Targeted Therapy in HR+ HER2− Metastatic Breast Cancer: Current Clinical Trials and Their Implications for CDK4/6 Inhibitor Therapy and beyond Treatment Options
Abstract
Simple Summary
Abstract
1. Introduction
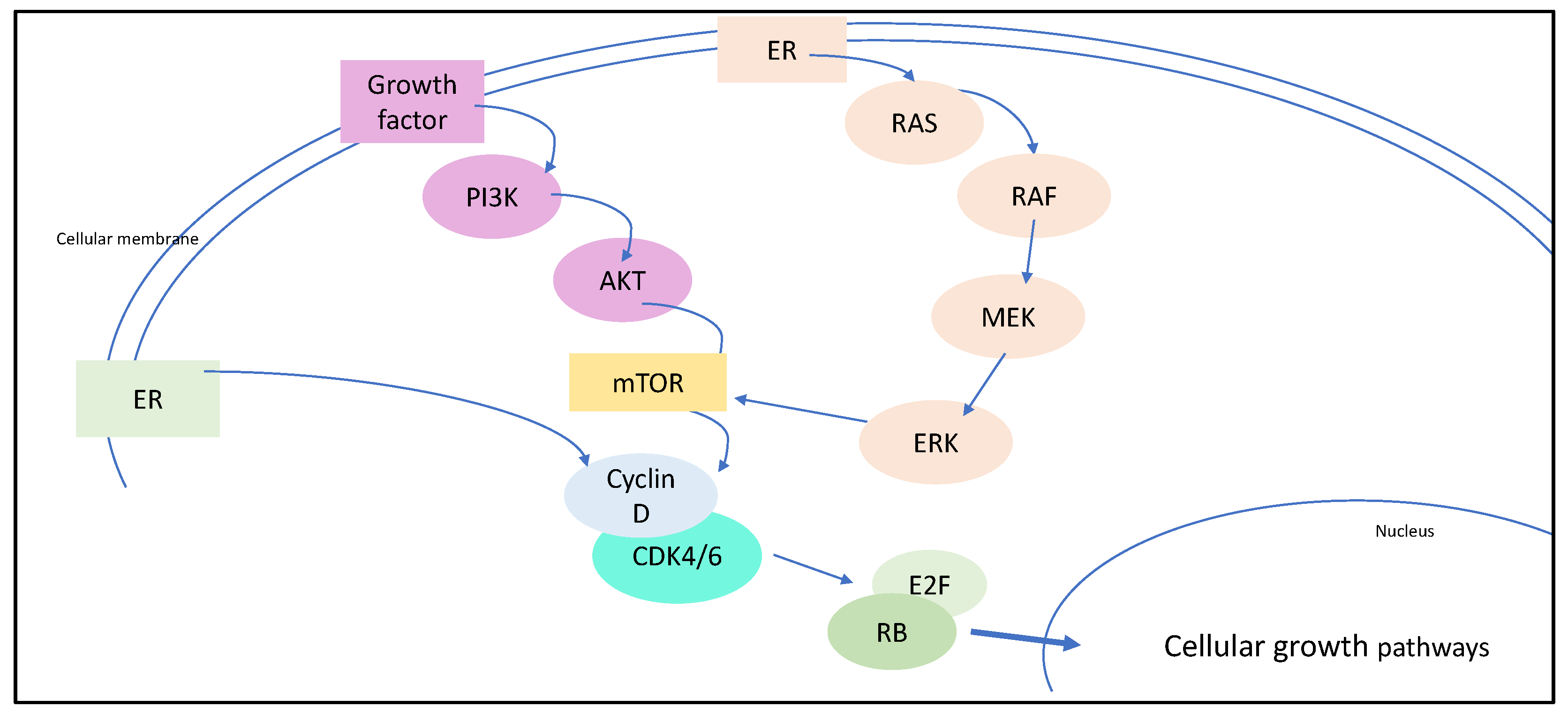
2. The Rationale of Targeting CDK4/6 for Breast Cancer Therapy
3. Clinical Impact and Real-Life Data on CDK4/6 Inhibitors
4. Adverse Events, Quality of Life, and Non-Compliance under CDK4/6 Inhibitors
5. Resistance to CDK4/6 Inhibitors
6. Alternative Therapeutic Approaches for Patients with CDK4/6 Inhibitor Resistance
6.1. PI3K Inhibitors
6.2. Everolimus
6.3. AKT Inhibitors
7. Conclusions and Perspective
8. Strengths and Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet 2005, 365, 1687–1717. [Google Scholar] [CrossRef]
- Anderson, W.F.; Chatterjee, N.; Ershler, W.B.; Brawley, O.W. Estrogen receptor breast cancer phenotypes in the Surveil-lance, Epidemiology, and End Results database. Breast Cancer Res. Treat. 2002, 76, 27–36. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Anderson, B.O.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Blair, S.L.; Burstein, H.J.; Dang, C.; Elias, A.D.; et al. Breast Cancer, Version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2020, 18, 452–478. [Google Scholar] [CrossRef] [PubMed]
- Caswell-Jin, J.; Plevritis, S.K.; Tian, L.; Cadham, C.; Xu, C.; Stout, N.K.; Sledge, G.W.; Mandelblatt, J.S.; Kurian, A.W. Change in Survival in Metastatic Breast Cancer with Treatment Advances: Meta-Analysis and Systematic Review. JNCI Cancer Spectr. 2018, 2, pky062. [Google Scholar] [CrossRef] [PubMed]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498. [Google Scholar] [CrossRef]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Henley, S.A.; Dick, F.A. The retinoblastoma family of proteins and their regulatory functions in the mammalian cell di-vision cycle. Cell Div. 2012, 7, 10. [Google Scholar] [CrossRef]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar] [CrossRef]
- Klein, M.E.; Kovatcheva, M.; Davis, L.E.; Tap, W.D.; Koff, A. CDK4/6 inhibitors: The mechanism of action may not be as simple as once thought. Cancer Cell 2018, 34, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Nunes, M.R.; Stearns, V. CDK4/6 Inhibitors: Game Changers in the Management of Hormone Receptor–Positive Advanced Breast Cancer? Oncol. Williston Park N 2018, 32, 216–222. [Google Scholar]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martin, M.; et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J. Clin. Oncol. 2018, 36, 2465–2472. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.; Cortes, J.; Ozyilkan, O.; Chen, S.-C.; Petrakova, K.; Manikhas, A.; Jerusalem, G.; Hegg, R.; Huober, J.; Chapman, S.C.; et al. nextMONARCH: Abemaciclib Monotherapy or Combined with Tamoxifen for Metastatic Breast Cancer. Clin. Breast Cancer 2020, 21, 181–190.e2. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huo, X.; Zhao, F.; Ren, D.; Ahmad, R.; Youan, X.; Du, F.; Zhao, J. Association of Cyclin-Dependent Kinases 4 and 6 In-hibitors With Survival in Patients With Hormone Receptor-Positive Metastatic Breast Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2020312. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, K.B.; Thomsen, I.M.N.; Berg, T.; Kodahl, A.R.; Jensen, A.B. Dose modifications of ribociclib and endocrine therapy for treatment of ER+ HER2− metastatic breast cancer. Breast Cancer Res. Treat. 2021, 188, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Denduluri, N.; Lyman, G.H.; Wang, Y.; Morrow, P.K.; Barron, R.; Patt, D.; Bhowmik, D.; Li, X.; Bhor, M.; Fox, P.; et al. Chem-otherapy Dose Intensity and Overall Survival Among Patients with Advanced Breast or Ovarian Cancer. Clin. Breast Cancer 2018, 18, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef]
- Goel, S.; Wang, Q.; Watt, A.C.; Tolaney, S.M.; Dillon, D.A.; Li, W.; Ramm, S.; Palmer, A.C.; Yuzugullu, H.; Varadan, V.; et al. Overcoming Therapeutic Resistance in HER2-Positive Breast Cancers with CDK4/6 Inhibitors. Cancer Cell 2016, 29, 255–269. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Wardley, A.M.; Zambelli, S.; Hilton, J.F.; Troso-Sandoval, T.A.; Ricci, F.; Im, S.-A.; Kim, S.-B.; Johnston, S.R.; Chan, A.; et al. Abemaciclib plus trastuzumab with or without fulvestrant versus trastuzumab plus standard-of-care chemo-therapy in women with hormone receptor-positive, HER2-positive advanced breast cancer (monarcHER): A randomised, open-label, phase 2 trial. Lancet Oncol. 2020, 21, 763–775. [Google Scholar] [CrossRef]
- Cretella, D.; Fumarola, C.; Bonelli, M.; Alfieri, R.; La Monica, S.; Digiacomo, G.; Cavazzoni, A.; Galetti, M.; Generali, D.; Petronini, P.G. Pre-treatment with the CDK4/6 inhibitor palbociclib improves the efficacy of paclitaxel in TNBC cells. Sci. Rep. 2019, 9, 13014. [Google Scholar]
- Di Maio, M.; Perrone, F.; Conte, P. Real-World Evidence in Oncology: Opportunities and Limitations. Oncol. 2019, 25, e746–e752. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef] [PubMed]
- Rossi, V.; Berchialla, P.; Giannarelli, D.; Nistico, C.; Ferretti, G.; Gasparro, S.; Russillo, M.; Gatania, G.; Vigna, L.; Mancusi, R.L.; et al. Should All Patients With HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor As First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MON-ARCH 3, FALCON, SWOG and FACT Trials. Cancers 2019, 11, 1661. [Google Scholar] [CrossRef] [PubMed]
- Van Ommen-Nijhof, A.; Konings, I.R.; Van Zeijl, C.J.J.; Uyl-de Groot, C.A.; Van der Noort, V.; Jager, A.; Sonke, G.S. Selecting the optimal position of CDK4/6 inhibitors in hormone receptor-positive advanced breast cancer—The SONIA study: Study protocol for a randomized controlled trial. BMC Cancer 2018, 18, 1146. [Google Scholar] [CrossRef]
- Giuliano, M.; Schettini, F.; Rognoni, C.; Milani, M.; Jerusalem, G.; Bachelot, T.; De Laurentiis, M.; Thomas, G.; De Placido, P.; Arpino, G.; et al. Endocrine treatment versus chemotherapy in postmenopausal women with hormone receptor-positive, HER2-negative, metastatic breast cancer: A systematic review and network meta-analysis. Lancet Oncol. 2019, 20, 1360–1369. [Google Scholar] [CrossRef]
- Gao, J.J.; Cheng, J.; Bloomquist, E.; Sanchez, J.; Wedam, S.B.; Singh, H.; Amiri-Kordestani, L.; Ibrahim, A.; Sridhara, R.; Goldberg, K.B.; et al. CDK4/6 inhibitor treatment for patients with hormone receptor-positive, HER2-negative, advanced or metastatic breast cancer: A US Food and Drug Administration pooled analysis. Lancet Oncol. 2020, 21, 250–260. [Google Scholar] [CrossRef]
