Comparison of Clinical Outcomes of Radical Prostatectomy versus IMRT with Long-Term Hormone Therapy for Relatively Young Patients with High- to Very High-Risk Localized Prostate Cancer
Abstract
:Simple Summary
Abstract
1. Introduction
2. Patients and Methods
2.1. Database
2.2. The Cohort
2.2.1. Inclusion Criteria
- (1)
- Tumor staging from cT1 to T3a, pretreatment PSA levels from 0 to more than 20 ng/mL, or grade group from 1 to 5 were defined as NCCN HR/VHR-LPC.
- (2)
- A newly diagnosed NCCN HR/VHR-LPC who received RP or IMRT.
- (3)
- No other cancer, clinical lymph node metastasis, or distant metastasis were named as LPC.
- (4)
- Removal of the entire prostate gland, seminal vesicles, and the surrounding lymph nodes was defined as standard surgical procedures of RP [24].
- (5)
- Standard IMRT was defined that pelvic lymph nodes receiving prophylactic doses of 45 Gy in 1.8 Gy per fraction, the seminal vesicles having 54 Gy, and the prostate receiving boost radiation dose to 72–81 Gy.
2.2.2. Exclusion Criteria
- (1)
- (2)
- IMRT without long-term (<1.5 years) ADT.
- (3)
- Patients with PC who did not receive standard RP or doses of IMRT after LPC diagnosis.
2.3. Covariates
2.4. Endpoints
2.5. Propensity Score Matching
2.6. Statistics
3. Results
3.1. Study Cohort after Propensity Scores Matching
3.2. Clinical Outcomes between the Two Therapeutic Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chang, S.C.; Chen, C.I.; Huang, C.C. Oncologic Outcomes of Radical Prostatectomy and High-Dose Intensity-Modulated Radiotherapy with Androgen-Deprivation Therapy for Relatively Young Patients with Unfavorable Intermediate-Risk Prostate Adenocarcinoma. Cancers 2021, 13, 1517. [Google Scholar] [CrossRef]
- Health Promotion Administration, Ministry of Health and Welfare. Taiwan Cancer Registry Annual Report; Ministry of Health and Welfare: Taipei City, Taiwan, 2018. [Google Scholar]
- Gomella, L.G.; Johannes, J.; Trabulsi, E.J. Current prostate cancer treatments: Effect on quality of life. Urology 2009, 73, S28–S35. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S.; ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part I: Risk Stratification, Shared Decision Making, and Care Options. J. Urol. 2018, 199, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Mottet, N.; van den Bergh, R.C.N.; Briers, E.; Van den Broeck, T.; Cumberbatch, M.G.; De Santis, M.; Fanti, S.; Fossati, N.; Gandaglia, G.; Gillessen, S.; et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- The National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Available online: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (accessed on 17 February 2021).
- Narang, A.K.; Gergis, C.; Robertson, S.P.; He, P.; Ram, A.N.; McNutt, T.R.; Griffith, E.; DeWeese, T.A.; Honig, S.; Singh, H.; et al. Very High-Risk Localized Prostate Cancer: Outcomes Following Definitive Radiation. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, G.; Warde, P.; Pickles, T.; Crook, J.; Brundage, M.; Souhami, L.; Lukka, H.; Genitourinary Radiation Oncologists of Canada. Pre-treatment risk stratification of prostate cancer patients: A critical review. Can. Urol. Assoc. J. 2012, 6, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agrawal, V.; Ma, X.; Hu, J.C.; Barbieri, C.E.; Nagar, H. Trends in Diagnosis and Disparities in Initial Management of High-Risk Prostate Cancer in the US. JAMA Netw. Open 2020, 3, e2014674. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.; Cole, A.P.; Krimphove, M.J.; Nabi, J.; Marchese, M.; Lipsitz, S.R.; Noldus, J.; Choueiri, T.K.; Kibel, A.S.; Trinh, Q.D. Comparative Effectiveness of Radical Prostatectomy Versus External Beam Radiation Therapy Plus Brachytherapy in Patients with High-risk Localized Prostate Cancer. Eur. Urol. 2019, 75, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Muscatelli, S.; Naslund, M.; Badiyan, S.N.; Kaiser, A.; Siddiqui, M.M. Evaluation of Cancer Specific Mortality with Surgery versus Radiation as Primary Therapy for Localized High Grade Prostate Cancer in Men Younger Than 60 Years. J. Urol. 2019, 201, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Boorjian, S.A.; Karnes, R.J.; Viterbo, R.; Rangel, L.J.; Bergstralh, E.J.; Horwitz, E.M.; Blute, M.L.; Buyyounouski, M.K. Long-term survival after radical prostatectomy versus external-beam radiotherapy for patients with high-risk prostate cancer. Cancer 2011, 117, 2883–2891. [Google Scholar] [CrossRef]
- Moris, L.; Cumberbatch, M.G.; Van den Broeck, T.; Gandaglia, G.; Fossati, N.; Kelly, B.; Pal, R.; Briers, E.; Cornford, P.; De Santis, M.; et al. Benefits and Risks of Primary Treatments for High-risk Localized and Locally Advanced Prostate Cancer: An International Multidisciplinary Systematic Review. Eur. Urol. 2020, 77, 614–627. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, X.; Gao, X.; Cui, M.; Xie, M.; Ma, M.; Qin, S.; Li, X.; Qi, X.; Bai, Y.; Wang, D. Survival outcomes of radical prostatectomy and external beam radiotherapy in clinically localized high-risk prostate cancer: A population-based, propensity score matched study. Cancer Manag. Res. 2018, 10, 1061–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiang, C.J.; You, S.L.; Chen, C.J.; Yang, Y.W.; Lo, W.C.; Lai, M.S. Quality assessment and improvement of nationwide cancer registration system in Taiwan: A review. Jpn. J. Clin. Oncol. 2015, 45, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.P.; Tsai, S.P.; Chung, W.S. A 10-year experience with universal health insurance in Taiwan: Measuring changes in health and health disparity. Ann. Intern. Med. 2008, 148, 258–267. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.C.; Chen, H.M.; Wu, S.Y. There Are No Differences in Positive Surgical Margin Rates or Biochemical Failure-Free Survival among Patients Receiving Open, Laparoscopic, or Robotic Radical Prostatectomy: A Nationwide Cohort Study from the National Cancer Database. Cancers 2020, 13, 106. [Google Scholar] [CrossRef]
- Chang, S.C.; Hsu, C.H.; Lin, Y.C.; Wu, S.Y. Effects of 1-Year Hospital Volume on Surgical Margin and Biochemical-Failure-Free Survival in Patients Undergoing Robotic versus Nonrobotic Radical Prostatectomy: A Nationwide Cohort Study from the National Taiwan Cancer Database. Cancers 2021, 13, 488. [Google Scholar] [CrossRef]
- Wu, S.Y.; Chang, S.C.; Chen, C.I.; Huang, C.C. Latest Comprehensive Medical Resource Consumption in Robot-Assisted versus Laparoscopic and Traditional Open Radical Prostatectomy: A Nationwide Population-Based Cohort Study. Cancers 2021, 13, 1564. [Google Scholar] [CrossRef]
- Wu, S.Y.; Fang, S.C.; Shih, H.J.; Wen, Y.C.; Shao, Y.J. Mortality associated with statins in men with advanced prostate cancer treated with androgen deprivation therapy. Eur. J. Cancer 2019, 112, 109–117. [Google Scholar] [CrossRef]
- Lepor, H. A review of surgical techniques for radical prostatectomy. Rev. Urol. 2005, 7 (Suppl. S2), S11–S17. [Google Scholar]
- Kupelian, P.A.; Elshaikh, M.; Reddy, C.A.; Zippe, C.; Klein, E.A. Comparison of the efficacy of local therapies for localized prostate cancer in the prostate-specific antigen era: A large single-institution experience with radical prostatectomy and external-beam radiotherapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2002, 20, 3376–3385. [Google Scholar] [CrossRef]
