (0.32–0.57), p < 0.0001 ORR: 94 vs. 79%, p < 0.0001, ≥VGPR 82% Vs. 54%, p < 0.0001 MRD 10−5: 33% vs. 9%, p < 0.0001 | PFS: 26.8 vs. 8.3 months HR: 0.34 (0.16–0.72), p = 0.0035 ORR: 89% vs. 68%, p = 0.0145, ≥VGPR 71% Vs. 29%, p = 0.0004 MRD 10−5: 26% vs. 0%, p = 0.0022 |
| ENDEAVOR 2016 [66] | Kd vs. Vd | 3 | 464 vs. 465 | 85% | 73% vs. 27% | PFS: NR vs. 10.2 months HR 0.439 (0.333–0.578), p < 0.0001 ORR: 79.2% vs. 66.0%, p = 0.0002, ≥VGPR 58.8%% vs. 29.5% | PFS: 8.8 vs. 6.0 months HR: 0.646 (0.453–0.921), p = 0.0075 ORR: 72.2%% vs. 58.4%, p = 0.0190, ≥VGPR: 46.4% vs. 30.2% |
| OPTIMISMM 2019 [24] | PVd vs. Vd | 3 | 281 vs. 278 | 68% | 71% vs. 29% | PFS: n.r. HR: 0.67 (0.48–0.94) ORR n.r. | PFS: 8.44 vs. 5.32 months HR: 0.56 (0.35–0.90) ORR: n.r. |
| BOSTON 2020 [25] | SVd vs. Vd | 3 | 195 vs. 207 | 90% | | 47% vs. 53% | PFS: n.r. HR: n.r. ORR: n.r. | PFS: n.r. HR: 0.67 (0.45–0.98) ORR: 77.3% vs. 55.8%, p = 0.0008 |
| CANDOR 2020 [26] | DaraKd vs. Kd | 3 | 312 vs. 154 | 49% | 68% vs. 32% | PFS: n.r. HR: 0.50 (0.28–0.90) ORR: n.r. | PFS: n.r. HR: 0.70 (0.36–1.40) ORR: n.r. |
| BELLINI 2020 [27] | VenVd vs. Vd | 3 | 194 vs. 97 | 90% | 81% vs. 19% | PFS: NR vs. 12.2 months HR: 0.54 (0.35- 0.84) ORR: n.r. | PFS: 9.0 vs. 11.4 HR: 1.21 (0.58- 2.52) ORR: n.r. |
| IKEMA 2021 [28] | IsaKd vs. Kd | 3 | 179 vs. 123 | 88% | 72% vs. 28% | PFS: NR vs. 19.45 months HR: 0·44 (0.27–0.73) ORR: n.r. | PFS: NR vs. 18.2 months HR: 0.72 (0.36–1.45) ORR: n.r. |
| APOLLO 2021 [30] | DaraPd vs. Pd | 3 | 151 vs. 153 | 69% | 65% vs. 35% | PFS: 21 vs. 7.4 months HR: 0.51 (0.32–0.81) ORR: n.r. | PFS: 5.8 vs. 4.0 months HR: 0.85 (0.49–1.44) ORR: n.r. |
| ELOQUENT-3 2018 [31] | EloPd vs. Pd | 2 | 60 vs. 57 | 73% | 68% vs. 32% | PFS: NR vs. 4.9 months HR: 0.56 (0.27–1.14) ORR: n.r. | PFS: 6.5 vs. 2.5 months HR: 0.52 (0.22–1.25) ORR: n.r. |
| ICARIA 2019 [67] | IsaPd vs. Pd | 3 | 154 vs. 153 | 79% | 75% vs. 25% | PFS: 11.6 vs. 7.4 months HR: 0.62 (0.42–0.93) ORR: 65% vs. 42.3%, p = 0.0012, ≥VGPR 32% vs. 9% | PFS: 7.5 vs. 3.7 months HR: 0.66 (0.33–1.28) ORR:50.0% vs. 16.7%, p = 0.0031, ≥VGPR 29.2% vs. 2.8% |
| HORIZON OP-106 2020 [33] | Single arm melflufen | 2 | 157 | 80% | 53% vs. 47% | PFS: 4.4 months HR: n.r. ORR: 31% | PFS: 3.1 months HR: n.r. ORR: 20% |
| DREAMM-2 2020 [34] | Belantamab–mafodotin 2.5 mg/kg vs. 3–4 mg/kg | 2 | 97 vs. 99 | n.r. | 45% | PFS: n.r. HR: n.r. ORR: n.r. | PFS: n.r. HR: n.r. ORR: 29.3% vs. 38.3% |
| SIRIUS 2016 [35] | Dara–d | 2 | 106 | 89% | 72% vs. 21% | PFS: n.r. HR: n.r. ORR: 29.4% | PFS: n.r. HR: n.r. ORR: 20% |