- Piezzo, M.; Chiodini, P.; Riemma, M.; Cocco, S.; Caputo, R.; Cianniello, D.; Di Gioia, G.; Di Lauro, V.; Di Rella, F.; Fusco, G.; et al. Progression-Free Survival and Overall Survival of CDK 4/6 Inhibitors Plus Endocrine Therapy in Metastatic Breast Cancer: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 6400. [Google Scholar] [CrossRef]
- Schettini, F.; Giuliano, M.; Giudici, F.; Conte, B.; De Placido, P.; Venturini, S.; Rognoni, C.; Di Leo, A.; Locci, M.; Jerusalem, G.; et al. Endocrine-Based Treatments in Clinically-Relevant Subgroups of Hormone Receptor-Positive/HER2-Negative Meta-static Breast Cancer: Systematic Review and Meta-Analysis. Cancers 2021, 13, 1458. [Google Scholar]
- Finn, R.S.; Cristofanilli, M.; Ettl, J.; Gelmon, K.A.; Colleoni, M.; Giorgetti, C.; Gauthier, E.; Liu, Y.; Lu, D.R.; Zhang, Z.; et al. Treatment effect of palbociclib plus endocrine therapy by prognostic and intrinsic subtype and biomarker analysis in pa-tients with bone-only disease: A joint analysis of PALOMA-2 and PALOMA-3 clinical trials. Breast Cancer Res. Treat. 2020, 184, 23–35. [Google Scholar] [CrossRef]
- Rugo, H.; Cristofanilli, M.; Loibl, S.; Harbeck, N.; Demichele, A.; Iwata, H.; Park, Y.H.; Brufsky, A.; Theall, K.P.; Huang, X.; et al. Predictors of efficacy in patients with hormone receptor–positive/ human epidermal growth factor receptor 2–negative advanced breast cancer (HR+/HER2− ABC): Subgroup analyses of PALOMA-3. Eur. J. Cancer 2020, 138, S7–S8. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Sahebjam, S.; Le Rhun, E.; Bachelot, T.; Kabos, P.; Awada, A.; Yardley, D.; Chan, A.; Conte, P.; Diéras, V.; et al. A Phase II Study of Abemaciclib in Patients with Brain Metastases Secondary to Hormone Receptor–Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 5310–5319. [Google Scholar] [CrossRef] [PubMed]
- Roncato, R.; Angelini, J.; Pani, A.; Cecchin, E.; Sartore-Bianchi, A.; Siena, S.; De Mattia, E.; Scaglione, F.; Toffoli, G. CDK4/6 Inhibitors in Breast Cancer Treatment: Potential Interactions with Drug, Gene, and Pathophysiological Conditions. Int. J. Mol. Sci. 2020, 21, 6350. [Google Scholar] [CrossRef] [PubMed]
- Hlevnjak, M.; Schulze, M.; Elgaafary, S.; Fremd, C.; Michel, L.; Beck, K.; Pfütze, K.; Richter, D.; Wolf, S.; Horak, P.; et al. CATCH: A Prospective Precision Oncology Trial in Metastatic Breast Cancer. JCO Precis. Oncol. 2021, 5, 676–686. [Google Scholar] [CrossRef]
- Richardson, D.; Zhan, L.; Mahtani, R.; McRoy, L.; Mitra, D.; Reynolds, M.; Odom, D.; Hollis, K.; Kaye, J.A.; Jones, C.; et al. A prospective observational study of patient-reported functioning and quality of life in advanced and metastatic breast cancer utilizing a novel mobile application. Breast Cancer Res. Treat. 2021, 187, 113–124. [Google Scholar] [CrossRef]
- Guo, L.; Hu, Y.; Chen, X.; Li, Q.; Wei, B.; Ma, X. Safety and efficacy profile of cyclin-dependent kinases 4/6 inhibitor palbo-ciclib in cancer therapy: A meta-analysis of clinical trials. Cancer Med. 2019, 8, 1389–1400. [Google Scholar] [CrossRef]
- Desnoyers, A.; Nadler, M.B.; Kumar, V.; Saleh, R.; Amir, E. Comparison of treatment-related adverse events of different Cyclin-dependent kinase 4/6 inhibitors in metastatic breast cancer: A network meta-analysis. