Copper-64-Labeled 1C1m-Fc, a New Tool for TEM-1 PET Imaging and Prediction of Lutetium-177-Labeled 1C1m-Fc Therapy Efficacy and Safety
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Fusion Protein Antibody
2.3. Conjugation
2.4. Characterization of the Immunoconjugates: Mass Spectrometry Analysis
2.5. Radiolabeling
2.6. In Vitro Studies: Radio-Immunoreactivity
2.7. In Vivo Characterization Studies
2.7.1. Animal Model
2.7.2. PET Imaging Study
2.7.3. Biodistribution Study
2.7.4. Murine Dosimetry
2.7.5. Dose Extrapolation to the 177Lu Compound
2.8. Statistical Analysis
3. Results
3.1. Conjugation
3.2. Radiolabeling
3.3. In Vitro Studies: Radio-Immunoreactivity
3.4. Imaging Study
3.5. Biodistribution Study
3.6. Murine Dosimetry
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Cianni, R.; Bagni, O. Theranostic Approaches in Nuclear Medicine: Current Status and Future Prospects. Expert Rev. Med. Devices 2020, 17, 331–343. [Google Scholar] [CrossRef]
- Keinänen, O.; Fung, K.; Brennan, J.M.; Zia, N.; Harris, M.; van Dam, E.; Biggin, C.; Hedt, A.; Stoner, J.; Donnelly, P.S.; et al. Harnessing64Cu/67Cu for a Theranostic Approach to Pretargeted Radioimmunotherapy. Proc. Natl. Acad. Sci. USA 2020, 117, 28316–28327. [Google Scholar] [CrossRef]
- Langbein, T.; Weber, W.A.; Eiber, M. Future of Theranostics: An Outlook on Precision Oncology in Nuclear Medicine. J. Nucl. Med. 2019, 60, 13S–19S. [Google Scholar] [CrossRef] [Green Version]
- Accorsi, R. Brain Single-Photon Emission CT Physics Principles. Am. J. Neuroradiol. 2008, 29, 1247–1256. [Google Scholar] [CrossRef] [Green Version]
- Jadvar, H.; Chen, X.; Cai, W.; Mahmood, U. Radiotheranostics in Cancer Diagnosis and Management. Radiology 2018, 286, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Teicher, B.A. CD248: A Therapeutic Target in Cancer and Fibrotic Diseases. Oncotarget 2019, 10, 993–1009. [Google Scholar] [CrossRef] [Green Version]
- MacFadyen, J.R.; Haworth, O.; Roberston, D.; Hardie, D.; Webster, M.T.; Morris, H.R.; Panicoc, M.; Sutton-Smith, M.; Dell, A.; van der Geer, P.; et al. Endosialin (TEM1, CD248) is a Marker of Stromal Fibroblasts and is not Selectively Expressed on Tumour Endothelium. FEBS Lett. 2005, 579, 2569–2575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomkowicz, B.; Rybinski, K.; Foley, B.; Ebel, W.; Kline, B.; Routhier, E.; Sass, P.; Nicolaides, N.C.; Grasso, L.; Zhou, Y. Interaction of Endosialin/TEM1 with Extracellular Matrix Proteins Mediates Cell Adhesion and Migration. Proc. Natl. Acad. Sci. USA 2007, 104, 17965–17970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simonavicius, N.; Robertson, D.; Bax, D.A.; Jones, C.; Huijbers, I.J.; Isacke, C. Endosialin (CD248) is a Marker of Tumor-Associated Pericytes in High-Grade Glioma. Mod. Pathol. 2008, 21, 308–315. [Google Scholar] [CrossRef]
