A Population-Based Study of Cardiovascular Disease Mortality in Italian Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Data Sources
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2013 Mortality and Causes of Death Collaborators. Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [Google Scholar] [CrossRef]
- Istituto Nazionale di Statistica. Available online: https://www.istat.it/it/archivio/240401 (accessed on 12 July 2021).
- Coviello, E.; Rashid, I.; Buzzoni, C.; Fusco, M.; AIRTUM Working Group. Aumenta la sopravvivenza per tutti i tumori, soprattutto nei maschi, ma e’ solo questione di PSA [Survival for all cancers is increasing, especially in men, but only thanks to PSA]. Epidemiol. Prev. 2012, 36, 372. (In Italian) [Google Scholar]
- AIOM-AIRTUM-SIAPEC-IAP. I Numeri del Cancro in Italia, 2020; Intermedia Editore: Brescia, Italy, 2020. [Google Scholar]
- Mazzutti, F.; Custódio, I.; Lima, M.; Carvalho, K.; Pereira, T.; Molina, M.; Canto, P.; Paiva, C.; Maia, Y. Breast Cancer Survivors Undergoing Endocrine Therapy Have a Worrying Risk Factor Profile for Cardiovascular Diseases. Nutrients 2021, 13, 1114. [Google Scholar] [CrossRef]
- Sturgeon, K.M.; Deng, L.; Bluethmann, S.M.; Zhou, S.; Trifiletti, D.M.; Jiang, C.; Kelly, S.; Zaorsky, N.G. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019, 40, 3889–3897. [Google Scholar] [CrossRef] [Green Version]
- Rubens, M.; Appunni, S.; Ramamoorthy, V.; Saxena, A.; Das, S.; Bhatt, C.; Boulanger, B.K.; Viamonte-Ros, A.; Veledar, E. Prevalence of Cardiovascular Risk Factors Among Cancer Patients in the United States. Metab. Syndr. Relat. Disord. 2019, 17, 397–405. [Google Scholar] [CrossRef]
- Michel, L.; Schadendorf, D.; Rassaf, T. Oncocardiology: New challenges, new opportunities. Herz 2020, 45, 619–625. [Google Scholar] [CrossRef]
- Armenian, S.H.; Xu, L.; Ky, B.; Sun, C.; Farol, L.T.; Pal, S.K.; Douglas, P.S.; Bhatia, S.; Chao, C. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J. Clin. Oncol. 2016, 34, 1122–1130. [Google Scholar] [CrossRef]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bluethmann, S.M.; Mariotto, A.B.; Rowland, J.H. Anticipating the “Silver Tsunami”: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol. Biomark. Prev. 2016, 25, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kline, R.M.; Arora, N.K.; Bradley, C.J.; Brauer, E.R.; Graves, D.L.; Lunsford, N.B.; McCabe, M.S.; Nasso, S.F.; Nekhlyudov, L.; Rowland, J.H.; et al. Long-Term Survivorship Care After Cancer Treatment—Summary of a 2017 National Cancer Policy Forum Workshop. J. Natl. Cancer Inst. 2018, 110, 1300–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Classificazione Statistica Internazionale delle Malattie e dei Problemi Sanitari Correlati. Decima Revisione. Volume 1 Versione 2008. A Cura del Ministero Della Salute—I Edizione—Anno 2000; Istituto Poligrafico e Zecca dello Stato, Libreria dello Stato: Roma, Italy, 2008. [Google Scholar]
- Zaorsky, N.G.; Churilla, T.M.; Egleston, B.; Fisher, S.G.; Ridge, J.A.; Horwitz, E.M.; Meyer, J.E. Causes of death among cancer patients. Ann. Oncol. 2017, 28, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- Garg, T.; Young, A.J.; Kost, K.A.; Danella, J.F.; Larson, S.; Nielsen, M.E.; Kirchner, H.L. Burden of Multiple Chronic Conditions among Patients with Urological Cancer. J. Urol. 2018, 199, 543–550. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, S.; Garmo, H.; Holmberg, L.; Adolfsson, J.; Stattin, P.; Van Hemelrijck, M. Risk and Timing of Cardiovascular Disease After Androgen-Deprivation Therapy in Men With Prostate Cancer. J. Clin. Oncol. 2015, 33, 1243–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi, M.; Tritto, R.; Tarantini, L.; Navazio, A.; Corrao, G. Adjuvant Hormonotherapy and Cardiovascular Risk in Post-Menopausal Women with Breast Cancer: A Large Population-Based Cohort Study. Cancers 2021, 13, 2254. [Google Scholar] [CrossRef]
- de Haas, E.C.; Oosting, S.F.; Lefrandt, J.D.; Wolffenbuttel, B.H.; Sleijfer, D.T.; Gietema, J.A. The metabolic syndrome in cancer survivors. Lancet Oncol. 2010, 11, 193–203. [Google Scholar] [CrossRef]
- Hamood, R.; Hamood, H.; Merhasin, I.; Keinan-Boker, L. Diabetes After Hormone Therapy in Breast Cancer Survivors: A Case-Cohort Study. J. Clin. Oncol. 2018, 36, 2061–2069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Earle, C.C.; Bae, S.J.; Fischer, H.D.; Yun, L.; Austin, P.C.; Rochon, P.A.; Anderson, G.M.; Lipscombe, L. Incidence of Diabetes in Colorectal Cancer Survivors. J. Natl. Cancer Inst. 2016, 108, djv402. [Google Scholar] [CrossRef] [Green Version]
- Crawley, D.; Garmo, H.; Rudman, S.; Stattin, P.; Häggström, C.; Zethelius, B.; Holmberg, L.; Adolfsson, J.; Van Hemelrijck, M. Association between duration and type of androgen deprivation therapy and risk of diabetes in men with prostate cancer. Int. J. Cancer 2016, 139, 2698–2704. [Google Scholar] [CrossRef] [Green Version]
- Vergès, B.; Walter, T.; Cariou, B. ENDOCRINE SIDE EFFECTS OF ANTI-CANCER DRUGS: Effects of anti-cancer targeted therapies on lipid and glucose metabolism. Eur. J. Endocrinol. 2014, 170, R43–R55. [Google Scholar] [CrossRef] [Green Version]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K.; International Agency for Research on Cancer Handbook Working Group. Body Fatness and Cancer—Viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoltzfus, K.C.; Zhang, Y.; Sturgeon, K.; Sinoway, L.I.; Trifiletti, D.M.; Chinchilli, V.M.; Zaorsky, N.G. Fatal heart disease among cancer patients. Nat. Commun. 2020, 11, 2011. [Google Scholar] [CrossRef] [PubMed]
- Koczwara, B.; Meng, R.; Miller, M.D.; Clark, R.A.; Kaambwa, B.; Marin, T.; Damarell, R.A.; Roder, D.M. Late mortality in people with cancer: A population-based Australian study. Med. J. Aust. 2021, 214, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Goldoni, C.A.; The IMPACT Working Group; Bonora, K.; Ciatto, S.; Giovannetti, L.; Patriarca, S.; Sapino, A.; Sarti, S.; Puliti, D.; Paci, E.; et al. Misclassification of breast cancer as cause of death in a service screening area. Cancer Causes Control. 2008, 20, 533–538. [Google Scholar] [CrossRef]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef] [PubMed]
- Canale, M.L.; Turazza, F.; Lestuzzi, C.; Parrini, I.; Camerini, A.; Russo, G.; Colivicchi, F.; Gabrielli, D.; Gulizia, M.M.; Oliva, S.; et al. Portrait of Italian Cardio-Oncology: Results of a Nationwide Associazione Nazionale Medici Cardiologi Ospedalieri (ANMCO) Survey. Front. Cardiovasc. Med. 2021, 8, 677544. [Google Scholar] [CrossRef]
- Kappel, C.; Rushton, M.; Johnson, C.; Aseyev, O.; Small, G.; Law, A.; Ivars, J.; Dent, S. Clinical Experience of Patients Referred to a Multidisciplinary Cardio-Oncology Clinic: An Observational Cohort Study. Curr. Oncol. 2019, 26, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Jovenaux, L.; Cautela, J.; Resseguier, N.; Pibarot, M.; Taouqi, M.; Orabona, M.; Pinto, J.; Peyrol, M.; Barraud, J.; Laine, M.; et al. Practices in management of cancer treatment-related cardiovascular toxicity: A cardio-oncology survey. Int. J. Cardiol. 