| EQUULEUS (MMY1001) 2017 [55] | DaraPd–arm | 1b | 103 | 85% | 75% vs. 25% | PFS: n.r. HR: n.r. ORR: 58.5% | PFS: n.r. HR: n.r. ORR: 59.1% |
| STORM 2019 ‡ [68] | Single arm Selinexor | 2 | 200 | n.r. | 61% | | PFS: 4.2 months HR: n.r. ORR: 29.5%, CBR 38.5% | PFS: 3.8 months HR: n.r. ORR: 20.5%, CBR: 35.2% |
| KarMMa 2021 [38] | Ice-cel CAR T cells | 2 | 128 | 87% | 59% vs. 41% | ORR ≥ 50% | ORR ≥ 50% |
| Trial Name | Combination | Phase | n | Prev. Lines of Therapy | Extramedullary Disease | PS vs. ST-EMD | Response |
|---|---|---|---|---|---|---|---|
| Short et al., 2011 [77] | Pd | 2 | 174 | 7.5% | 0% vs. 100% (only treatment emergent ST-EMD) | ORR 30% | |
| Storm subgroup analysis 2019 [74] | Single arm selinexor | 2 | 122 | Median 7 | 22.1% | 18.5 vs. 81.5% | ORR 18.5% |
| SIRIUS 2016 [35] | Dara–d | 2 | 106 | ≥3, median 5 | 13% | n.r. | 21.4% |
| Usmani 2016 [79] | Dara single agent | Joint analysis of GEN01 + Sirius | 148 | Median 5 (2–14) | 12% | n.r. | ORR 16.7% |
| HORIZON OP-106 2020 [50] | Single arm melflufen | 2 | 157 | ≥2, median 5 | 35% | n.r. | ORR 24% PFS 2.9 months (2.0–3.8) |
| LEGEND-2 2020 [78] | Cilta-cel CAR T cells | 1 | 57 | Median 3 (1–9) | 30% | n.r. | ORR 82% |
| DREAMM-2 2020 [34] | Belantamab–mafodotin 2.5 mg/kg vs. 3.4 mg/kg | 2 | 97 vs. 99 | ≥3, more than 4: 84% vs. 83% | 20% | n.r. | ORR 9.1% vs. 5.6% |
| ICARIA-MM subgroup analysis 2020 [75] | IsaPd vs. Pd | 3 | 307 | ≥2, 3.5 (2–13) vs. 5.5 (2–6) | 8% | IsaPd: 28.6% vs. 71.4% Pd 20% vs. 80% | ORR 50% vs. 10% PFS 4.57 vs. 1.56 HR 0.219 (0.07–0.689) |
| Zhou et al., 2020 [76] | Carfilzomib-based regimens | Retrospective analysis | 45 | Median 4 (1–9) | 100% | 44% vs. 56% | EMD: ORR 27%, CBR 54% KRd: ORR 76% CBR 88% |
| KarMMa 2021 [38] | Ice-cel CAR T cells | 2 | 128 | ≥3, median 6 (3–16) | 39% | n.a. | ORR 70% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeryczynski, G.; Bolomsky, A.; Agis, H.; Krauth, M.-T. Stratification for RRMM and Risk-Adapted Therapy: Sequencing of Therapies in RRMM. Cancers 2021, 13, 5886. https://doi.org/10.3390/cancers13235886
Jeryczynski G, Bolomsky A, Agis H, Krauth M-T. Stratification for RRMM and Risk-Adapted Therapy: Sequencing of Therapies in RRMM. Cancers. 2021; 13(23):5886. https://doi.org/10.3390/cancers13235886
Chicago/Turabian StyleJeryczynski, Georg, Arnold Bolomsky, Hermine Agis, and Maria-Theresa Krauth. 2021. "Stratification for RRMM and Risk-Adapted Therapy: Sequencing of Therapies in RRMM" Cancers 13, no. 23: 5886. https://doi.org/10.3390/cancers13235886