- MacFadyen, J.; Savage, K.; Wienke, D.; Isacke, C.M. Endosialin Is Expressed on Stromal Fibroblasts and CNS Pericytes in Mouse Embryos and is Downregulated during Development. Gene Expr. Patterns 2007, 7, 363–369. [Google Scholar] [CrossRef]
- Davies, G.; Cunnick, G.H.; Mansel, R.E.; Mason, M.D.; Jiang, W.G. Levels of Expression of Endothelial Markers Specific to Tumour-Associated Endothelial Cells and their Correlation with Prognosis in Patients with Breast Cancer. Clin. Exp. Metastasis 2004, 21, 31–37. [Google Scholar] [CrossRef]
- O’Shannessy, D.J.J.; Somers, E.B.; Chandrasekaran, L.K.; Nicolaides, N.C.; Bordeaux, J.; Gustavson, M.D. Influence of Tumor Microenvironment on Prognosis in Colorectal Cancer: Tissue Architecture-Dependent Signature of Endosialin (TEM-1) and Associated Proteins. Oncotarget 2014, 5, 3983–3995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maia, M.; Conway, E. CD248: Reviewing its Role in Health and Disease. Curr. Drug Targets 2012, 13, 432–439. [Google Scholar] [CrossRef]
- Rouleau, C.; Gianolio, D.A.; Smale, R.; Roth, S.D.; Krumbholz, R.; Harper, J.; Munroe, K.J.; Green, T.L.; Horten, B.C.; Schmid, S.M.; et al. Anti-Endosialin Antibody–Drug Conjugate: Potential in Sarcoma and Other Malignancies. Mol. Cancer Ther. 2015, 14, 2081–2089. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Teicher, B.A.; Hassan, R. Antibody–Drug Conjugates for Cancer Therapy. Lancet Oncol. 2016, 17, e254–e262. [Google Scholar] [CrossRef]
- Li, C.; Wang, J.; Hu, J.; Feng, Y.; Hasegawa, K.; Peng, X.; Duan, X.; Zhao, A.; Mikitsh, J.L.; Muzykantov, V.R.; et al. Development, Optimization, and Validation of Novel anti-TEM1/CD248 Affinity Agent for Optical Imaging in Cancer. Oncotarget 2014, 5, 6994–7012. [Google Scholar] [CrossRef] [Green Version]
- Zhao, A.; Nunez-Cruz, S.; Li, C.; Coukos, G.; Siegel, D.L.; Scholler, N. Rapid Isolation of High-Affinity Human Antibodies Against the Tumor Vascular Marker Endosialin/TEM1, Using a Paired Yeast-Display/Secretory Scfv Library Platform. J. Immunol. Methods 2011, 363, 221–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicone, F.; Denoel, T.; Gnesin, S.; Riggi, N.; Irving, M.; Jakka, G.; Schaefer, N.; Viertl, D.; Coukos, G.; Prior, J.O. Preclinical Evaluation and Dosimetry of [(111)In]CHX-DTPA-scFv78-Fc Targeting Endosialin/Tumor Endothelial Marker 1 (TEM1). Mol. Imaging Biol. 2020, 22, 979–991. [Google Scholar] [CrossRef] [Green Version]
- Chacko, A.M.; Li, C.; Nayak, M.; Mikitsh, J.L.; Hu, J.; Hou, C.; Grasso, L.; Nicolaides, N.C.; Muzykantov, V.R.; Divgi, C.R.; et al. Development of 124I Immuno-PET Targeting Tumor Vascular TEM1/Endosialin. J. Nucl. Med. 2014, 55, 500–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, S.E.; Zheleznyak, A.; Studer, M.; O’Shannessy, D.J.; Lapi, S.E.; Van Tine, B.A. Development of 89Zr-Ontuxizumab for in vivo TEM-1/endosialin PET Applications. Oncotarget 2016, 7, 13082–13092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grothey, A.; Strosberg, J.R.; Renfro, L.A.; Hurwitz, H.I.; Marshall, J.L.; Safran, H.; Guarino, M.J.; Kim, J.P.; Hecht, J.R.; Weil, S.C.; et al. A Randomized, Double-Blind, Placebo-Controlled Phase II Study of the Efficacy and Safety of Monotherapy Ontuxizumab (MORAb-004) Plus Best Supportive Care in Patients with Chemorefractory Metastatic Colorectal Cancer. Clin. Cancer Res. 2018, 24, 316–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norris, R.E.; Fox, E.; Reid, J.M.; Ralya, A.; Liu, X.W.; Minard, C.; Weigel, B.J. Phase 1 Trial of Ontuxizumab (MORAb-004) in Children with Relapsed or Refractory Solid Tumors: A Report from the Children’s Oncology Group Phase 1 Pilot Consortium (ADVL1213). Pediatr. Blood Cancer. 2018, 65, e26944. [Google Scholar] [CrossRef] [PubMed]
- Diaz, L.A.; Coughlin, C.M.; Weil, S.C.; Fishel, J.; Gounder, M.M.; Lawrence, S.; Azad, N.; O’Shannessy, D.J.; Grasso, L.; Wustner, J.; et al. A First-in-Human Phase I Study of MORAb-004, a Monoclonal Antibody to Endosialin in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015, 21, 1281–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgeois, M.; Bailly, C.; Frindel, M.; Guérard, F.; Chérel, M.; Faivre-Chauvet, A.; Kraeber-Bodéré, F.; Bodet-Milin, C. Radioimmunoconjugates for Treating Cancer: Recent Advances and Current Opportunities. Expert Opin. Biol. Ther. 2017, 17, 813–819. [Google Scholar] [CrossRef]
- Puvvada, S.D.; Guillen-Rodriguez, J.M.; Yan, J.; Inclan, L.; Heard, K.; Rivera, X.I.; Answer, F.; Mahadevan, D.; Schatz, J.H.; Persky, D.O. Yttrium-90-Ibritumomab Tiuxetan (Zevalin (R)) Radioimmunotherapy after Cytoreduction with ESHAP Chemotherapy in Patients with Relapsed Follicular Non-Hodgkin Lymphoma: Final Results of a Phase II Study. Oncol. Basel 2018, 94, 274–280. [Google Scholar] [CrossRef]