- Kupelian, P.A.; Potters, L.; Khuntia, D.; Ciezki, J.P.; Reddy, C.A.; Reuther, A.M.; Carlson, T.P.; Klein, E.A. Radical prostatectomy, external beam radiotherapy or < =72 Gy, external beam radiotherapy > or =72 Gy, permanent seed implantation, or combined seeds/external beam radiotherapy for stage T1-T2 prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 25–33. [Google Scholar] [CrossRef]
- Kupelian, P.; Kuban, D.; Thames, H.; Levy, L.; Horwitz, E.; Martinez, A.; Michalski, J.; Pisansky, T.; Sandler, H.; Shipley, W.; et al. Improved biochemical relapse-free survival with increased external radiation doses in patients with localized prostate cancer: The combined experience of nine institutions in patients treated in 1994 and 1995. Int. J. Radiat. Oncol. Biol. Phys. 2005, 61, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Cookson, M.S.; Aus, G.; Burnett, A.L.; Canby-Hagino, E.D.; D’Amico, A.V.; Dmochowski, R.R.; Eton, D.T.; Forman, J.D.; Goldenberg, S.L.; Hernandez, J.; et al. Variation in the definition of biochemical recurrence in patients treated for localized prostate cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel report and recommendations for a standard in the reporting of surgical outcomes. J. Urol. 2007, 177, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Roach, M., 3rd; Hanks, G.; Thames, H., Jr.; Schellhammer, P.; Shipley, W.U.; Sokol, G.H.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Charlson, M.; Szatrowski, T.P.; Peterson, J.; Gold, J. Validation of a combined comorbidity index. J. Clin. Epidemiol. 1994, 47, 1245–1251. [Google Scholar] [CrossRef]
- Chen, J.H.; Yen, Y.C.; Yang, H.C.; Liu, S.H.; Yuan, S.P.; Wu, L.L.; Lee, F.P.; Lin, K.C.; Lai, M.T.; Wu, C.C.; et al. Curative-Intent Aggressive Treatment Improves Survival in Elderly Patients With Locally Advanced Head and Neck Squamous Cell Carcinoma and High Comorbidity Index. Medicine 2016, 95, e3268. [Google Scholar] [CrossRef]
- Rassen, J.A.; Shelat, A.A.; Myers, J.; Glynn, R.J.; Rothman, K.J.; Schneeweiss, S. One-to-many propensity score matching in cohort studies. Pharm. Drug Saf. 2012, 21 (Suppl. S2), 69–80. [Google Scholar] [CrossRef]
- Austin, P.C. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 2011, 10, 150–161. [Google Scholar] [CrossRef] [Green Version]
- Austin, P.C. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat. Med. 2014, 33, 1242–1258. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.C.; Liu, H.E.; Kao, Y.W.; Qin, L.; Lin, K.C.; Fang, C.Y.; Tsai, L.L.; Shia, B.C.; Wu, S.Y. Definitive radiotherapy or surgery for early oral squamous cell carcinoma in old and very old patients: A propensity-score-matched, nationwide, population-based cohort study. Radiother. Oncol. 2020, 151, 214–221. [Google Scholar] [CrossRef]
- Simmons, M.N.; Stephenson, A.J.; Klein, E.A. Natural history of biochemical recurrence after radical prostatectomy: Risk assessment for secondary therapy. Eur. Urol. 2007, 51, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Ames, S.C.; Tan, W.W.; Ames, G.E.; Stone, R.L.; Rizzo, T.D., Jr.; Heckman, M.G.; Crook, J.E.; Clark, M.M.; Rummans, T.A.; Werch, C.E. Quality of life of men with biochemical recurrence of prostate cancer. J. Psychosoc. Oncol. 2008, 26, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Berge, V.; Baco, E.; Dahl, A.A.; Karlsen, S.J. Health-related quality of life after salvage high-intensity focused ultrasound (HIFU) treatment for locally radiorecurrent prostate cancer. Int. J. Urol. 2011, 18, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Uchio, E.M.; Aslan, M.; Wells, C.K.; Calderone, J.; Concato, J. Impact of biochemical recurrence in prostate cancer among US veterans. Arch. Intern. Med. 2010, 170, 1390–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trock, B.J.; Han, M.; Freedland, S.J.; Humphreys, E.B.; DeWeese, T.L.; Partin, A.W.; Walsh, P.C. Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008, 299, 2760–2769. [Google Scholar] [CrossRef] [Green Version]