2017, 241, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Pareek, N.; Cevallos, J.; Moliner, P.; Shah, M.; Tan, L.L.; Chambers, V.; Baksi, A.J.; Khattar, R.S.; Sharma, R.; Rosen, S.D.; et al. Activity and outcomes of a cardio-oncology service in the United Kingdom-a five-year experience. Eur. J. Heart Fail. 2018, 20, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Strongman, H.; Gadd, S.; Matthews, A.; Mansfield, K.E.; Stanway, S.; Lyon, A.R.; Dos-Santos-Silva, I.; Smeeth, L.; Bhaskaran, K. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: A population-based cohort study using multiple linked UK electronic health records databases. Lancet 2019, 394, 1041–1054. [Google Scholar] [CrossRef] [Green Version]



| Cancer Patients | No. of Deaths | CVD Deaths | Cancer Deaths | ||||
|---|---|---|---|---|---|---|---|
| No. | No. | % | No. | % | No. | % | |
| All | 67,163 | 38,272 | 57.0 | 4466 | 6.6 | 28,579 | 42.6 |
| Sex | |||||||
| Male | 34,918 | 21,857 | 62.6 | 2445 | 7.0 | 16,345 | 46.8 |
| Female | 32,245 | 16,415 | 50.9 | 2021 | 6.3 | 12,234 | 37.9 |
| Age at diagnosis | |||||||
| 0–19 | 425 | 75 | 17.6 | 0 | 0.0 | 75 | 17.6 |
| 20–39 | 2997 | 495 | 16.5 | 3 | 0.1 | 443 | 14.8 |
| 40–59 | 14,829 | 4597 | 31.0 | 122 | 0.8 | 4094 | 27.6 |
| 60–79 | 34,997 | 21,170 | 60.5 | 2525 | 7.2 | 15,674 | 44.8 |
| 80+ | 13,915 | 11,935 | 85.8 | 1816 | 13.1 | 8293 | 59.6 |
| Year of diagnosis | |||||||
| 1996–1999 | 9965 | 8008 | 80.4 | 1135 | 11.4 | 5900 | 59.2 |
| 2000–2004 | 14,050 | 10,126 | 72.1 | 1413 | 10.1 | 7422 | 52.8 |
| 2005–2009 | 14,332 | 8694 | 60.7 | 1055 | 7.4 | 6346 | 44.3 |
| 2010–2014 | 14,425 | 6970 | 48.3 | 623 | 4.3 | 5202 | 36.1 |
| 2015–2019 | 14,391 | 4474 | 31.1 | 240 | 1.7 | 3709 | 25.8 |
| Year of death * | |||||||
| 1996–1999 | 9965 | 3605 | 36.2 | 189 | 1.9 | 3268 | 32.8 |
| 2000–2004 | 20,410 | 7300 | 35.8 | 630 | 3.1 | 6230 | 30.5 |
| 2005–2009 | 27,442 | 8445 | 30.8 | 1012 | 3.7 | 6527 | 23.8 |
| 2010–2014 | 33,422 | 9051 | 27.1 | 1230 | 3.7 | 6101 | 18.3 |
| 2015–2019 | 38,762 | 9871 | 25.5 | 1405 | 3.6 | 6453 | 16.6 |
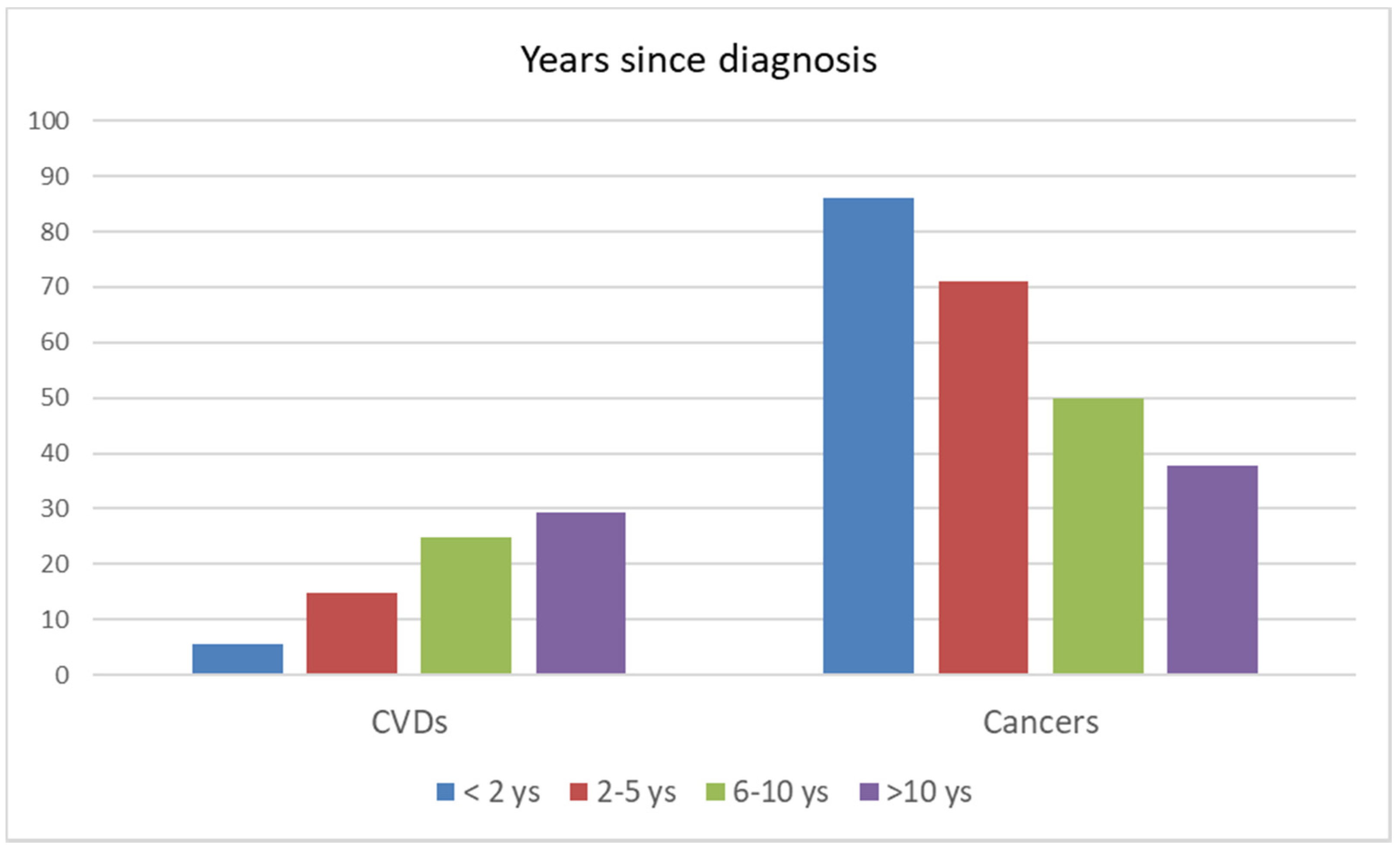
| Years since diagnosis | |||||||
| <2 years | 27,526 | 22,520 | 81.8 | 1230 | 4.5 | 19,401 | 70.5 |
| 2–5 years | 15,366 | 8158 | 53.1 | 1210 | 7.9 | 5789 | 37.7 |
| 6–10 years | 11,391 | 4371 | 38.4 | 1085 | 9.5 | 2176 | 19.1 |
| >10 years | 12,880 | 3223 | 25.0 | 941 | 7.3 | 1213 | 9.4 |
| 10 main tumor sites | |||||||
| Breast | 10,295 | 3384 | 32.9 | 730 | 7.1 | 1963 | 19.1 |
| Colorectum | 7737 | 4668 | 60.3 | 698 | 9.0 | 3303 | 42.7 |
| Lung | 7306 | 6487 | 88.8 | 238 | 3.3 | 5668 | 77.6 |
| Prostate | 6452 | 2769 | 42.9 | 662 | 10.3 | 1511 | 23.4 |
| Bladder | 4438 | 2443 | 55.0 | 512 | 11.5 | 1365 | 30.8 |
| Stomach | 3298 | 2733 | 82.9 | 212 | 6.4 | 2298 | 69.7 |
| Non-Hodgkin’s lymphoma | 2679 | 1338 | 49.9 | 176 | 6.6 | 920 | 34.3 |
| Pancreas | 2343 | 2134 | 91,7 | 45 | 1.9 | 2034 | 86.8 |
| Thyroid | 2300 | 249 | 10.8 | 47 | 2.0 | 152 | 6.6 |
| Melanoma | 2038 | 459 | 22.0 | 108 | 5.2 | 271 | 13.0 |
| Year of Diagnosis | No. | Expected | SMR | 95% CI |
|---|---|---|---|---|
| 1996–1999 | 1135 | 1300.64 | 0.87 | (0.82–0.92) |
| 2000–2004 | 1413 | 1570.77 | 0.89 | (0.85–0.94) |
| 2005–2009 | 1055 | 1137.27 | 0.92 | (0.87–0.98) |
| 2010–2014 | 623 | 677.07 | 0.92 | (0.85–0.99) |
| 2015–2019 | 240 | 250.67 | 0.95 | (0.84–1.08) |
| Sex | ||||
| Male | 2445 | 2722.58 | 0.89 | (0.86–0.93) |
| Female | 2021 | 2213.85 | 0.91 | (0.87–0.95) |
| Age | ||||
| 0–19 | 0 | 0.08 | 0.0 | - |
| 20–39 | 3 | 3.45 | 0.87 | (0.28–2.69) |
| 40–59 | 122 | 131.30 | 0.92 | (0.77–1.10) |
| 60–79 | 2525 | 2787.79 | 0.90 | (0.87–0.94) |
| 80+ | 1816 | 2013.81 | 0.90 | (0.86–0.94) |
| Years from diagnosis | ||||
| <2 years | 1230 | 936.68 | 1.31 | (1.24–1.39) |
| 2–5 years | 1210 | 1537.98 | 0.78 | (0.74–0.83) |
| 6–10 years | 1085 | 1297.39 | 0.84 | (0.79–0.88) |
| >10 years | 941 | 1164.37 | 0.81 | (0.76–0.86) |
| All cases | 4466 | 4936.43 | 0.90 | (0.88–0.93) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangone, L.; Mancuso, P.; Tarantini, L.; Larocca, M.; Bisceglia, I.; Damato, A.; Giorgi Rossi, P.; Navazio, A.; Pinto, C. A Population-Based Study of Cardiovascular Disease Mortality in Italian Cancer Patients. Cancers 2021, 13, 5903. https://doi.org/10.3390/cancers13235903
Mangone L, Mancuso P, Tarantini L, Larocca M, Bisceglia I, Damato A, Giorgi Rossi P, Navazio A, Pinto C. A Population-Based Study of Cardiovascular Disease Mortality in Italian Cancer Patients. Cancers. 2021; 13(23):5903. https://doi.org/10.3390/cancers13235903
Chicago/Turabian StyleMangone, Lucia, Pamela Mancuso, Luigi Tarantini, Mario Larocca, Isabella Bisceglia, Angela Damato, Paolo Giorgi Rossi, Alessandro Navazio, and Carmine Pinto. 2021. "A Population-Based Study of Cardiovascular Disease Mortality in Italian Cancer Patients" Cancers 13, no. 23: 5903. https://doi.org/10.3390/cancers13235903