- Caserta, E.; Chea, J.; Minnix, M.; Poku, E.K.; Viola, D.; Vonderfecht, S.; Yazaki, P.; Crow, D.; Khalife, J.; Sanchez, J.F.; et al. Copper 64–Labeled Daratumumab as a PET/CT Imaging Tracer for Multiple Myeloma. Blood 2018, 131, 741–745. [Google Scholar] [CrossRef]
- Delage, J.A.; Faivre-Chauvet, A.; Fierle, J.K.; Gnesin, S.; Schaefer, N.; Coukos, G.; Dunn, M.; Viertl, D.S.; Prior, J.O. (177) Lu Radiolabeling and Preclinical Theranostic Study of 1C1m-Fc: An Anti-TEM-1 scFv-Fc Fusion Protein in Soft Tissue Sarcoma. EJNMMI Res. 2020, 10, 98. [Google Scholar] [CrossRef]
- Delage, J.A.; Faivre-Chauvet, A.; Barbet, J.; Fierle, J.K.; Schaefer, N.; Coukos, G.; Dunn, M.; Viertl, D.S.; Prior, J.O. Impact of DOTA Conjugation on Pharmacokinetics and Immunoreactivity of [(177)Lu]Lu-1C1m-Fc, an Anti TEM-1 Fusion Protein Antibody in a TEM-1 Positive Tumor Mouse Model. Pharmaceutics 2021, 13, 96. [Google Scholar] [CrossRef]
- Navarro, A.S.; Le Bihan, T.; Le Saec, P.; Bris, N.L.; Bailly, C.; Sai-Maurel, C.; Bourgeois, M.; Chérel, M.; Tripier, R.; Faivre-Chauvet, A. TE1PA as Innovating Chelator for (64)Cu Immuno-TEP Imaging: A Comparative In Vivo Study with DOTA/NOTA by Conjugation on 9E7.4 mAb in a Syngeneic Multiple Myeloma Model. Bioconjug. Chem. 2019, 30, 2393–2403. [Google Scholar] [CrossRef]
- Mortimer, J.E.; Bading, J.R.; Colcher, D.M.; Conti, P.S.; Frankel, P.H.; Carroll, M.I.; Tong, S.; Poku, E.; Miles, J.K.; Shively, J.E.; et al. Functional Imaging of Human Epidermal Growth Factor Receptor 2-Positive Metastatic Breast Cancer Using (64)Cu-DOTA-trastuzumab PET. J. Nucl. Med. 2014, 55, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Fierle, J.K.; Abram-Saliba, J.; Brioschi, M.; Detiani, M.; Coukos, G.; Dunn, S.M. Integrating SpyCatcher/SpyTag Covalent Fusion Technology into Phage Display Workflows for Rapid Antibody Discovery. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Li, D.; Park, R.; Liu, R.; Xia, Z.; Guo, J.; Krasnoperov, V.; Gill, P.S.; Li, Z.; Shan, H.; et al. PET Imaging of Colorectal and Breast Cancer by Targeting EphB4 Receptor with 64Cu-Labeled hAb47 and hAb131 Antibodies. J. Nucl. Med. 2013, 54, 1094–1100. [Google Scholar] [CrossRef] [Green Version]
- Christian, S.; Winkler, R.; Helfrich, I.; Boos, A.M.; Besemfelder, E.; Schadendorf, D.; Augustin, H.G. Endosialin (Tem1) Is a Marker of Tumor-Associated Myofibroblasts and Tumor Vessel-Associated Mural Cells. Am. J. Pathol. 2008, 172, 486–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, S.; Ahorn, H.; Koehler, A.; Eisenhaber, F.; Rodi, H.-P.; Garin-Chesa, P.; Park, J.E.; Rettig, W.J.; Lenter, M.C. Molecular Cloning and Characterization of Endosialin, a C-type Lectin-like Cell Surface Receptor of Tumor Endothelium. J. Biol. Chem. 2001, 276, 7408–7414. [Google Scholar] [CrossRef] [Green Version]
- Qin, C.; Liu, H.; Chen, K.; Hu, X.; Ma, X.; Lan, X.; Zhang, Y.; Cheng, Z. Theranostics of Malignant Melanoma with 64CuCl2. J. Nucl. Med. 2014, 55, 812–817. [Google Scholar] [CrossRef] [Green Version]
- Chakravarty, R.; Chakraborty, S.; Dash, A. 64Cu2+ Ions as PET Probe: An Emerging Paradigm in Molecular Imaging of Cancer. Mol. Pharm. 2016, 13, 3601–3612. [Google Scholar] [CrossRef]
- Johnbeck, C.B.; Knigge, U.; Loft, A.; Berthelsen, A.K.; Mortensen, J.; Oturai, P.; Langer, S.W.; Elema, D.R.; Kjaer, A. Head-to-Head Comparison of 64Cu-DOTATATE and 68Ga-DOTATOC PET/CT: A Prospective Study of 59 Patients with Neuroendocrine Tumors. J. Nucl. Med. 2017, 58, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Piccardo, A.; Paparo, F.; Puntoni, M.; Righi, S.; Bottoni, G.; Bacigalupo, L.; Zanardi, S.; DeCensi, A.; Ferrarazzo, G.; Gambaro, M.; et al. 64CuCl2 PET/CT in Prostate Cancer Relapse. J. Nucl. Med. 2017, 59, 444–451. [Google Scholar] [CrossRef] [Green Version]
- Boschi, A.; Martini, P.; Janevik-Ivanovska, E.; Duatti, A. The Emerging Role of Copper-64 Radiopharmaceuticals as Cancer Theranostics. Drug Discov. Today 2018, 23, 1489–1501. [Google Scholar] [CrossRef] [PubMed]
- Wadas, T.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef] [Green Version]
- Dolznig, H.; Schweifer, N.; Puri, C.; Kraut, N.; Rettig, W.J.; Kerjaschki, D.; Garin-Chesa, P. Characterization of Cancer Stroma Markers: In Silico Analysis of an mRNA Expression Database for Fibroblast Activation Protein and Endosialin. Cancer Immun. 2005, 5, 10. [Google Scholar]
- Opavsky, R.; Haviernik, P.; Jurkovicova, D.; Garin, M.T.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Bies, J.; Garfield, S.; Pastorekova, S.; et al. Molecular Characterization of the Mouse Tem1/Endosialin Gene Regulated by Cell Density In Vitro and Expressed in Normal Tissues In Vivo. J. Biol. Chem. 2001, 276, 38795–38807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinne, S.S.; Leitao, C.D.; Gentry, J.; Mitran, B.; Abouzayed, A.; Tolmachev, V.; Ståhl, S.; Löfblom, J.; Orlova, A. Increase in Negative Charge of 68Ga/Chelator Complex Reduces Unspecific Hepatic Uptake but does not Improve Imaging Properties of HER3-targeting Affibody Molecules. Sci. Rep. 2019, 9, 17710. [Google Scholar] [CrossRef]
- Cooper, M.S.; Ma, M.T.; Sunassee, K.; Shaw, K.P.; Williams, J.D.; Paul, R.L.; Donnelly, P.S.; Blower, P.J. Comparison of (64)Cu-Complexing Bifunctional Chelators for Radioimmunoconjugation: Labeling Efficiency, Specific Activity, and In Vitro/In Vivo Stability. Bioconjug. Chem. 2012, 23, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Grunberg, J.; Jeger, S.; Sarko, D.; Dennler, P.; Zimmermann, K.; Mier, W.; Schibli, R. DOTA-Functionalized Polylysine: A High Number of DOTA Chelates Positively Influences the Biodistribution of Enzymatic Conjugated Anti-Tumor Antibody chCE7agl. PLoS ONE 2013, 8, e60350. [Google Scholar] [CrossRef] [Green Version]
- Capasso, E.; Durzu, S.; Piras, S.; Zandieh, S.; Knoll, P.; Haug, A.; Hacker, M.; Meleddu, C.; Mirzaei, S. Role of 64CuCl2 PET/CT in Staging of Prostate Cancer. Ann. Nucl. Med. 2015, 29, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Anderson, C.J. Chelators for Copper Radionuclides in Positron Emission Tomography Radiopharmaceuticals. J. Label. Compd. Radiopharm. 2014, 57, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Frindel, M.; Camus, N.; Rauscher, A.; Bourgeois, M.; Alliot, C.; Barré, L.; Gestin, J.-F.; Tripier, R.; Faivre-Chauvet, A. Radiolabeling of HTE1PA: A New Monopicolinate Cyclam Derivative for Cu-64 Phenotypic Imaging. In vitro and In Vivo Stability Studies in Mice. Nucl. Med. Biol. 2014, 41, e49–e57. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, A.; Gano, L.; Melo, R.; Mendes, F.; Oliveira, M.C.; Denoël, T.; Schaefer, N.; Viertl, D.; Fierle, J.; Coukos, G.; et al. Biological Evaluation of New TEM1 Targeting Recombinant Antibodies for Radioimmunotherapy: In Vitro, In Vivo and in Silico Studies. Eur. J. Pharm. Biopharm. 2021, 158, 233–244. [Google Scholar] [CrossRef]
- Pérez-Debén, S.; Gonzalez-Martin, R.; Palomar, A.; Quiñonero, A.; Salsano, S.; Dominguez, F. Copper and Lead Exposures Disturb Reproductive Features of Primary Endometrial Stromal and Epithelial Cells. Reprod. Toxicol. 2020, 93, 106–117. [Google Scholar] [CrossRef]
- Cai, H.; Li, Z.; Huang, C.-W.; Park, R.; Shahinian, A.H.; Conti, P.S. An Improved Synthesis and Biological Evaluation of a New Cage-Like Bifunctional Chelator, 4-((8-amino-3,6,10,13,16,19-hexaazabicyclo[6.6.6]icosane-1-ylamino)methyl)benzoic acid, for 64Cu Radiopharmaceuticals. Nucl. Med. Biol. 2010, 37, 57–65. [Google Scholar] [CrossRef]