- Roberts, W.B.; Han, M. Clinical significance and treatment of biochemical recurrence after definitive therapy for localized prostate cancer. Surg. Oncol. 2009, 18, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Artibani, W.; Porcaro, A.B.; De Marco, V.; Cerruto, M.A.; Siracusano, S. Management of Biochemical Recurrence after Primary Curative Treatment for Prostate Cancer: A Review. Urol. Int. 2018, 100, 251–262. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Cathcart, P.; McCartan, N.; Kirkham, A.; Allen, C.; Freeman, A.; Emberton, M. Focal salvage therapy for localized prostate cancer recurrence after external beam radiotherapy: A pilot study. Cancer 2012, 118, 4148–4155. [Google Scholar] [CrossRef] [PubMed]
- Zaorsky, N.G.; Calais, J.; Fanti, S.; Tilki, D.; Dorff, T.; Spratt, D.E.; Kishan, A.U. Salvage therapy for prostate cancer after radical prostatectomy. Nat. Rev. Urol. 2021, 18, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Westover, K.; Chen, M.H.; Moul, J.; Robertson, C.; Polascik, T.; Dosoretz, D.; Katin, M.; Salenius, S.; D’Amico, A.V. Radical prostatectomy vs radiation therapy and androgen-suppression therapy in high-risk prostate cancer. BJU Int. 2012, 110, 1116–1121. [Google Scholar] [CrossRef]
- Greenberger, B.A.; Zaorsky, N.G.; Den, R.B. Comparison of Radical Prostatectomy Versus Radiation and Androgen Deprivation Therapy Strategies as Primary Treatment for High-risk Localized Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. Focus 2020, 6, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Bekelman, J.E.; Rumble, R.B.; Chen, R.C.; Pisansky, T.M.; Finelli, A.; Feifer, A.; Nguyen, P.L.; Loblaw, D.A.; Tagawa, S.T.; Gillessen, S.; et al. Clinically Localized Prostate Cancer: ASCO Clinical Practice Guideline Endorsement of an American Urological Association/American Society for Radiation Oncology/Society of Urologic Oncology Guideline. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2018, 36, 3251–3258. [Google Scholar] [CrossRef] [PubMed]
- Sanda, M.G.; Cadeddu, J.A.; Kirkby, E.; Chen, R.C.; Crispino, T.; Fontanarosa, J.; Freedland, S.J.; Greene, K.; Klotz, L.H.; Makarov, D.V.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO/SUO Guideline. Part II: Recommended Approaches and Details of Specific Care Options. J. Urol. 2018, 199, 990–997. [Google Scholar] [CrossRef]
- Epstein, J.I.; Zelefsky, M.J.; Sjoberg, D.D.; Nelson, J.B.; Egevad, L.; Magi-Galluzzi, C.; Vickers, A.J.; Parwani, A.V.; Reuter, V.E.; Fine, S.W.; et al. A Contemporary Prostate Cancer Grading System: A Validated Alternative to the Gleason Score. Eur. Urol. 2016, 69, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Xie, M.; Gao, X.S.; Ma, M.W.; Gu, X.B.; Li, H.Z.; Lyu, F.; Bai, Y.; Chen, J.Y.; Ren, X.Y.; Liu, M.Z. Population-Based Comparison of Different Risk Stratification Systems Among Prostate Cancer Patients. Front. Oncol. 2021, 11, 646073. [Google Scholar] [CrossRef]
- Barzi, A.; Klein, E.A.; Dorff, T.B.; Quinn, D.I.; Sadeghi, S. Prostatectomy at high-volume centers improves outcomes and lowers the costs of care for prostate cancer. Prostate Cancer Prostatic Dis. 2016, 19, 84–91. [Google Scholar] [CrossRef]