- Shi, X.; Xing, H.; Yang, X.; Li, F.; Yao, S.; Zhang, H.; Zhao, H.; Hacker, M.; Huo, L.; Li, X. Fibroblast Imaging of Hepatic Carcinoma with 68Ga-FAPI-04 PET/CT: A Pilot Study in Patients with Suspected Hepatic Nodules. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 196–203. [Google Scholar] [CrossRef]
- Dendl, K.; Koerber, S.A.; Finck, R.; Mokoala, K.M.G.; Staudinger, F.; Schillings, L.; Heger, U.; Röhrich, M.; Kratochwil, C.; Sathekge, M.; et al. 68Ga-FAPI-PET/CT in Patients with Various Gynecological Malignancies. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4089–4100. [Google Scholar] [CrossRef]
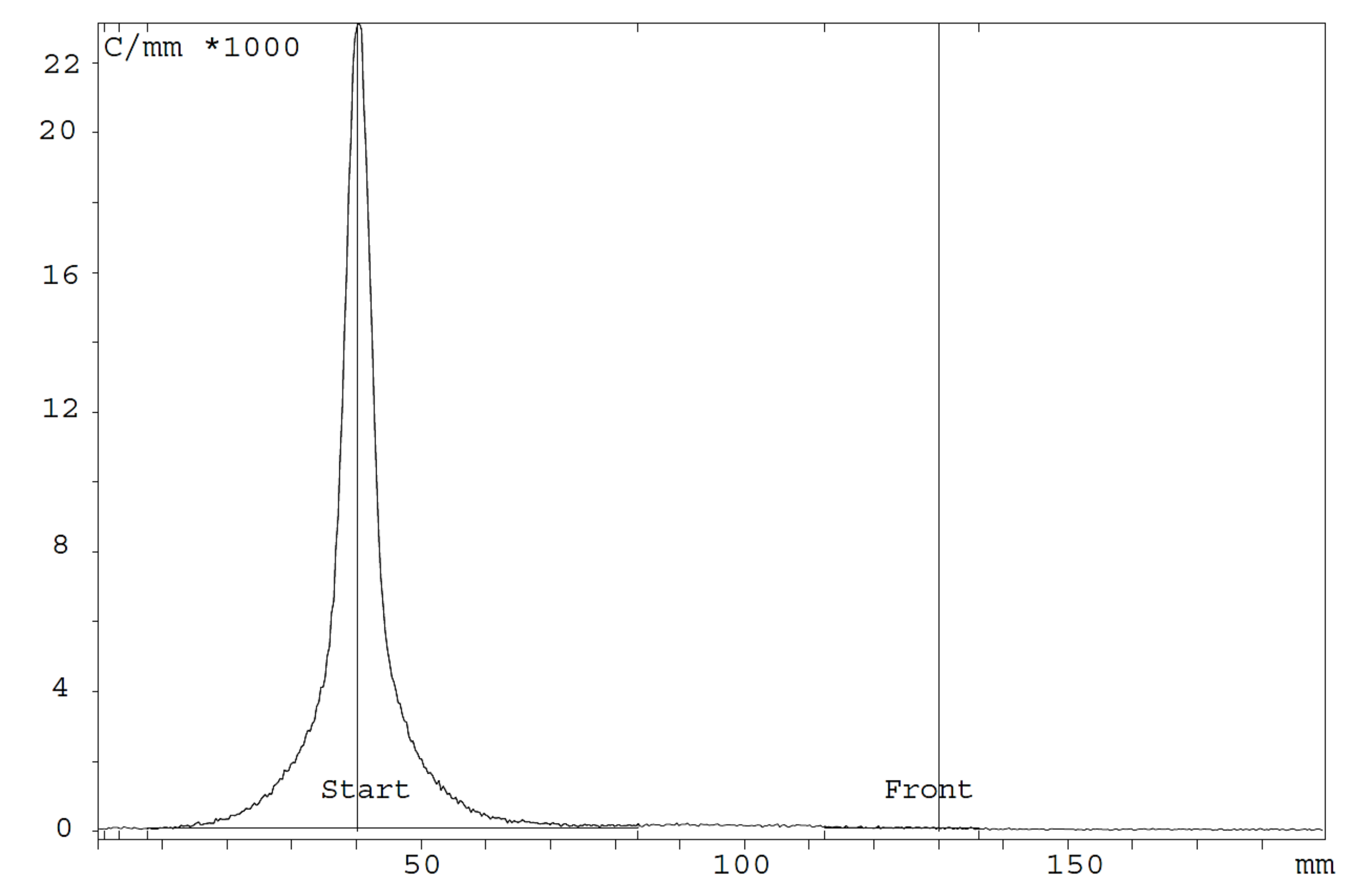

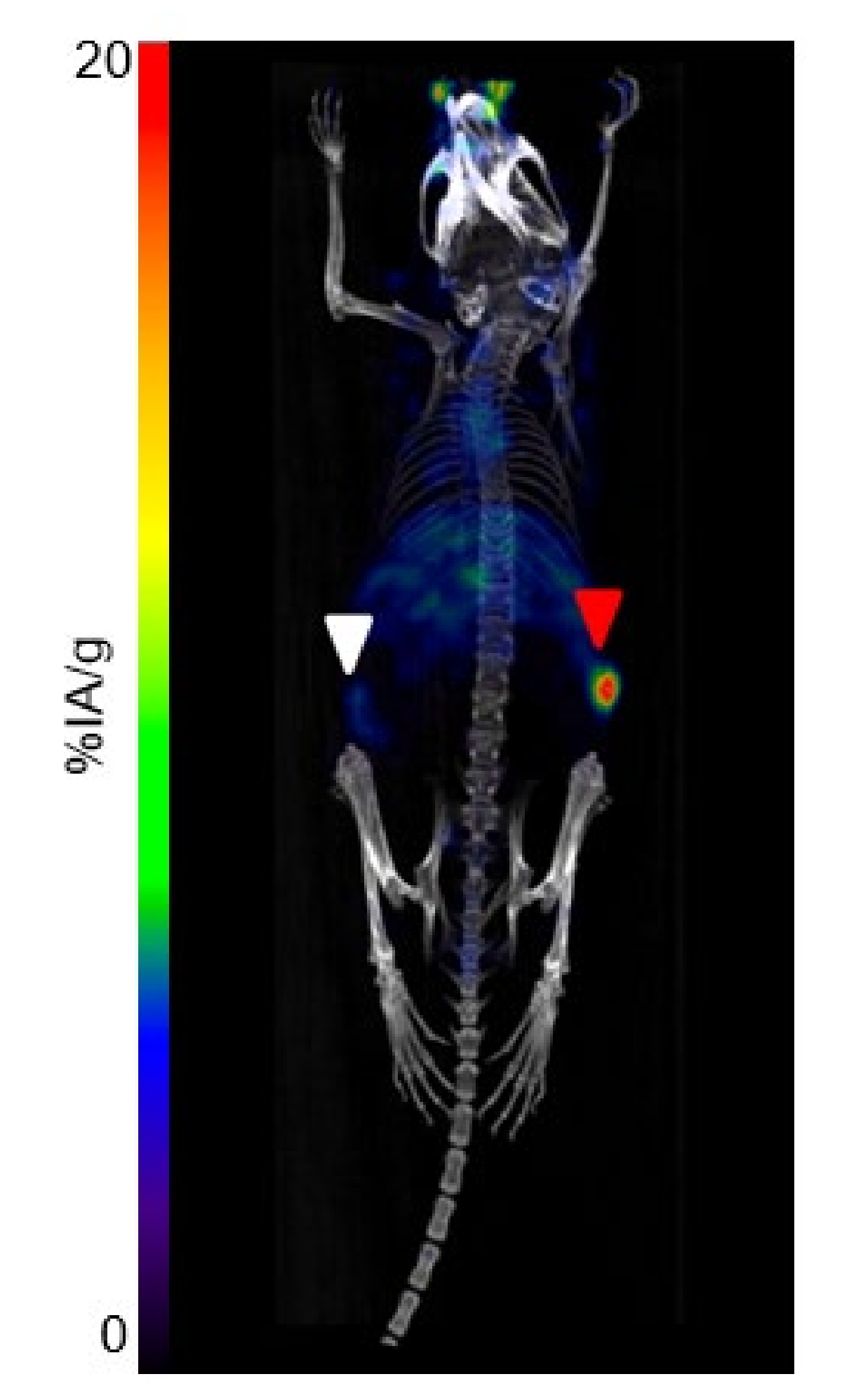

| Immunoreactivity (%) ± SD | 4 h | 24 h | 48 h |
|---|---|---|---|
| [64Cu]Cu-3DOTA-1C1m-Fc | 76 ± 1.4 (n = 2) | 70 (n = 1) | NA |
| [64Cu]Cu-4DOTA-1C1m-Fc | 75 ± 15 (n = 3) | 77 ± 14 (n = 3) | 72 ± 13 (n = 3) |
| Ratio | 4 h | 24 h | 48 h |
|---|---|---|---|
| Tumor/Liver | 1.1 | 1.9 | 1.8 |
| Tumor/Spleen | 1.1 | 2.2 | 2.5 |
| Tumor/Blood | 0.6 | 2.5 | 3.6 |
| Tumor/Kidney | 1.3 | 2.5 | 2.6 |
| Tumor/Lungs | 1.1 | 2.8 | 3.3 |
| Tumor/Heart | 1.8 | 5.4 | 5.8 |
| Tumor/Muscle | 7.2 | 8.0 | 7.3 |
| Tumor/Bone | 3.2 | 5.3 | 5.1 |
| Tumor/Small Intestine | 3.6 | 6.0 | 4.9 |
| Tumor/Stomach | 4.3 | 5.0 | 4.5 |
| Tumor/Colon | 3.3 | 4.3 | 3.2 |
| Tumor/Ovaries | 2.0 | 2.7 | 3.6 |
| Tumor/Uterus | 1.2 | 1.6 | 1.4 |
| Organ | Mean Organ Mass (g) | TIAC (MBq.h/MBq) | Absorbed Dose (mGy/MBq) | ||||
|---|---|---|---|---|---|---|---|
| Mean | Mean − SD | Mean + SD | Mean | Mean − SD | Mean + SD | ||
| Brain | 30 | 20.7 | 39.3 | ||||
| Large Intestine * | 0.25 | 0.264 | 0.199 | 0.321 | 110 | 81.1 | 137 |
| Small Intestine * | 0.58 | 0.422 | 0.263 | 0.565 | 87.4 | 57.3 | 115 |
| Stomach Wall * | 0.11 | 0.093 | 0.078 | 0.108 | 98.3 | 78.2 | 118 |
| Heart * | 0.14 | 0.182 | 0.169 | 0.194 | 137 | 118 | 155 |
| Kidneys * | 0.27 | 0.569 | 0.535 | 0.601 | 167 | 149 | 184 |
| Liver * | 1.01 | 2.613 | 2.242 | 2.932 | 184 | 152 | 212 |
| Lungs * | 0.14 | 0.328 | 0.248 | 0.407 | 159 | 119 | 198 |
| Pancreas | 38.2 | 27.8 | 48.4 | ||||
| Skeleton | 33.2 | 23.2 | 43 | ||||
| Spleen * | 0.08 | 0.189 | 0.174 | 0.204 | 169 | 149 | 189 |
| Testes | 30.1 | 20.7 | 39.4 | ||||
| Thyroid | 30.8 | 21.2 | 40.3 | ||||
| Urinary Bladder | 30.6 | 21.1 | 40 | ||||
| Total Body | 17.8 | 13.349 | 10.311 | 16.631 | 46.1 | 34.5 | 57.3 |
| Rest of the body * | 15.2 | 7.06 | 4.857 | 9.25 | |||
| Uterus *,s | 0.09 | 0.246 | 0.108 | 0.363 | 178 | 78.4 | 263 |
| Ovaries *,s | 0.04 | 0.054 | 0.034 | 0.073 | 85.1 | 53.6 | 115 |