- Patel, S.A.; Goyal, S.; Liu, Y.; Moghanaki, D.; Patel, P.R.; Hanasoge, S.; Dhere, V.R.; Shelton, J.W.; Godette, K.D.; Jani, A.B.; et al. Analysis of Radiation Facility Volume and Survival in Men With Lymph Node-Positive Prostate Cancer Treated With Radiation and Androgen Deprivation Therapy. JAMA Netw. Open 2020, 3, e2025143. [Google Scholar] [CrossRef]
- Ermann, D.A.; Vardell, V.A.; Kallam, A.; Silberstein, P.T.; Armitage, J.O. Academic Centers Compared With Nonacademic Centers for Patients With International Prognostic Index Risk-stratified Diffuse Large B-cell Lymphoma: A Survival Outcomes Analysis. Clin. Lymphoma Myeloma Leuk. 2020, 20, e174–e183. [Google Scholar] [CrossRef]
- Stolzenbach, L.F.; Deuker, M.; Colla-Ruvolo, C.; Nocera, L.; Tian, Z.; Maurer, T.; Tilki, D.; Briganti, A.; Saad, F.; Mirone, V.; et al. Differences between rural and urban prostate cancer patients. World J. Urol. 2021, 39, 2507–2514. [Google Scholar] [CrossRef]
- Walsh, P.C. Geographic patterns of prostate cancer mortality and variations in access to medical care in the United States. J. Urol. 2005, 174, 1294–1295. [Google Scholar] [CrossRef]
- Buwenge, M.; Scirocco, E.; Deodato, F.; Macchia, G.; Ntreta, M.; Bisello, S.; Siepe, G.; Cilla, S.; Alitto, A.R.; Valentini, V.; et al. Radiotherapy of prostate cancer: Impact of treatment characteristics on the incidence of second tumors. BMC Cancer 2020, 20, 90. [Google Scholar] [CrossRef] [PubMed]
- Bezak, E.; Takam, R.; Yeoh, E.; Marcu, L.G. The risk of second primary cancers due to peripheral photon and neutron doses received during prostate cancer external beam radiation therapy. Phys. Med. 2017, 42, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Hegemann, N.S.; Schlesinger-Raab, A.; Ganswindt, U.; Horl, C.; Combs, S.E.; Holzel, D.; Gschwend, J.E.; Stief, C.; Belka, C.; Engel, J. Risk of second cancer following radiotherapy for prostate cancer: A population-based analysis. Radiat Oncol. 2017, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Hall, E.J.; Wuu, C.S. Radiation-induced second cancers: The impact of 3D-CRT and IMRT. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 83–88. [Google Scholar] [CrossRef]
- Marcu, L.G. Photons—Radiobiological issues related to the risk of second malignancies. Phys. Med. 2017, 42, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Uemura, H.; Ye, D.; Kanesvaran, R.; Chiong, E.; Lojanapiwat, B.; Pu, Y.S.; Rawal, S.K.; Abdul Razack, A.H.; Zeng, H.; Chung, B.H.; et al. United in Fight against prOstate cancer (UFO) registry: First results from a large, multi-centre, prospective, longitudinal cohort study of advanced prostate cancer in Asia. BJU Int. 2020, 125, 541–552. [Google Scholar] [CrossRef]
- Nguyen-Nielsen, M.; Moller, H.; Tjonneland, A.; Borre, M. Patient-reported outcome measures after treatment for prostate cancer: Results from the Danish Prostate Cancer Registry (DAPROCAdata). Cancer Epidemiol. 2020, 64, 101623. [Google Scholar] [CrossRef]
- Aas, K.; Berge, V.; Myklebust, T.A.; Fossa, S.D. Comparative Survival Outcomes of High-risk Prostate Cancer Treated with Radical Prostatectomy or Definitive Radiotherapy Regimens. Eur. Urol. Open Sci. 2021, 26, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Berry, S.D.; Ngo, L.; Samelson, E.J.; Kiel, D.P. Competing risk of death: An important consideration in studies of older adults. J. Am. Geriatr Soc. 2010, 58, 783–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lau, B.; Cole, S.R.; Gange, S.J. Competing risk regression models for epidemiologic data. Am. J. Epidemiol. 2009, 170, 244–256. [Google Scholar] [CrossRef] [PubMed]