| Tumor *,s | 0.11 | 0.377 | 0.141 | 0.613 | 225 | 84.2 | 366 |
| Target Organs | 1: [177Lu]Lu-1C1m-Fc TIAC and Absorbed Dose Extrapolation from [64Cu]Cu-1C1m-Fc Biodistribution Data | 2: [177Lu]Lu-1DOTA-1C1m-Fc Absorbed Dose (mGy/MBq) AD2 Data from [28] | 3: Difference Ratio between AD1 and AD2 (%) | 4: [177Lu]Lu-3DOTA-1C1m-Fc Absorbed Dose (mGy/MBq) AD3 Data from [27] | 5: Difference Ratio between AD1 and AD3 (%) | |
|---|---|---|---|---|---|---|
| TIAC (MBq-h/MBq) | Absorbed Dose (mGy/MBq) AD1 | |||||
| Brain | 485 | 408 | 19 | |||
| Large Intestine * | 2.921 | 1490 | 703 | 112 | 328 | 354 |
| Small Intestine * | 5.844 | 1350 | 577 | 134 | 438 | 208 |
| Stomach Wall * | 0.803 | 1130 | 1660 | −32 | 1150 | −2 |
| Heart * | 0.45 | 904 | 1110 | −19 | 363 | 159 |
| Kidneys * | 1.99 | 1070 | 1320 | −19 | 705 | 52 |
| Liver * | 16.559 | 1630 | 1790 | −9 | 2230 | −27 |
| Lungs * | 0.864 | 976 | 983 | −1 | 539 | 81 |
| Pancreas | 512 | 441 | 16 | |||
| Skeleton | 492 | 418 | 18 | |||
| Spleen * | 0.672 | 1090 | 1180 | −8 | 1200 | −9 |
| Testes | 485 | 409 | 19 | |||
| Thyroid | 486 | 409 | 19 | |||
| Urinary Bladder | 486 | 534 | −9 | |||
| Total Body | 130.205 | 590 | 549 | 7 | ||
| Rest of the body * | 100.102 | |||||
| Uterus *,s | 3.31 | 2890 | 1830 | 58 | 1500 | 93 |
| Ovaries *,s | 0.272 | 570 | 742 | −23 | ||
| Tumor *,s | 5.294 | 3850 | 2530 | 52 | 1820 | 111 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delage, J.A.; Gnesin, S.; Prior, J.O.; Barbet, J.; Le Saëc, P.; Marionneau-Lambot, S.; Gouard, S.; Chérel, M.; Bourgeois, M.; Schaefer, N.; et al. Copper-64-Labeled 1C1m-Fc, a New Tool for TEM-1 PET Imaging and Prediction of Lutetium-177-Labeled 1C1m-Fc Therapy Efficacy and Safety. Cancers 2021, 13, 5936. https://doi.org/10.3390/cancers13235936
Delage JA, Gnesin S, Prior JO, Barbet J, Le Saëc P, Marionneau-Lambot S, Gouard S, Chérel M, Bourgeois M, Schaefer N, et al. Copper-64-Labeled 1C1m-Fc, a New Tool for TEM-1 PET Imaging and Prediction of Lutetium-177-Labeled 1C1m-Fc Therapy Efficacy and Safety. Cancers. 2021; 13(23):5936. https://doi.org/10.3390/cancers13235936
Chicago/Turabian StyleDelage, Judith Anna, Silvano Gnesin, John O. Prior, Jacques Barbet, Patricia Le Saëc, Séverine Marionneau-Lambot, Sébastien Gouard, Michel Chérel, Mickael Bourgeois, Niklaus Schaefer, and et al. 2021. "Copper-64-Labeled 1C1m-Fc, a New Tool for TEM-1 PET Imaging and Prediction of Lutetium-177-Labeled 1C1m-Fc Therapy Efficacy and Safety" Cancers 13, no. 23: 5936. https://doi.org/10.3390/cancers13235936