| Prostatectomy N = 481 | High-Dose IMRT + Long-Term ADT N = 215 | |||||
|---|---|---|---|---|---|---|
| n | (%) | N | (%) | p-Value | ||
| Age | Mean (SD) | 62.6 | (2.3) | 62.3 | (2.3) | 0.3378 |
| Median (IQR, Q1–Q3) | 63 | (58–65) | 63 | (59–65) | ||
| 20–59 | 73 | (15.2) | 29 | (13.5) | 0.9833 | |
| 60–65 | 408 | (84.8) | 186 | (86.5) | ||
| Years of diagnosis | 2011–2012 | 67 | (13.9) | 30 | (14.0) | 0.9953 |
| 2013 | 79 | (16.4) | 32 | (14.9) | ||
| 2014 | 96 | (20.0) | 42 | (19.5) | ||
| 2015 | 120 | (24.9) | 54 | (25.1) | ||
| 2016 | 119 | (24.7) | 57 | (26.5) | ||
| CCI scores | 0 | 220 | (45.7) | 99 | (46.0) | 0.9279 |
| 1 | 124 | (25.8) | 53 | (24.7) | ||
| 2+ | 137 | (28.5) | 63 | (29.3) | ||
| Myocardial infarction | 5 | (1.0) | 4 | (1.9) | 0.7702 | |
| Congestive heart failure | 20 | (4.2) | 8 | (3.7) | 0.4891 | |
| Peripheral vascular disease | 10 | (2.1) | 5 | (2.3) | 0.8838 | |
| Cerebrovascular disease | 26 | (5.4) | 18 | (8.4) | 0.3550 | |
| Chronic pulmonary disease | 60 | (12.5) | 24 | (11.2) | 0.5333 | |
| Diabetes | 132 | (27.4) | 56 | (26.0) | 0.5507 | |
| Hypertension | 244 | (50.7) | 115 | (53.5) | 0.7777 | |
| Income | Very low | 155 | (32.2) | 68 | (31.6) | 0.6764 |
| Low | 175 | (36.4) | 81 | (37.7) | ||
| Middle | 98 | (20.4) | 38 | (17.7) | ||
| High | 53 | (11.0) | 28 | (13.0) | ||
| Hospital area | North | 259 | (53.8) | 109 | (50.7) | 0.9569 |
| Central | 103 | (21.4) | 45 | (20.9) | ||
| South | 110 | (22.9) | 54 | (25.1) | ||
| East | 9 | (1.9) | 7 | (3.3) | ||
| Hospital level | Medical center | 289 | (60.1) | 113 | (52.6) | 0.5018 |
| Others | 192 | (39.9) | 102 | (47.4) | ||
| cT-stage | cT1 | 176 | (36.6) | 75 | (34.9) | 0.9798 |
| cT2a | 115 | (23.9) | 52 | (24.2) | ||
| cT2b | 24 | (5.0) | 10 | (4.7) | ||
| cT2c | 139 | (28.9) | 57 | (26.5) | ||
| cT3a | 27 | (5.6) | 21 | (9.8) | ||
| Gleason score | ≤5 | 5 | (1.0) | 6 | (2.8) | 0.6573 |
| 6 | 58 | (12.1) | 54 | (25.1) | ||
| 7 | 289 | (60.1) | 106 | (49.3) | ||
| 8 | 86 | (17.9) | 31 | (14.4) | ||
| 9+ | 33 | (6.9) | 13 | (6.0) | ||
| Missing | 10 | (2.1) | 5 | (2.3) | ||
| Grade group | 1–2 | 12 | (2.5) | 5 | (2.3) | 0.2556 |
| 3 | 60 | (12.5) | 59 | (27.4) | ||
| 4 | 360 | (74.8) | 134 | (62.3) | ||
| 5 | 49 | (10.2) | 17 | (7.9) | ||
| Preoperative PSA (ng/mL) | 0–5 | 81 | (16.8) | 31 | (14.4) | 0.6906 |
| 5–10 | 148 | (30.8) | 56 | (26.0) | ||
| 10–20 | 160 | (33.3) | 65 | (30.2) | ||
| 20+ | 45 | (9.4) | 43 | (20.0) | ||
| Missing | 47 | (9.8) | 20 | (9.3) | ||
| EAU risk group | Localized intermediate | 203 | (42.2) | 77 | (35.8) | 0.8815 |
| Localized high | 242 | (50.3) | 111 | (51.6) | ||
| Localized advanced | 36 | (7.5) | 27 | (12.6) | ||
| Follow-up time, months | Mean (SD) | 60.2 | (17.6) | 59.9 | (17.3) | |
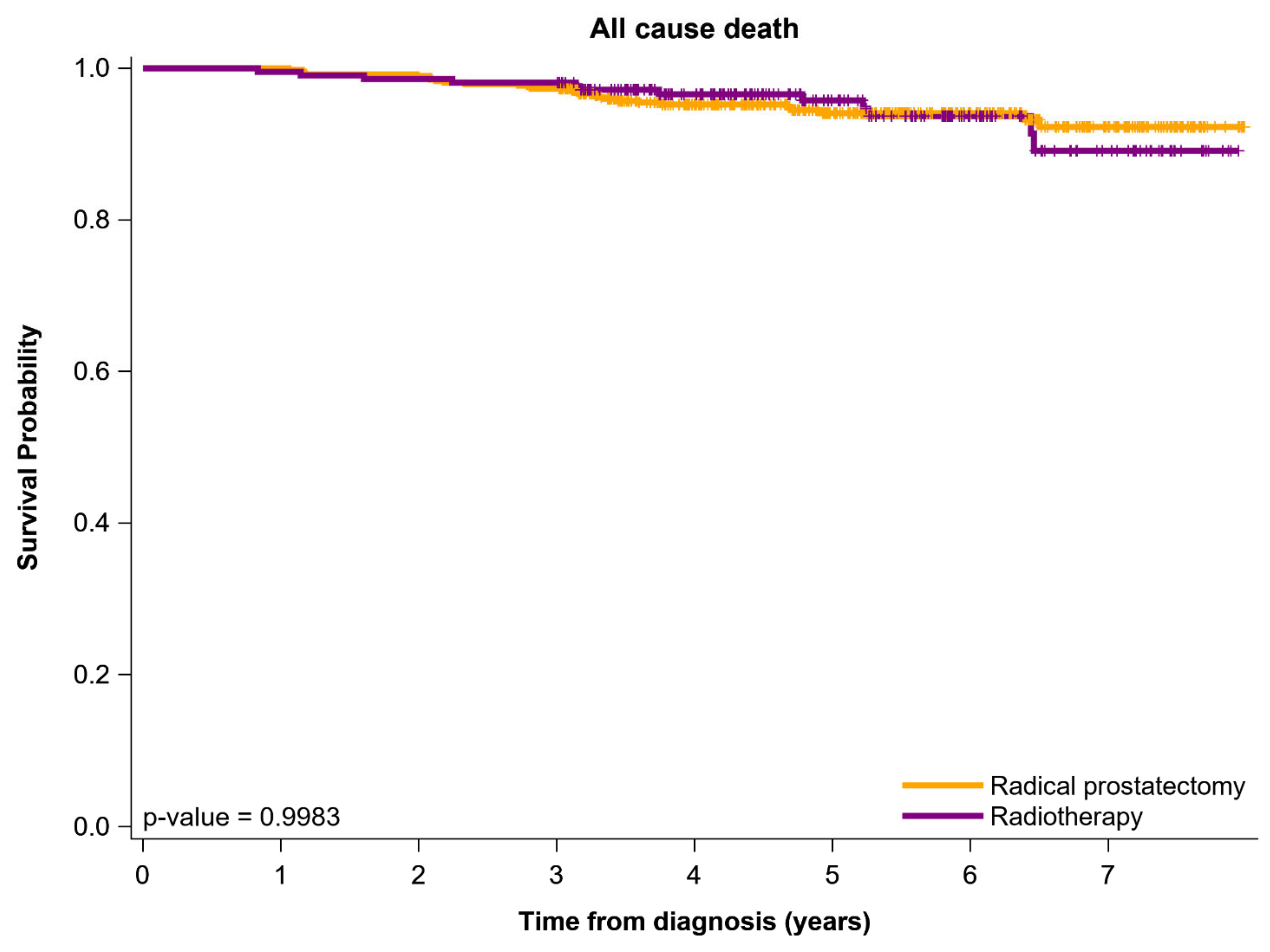
| All-cause death | 27 | (5.6) | 12 | (5.6) | 0.5704 | |
| Biochemical recurrence | 102 | (21.2) | 84 | (39.1) | <0.0001 | |
| Locoregional recurrence | 27 | (5.6) | 14 | (6.5) | 0.9982 | |
| Distant metastasis | 31 | (6.4) | 16 | (7.4) | 1.0000 | |
| Covariates | Adjusted HR * | (95% CI) | p-Value | |
|---|---|---|---|---|
| Curative treatment | Radical prostatectomy | ref | 0.5640 | |
| High-dose IMRT + long-term ADT | 1.20 | (0.65–2.24) | ||
| Age | 20–59 | ref | 0.6834 | |
| 60–65 | 1.18 | (0.54–2.58) | ||
| Years of diagnosis | 2011–2012 | ref | 0.4500 | |
| 2013 | 1.30 | (0.59–2.87) | ||
| 2014 | 0.55 | (0.21–1.45) | ||
| 2015 | 0.92 | (0.37–2.26) | ||
| 2016 | 0.82 | (0.31–2.18) | ||
| CCI scores | 0 | ref | 0.1043 | |
| 1 | 1.10 | (0.51–2.38) | ||
| 2+ | 2.30 | (0.96–5.54) | ||
| Congestive heart failure | 1.37 | (0.44–4.32) | 0.5865 | |
| Peripheral vascular disease | 0.00 | - | 0.9797 | |
| Cerebrovascular disease | 1.10 | (0.48–2.53) | 0.8199 | |
| Chronic pulmonary disease | 0.60 | (0.22–1.69) | 0.3353 | |
| Diabetes | 1.20 | (0.59–2.45) | 0.6142 | |
| Hypertension | 1.37 | (0.78–2.40) | 0.2762 | |
| Income | Very low | ref | 0.3395 | |
| Low | 1.41 | (0.74–2.68) | ||
| Middle | 0.94 | (0.43–2.08) | ||
| High | 0.64 | (0.24–1.69) | ||
| Hospital level | Academic centers | ref | 0.0129 | |
| Nonacademic centers | 2.01 | (1.16–3.50) | ||
| Hospital area | North | ref | 0.0026 | |
| Central | 1.64 | (0.82–3.28) | ||
| South | 2.20 | (1.13–4.31) | ||
| East | 7.68 | (2.51–23.55) | ||
| cT-stage | cT1 | ref | 0.2690 | |
| cT2a | 1.02 | (0.51–2.02) | ||
| cT2b | 0.67 | (0.22–2.02) | ||
| cT2c | 0.47 | (0.21–1.07) | ||
| cT3a | 0.38 | (0.08–1.74) | ||
| EAU risk group | Localized intermediate | ref | 0.0454 | |
| Localized high | 1.57 | (0.81–3.06) | ||
| Localized advanced | 2.55 | (1.36–5.18) |
| Covariates | Adjusted HR * | (95% CI) | p-Value | |
|---|---|---|---|---|
| Curative treatment | Radical prostatectomy | ref | <0.0001 | |
| IMRT + long-term ADT | 2.03 | (1.56–2.65) | ||
| Age | 20–59 | ref | 0.6054 | |
| 60–69 | 1.08 | (0.82–1.42) | ||
| 70–80 | ||||
| 80+ | ||||
| Years of diagnosis | 2011–2012 | ref | 0.3193 | |
| 2013 | 0.81 | (0.56–1.16) | ||
| 2014 | 0.89 | (0.62–1.27) | ||
| 2015 | 0.85 | (0.59–1.22) | ||
| 2016 | 0.67 | (0.45–0.98) | ||
| CCI scores | 0 | ref | 0.6576 | |
| 1 | 1.12 | (0.83–1.52) | ||
| 2+ | 1.20 | (0.79–1.83) | ||
| Congestive heart failure | 0.74 | (0.38–1.44) | 0.3760 | |
| Peripheral vascular disease | 0.51 | (0.16–1.64) | 0.2569 | |
| Cerebrovascular disease | 1.07 | (0.67–1.70) | 0.7711 | |
| Chronic pulmonary disease | 0.92 | (0.58–1.45) | 0.7106 | |
| Diabetes | 0.97 | (0.68–1.37) | 0.8568 | |
| Hypertension | 0.93 | (0.73–1.17) | 0.5365 | |
| Income | Low | ref | 0.6583 | |
| Very Low | 1.06 | (0.78–1.43) | ||
| Middle | 1.13 | (0.82–1.55) | ||
| High | 0.91 | (0.66–1.26) | ||
| Hospital level | Academic centers | ref | 0.0073 | |
| Nonacademic centers | 1.37 | (1.09–1.73) | ||
| Hospital area | North | ref | 0.1560 | |
| Central | 1.05 | (0.93–1.79) | ||
| South | 1.11 | (0.83–1.49) | ||
| East | 1.50 | (0.70–3.18) | ||
| cT-stage | cT1 | ref | 0.4036 | |
| cT2a | 1.04 | (0.78–1.39) | ||
| cT2b | 1.08 | (0.71–1.64) | ||
| cT2c | 1.18 | (0.41–1.81) | ||
| cT3a | 1.26 | (0.24–1.99) | ||
| EAU risk group | Localized intermediate | ref | <0.0001 | |
| localized-high | 2.18 | (1.63–2.91) | ||
| Localized advanced | 3.41 | (1.59–7.32) |
| Covariates | Adjusted HR * | (95% CI) | p-Value | |
|---|---|---|---|---|
| Curative treatment | Radical prostatectomy | ref | 0.3524 | |
| IMRT + long-term ADT | 0.88 | (0.67–1.06) | ||
| Age | 20–59 | ref | 0.5068 | |
| 60–65 | 0.87 | (0.57–1.32) | ||
| Years of diagnosis | 2011–2012 | ref | 0.6379 | |
| 2013 | 1.57 | (0.81–3.03) | ||
| 2014 | 1.26 | (0.66–2.41) | ||
| 2015 | 1.38 | (0.72–2.63) | ||
| 2016 | 1.08 | (0.53–2.20) | ||
| CCI scores | 0 | ref | 0.1806 | |
| 1 | 0.58 | (0.33–1.04) | ||
| 2+ | 0.66 | (0.32–1.36) | ||
| Congestive heart failure | 2.10 | (0.73–6.01) | 0.1665 | |
| Peripheral vascular disease | 0.90 | (0.67–1.31) | 0.4021 | |
| Cerebrovascular disease | 1.61 | (0.70–3.71) | 0.2584 | |
| Chronic pulmonary disease | 0.90 | (0.37–2.20) | 0.8124 | |
| Diabetes | 1.32 | (0.75–2.33) | 0.3324 | |
| Hypertension | 0.80 | (0.54–1.20) | 0.2893 | |
| Income | Very Low | ref | 0.1690 | |
| Low | 0.74 | (0.44–1.27) | ||
| Middle | 1.05 | (0.63–1.72) | ||
| High | 0.61 | (0.36–1.04) | ||
| Hospital level | Academic centers | ref | 0.0456 | |
| Nonacademic centers | 1.05 | (1.00–1.42) | ||
| Hospital area | North | ref | 0.9213 | |
| Central | 0.90 | (0.58–1.40) | ||
| South | 1.04 | (0.62–1.76) | ||
| East | 0.62 | (0.08–4.54) | ||
| cT-stage | cT1 | ref | 0.2812 | |
| cT2a | 1.00 | (0.63–1.60) | ||
| cT2b | 1.03 | (0.43–1.60) | ||
| cT2c | 1.06 | (0.40–1.63) | ||
| cT3a | 1.11 | (0.51–1.78) | ||
| EAU risk group | Localized intermediate | ref | 0.0077 | |
| Localized high | 1.68 | (1.08–2.62) | ||
| Localized advanced | 5.32 | (1.44–19.72) |
| Covariates | Adjusted HR * | (95% CI) | p-Value | |
|---|---|---|---|---|
| Curative treatment | Radical prostatectomy | ref | 0.9176 | |
| IMRT + long-term ADT | 1.03 | (0.56–1.90) | ||
| Age | 20–59 | ref | 0.7536 | |
| 60–69 | 1.10 | (0.62–1.95) | ||
| 70–80 | ||||
| 80+ | ||||
| Years of diagnosis | 2011–2012 | ref | 0.2664 | |
| 2013 | 1.66 | (0.83–3.31) | ||
| 2014 | 0.91 | (0.42–1.95) | ||
| 2015 | 0.76 | (0.35–1.67) | ||
| 2016 | 0.97 | (0.44–2.16) | ||
| CCI scores | 0 | ref | 0.4698 | |
| 1 | 1.22 | (0.63–2.36) | ||
| 2+ | 1.70 | (0.72–4.02) | ||
| Congestive heart failure | 0.57 | (0.14–2.40) | 0.4450 | |
| Peripheral vascular disease | 1.45 | (0.39–5.42) | 0.5775 | |
| Cerebrovascular disease | 1.21 | (0.56–2.60) | 0.6237 | |
| Chronic pulmonary disease | 0.98 | (0.43–2.25) | 0.9626 | |
| Diabetes | 1.64 | (0.85–3.19) | 0.1424 | |
| Hypertension | 1.16 | (0.73–1.84) | 0.5247 | |
| Income | Very Low | ref | 0.8722 | |
| Low | 1.29 | (0.69–2.41) | ||
| Middle | 1.23 | (0.63–2.40) | ||
| High | 1.20 | (0.60–2.41) | ||
| Hospital level | Medical center | ref | 0.1107 | |
| Others | 1.49 | (0.91–2.43) | ||
| Hospital area | North | ref | 0.2710 | |
| Central | 1.59 | (0.92–2.75) | ||
| South | 2.07 | (0.88–3.62) | ||
| East | 3.85 | (0.81–4.71) | ||
| cT-stage | cT1 | ref | 0.4248 | |
| cT2a | 1.00 | (0.55–1.67) | ||
| cT2b | 1.03 | (0.51–1.12) | ||
| cT2c | 1.08 | (0.69–1.61) | ||
| cT3a | 1.09 | (0.67–2.82) | ||
| EAU risk group | Localized intermediate | ref | 0.0114 | |
| localized high | 1.26 | (1.08–3.17) | ||
| Localized advanced | 3.43 | (1.58–4.43) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, H.-J.; Chang, S.-C.; Hsu, C.-H.; Lin, Y.-C.; Hung, C.-H.; Wu, S.-Y. Comparison of Clinical Outcomes of Radical Prostatectomy versus IMRT with Long-Term Hormone Therapy for Relatively Young Patients with High- to Very High-Risk Localized Prostate Cancer. Cancers 2021, 13, 5986. https://doi.org/10.3390/cancers13235986
Shih H-J, Chang S-C, Hsu C-H, Lin Y-C, Hung C-H, Wu S-Y. Comparison of Clinical Outcomes of Radical Prostatectomy versus IMRT with Long-Term Hormone Therapy for Relatively Young Patients with High- to Very High-Risk Localized Prostate Cancer. Cancers. 2021; 13(23):5986. https://doi.org/10.3390/cancers13235986
Chicago/Turabian StyleShih, Hung-Jen, Shyh-Chyi Chang, Chia-Hao Hsu, Yi-Chu Lin, Chu-Hsuan Hung, and Szu-Yuan Wu. 2021. "Comparison of Clinical Outcomes of Radical Prostatectomy versus IMRT with Long-Term Hormone Therapy for Relatively Young Patients with High- to Very High-Risk Localized Prostate Cancer" Cancers 13, no. 23: 5986. https://doi.org/10.3390/cancers13235986
APA StyleShih, H.-J., Chang, S.-C., Hsu, C.-H., Lin, Y.-C., Hung, C.-H., & Wu, S.-Y. (2021). Comparison of Clinical Outcomes of Radical Prostatectomy versus IMRT with Long-Term Hormone Therapy for Relatively Young Patients with High- to Very High-Risk Localized Prostate Cancer. Cancers, 13(23), 5986. https://doi.org/10.3390/cancers13235986






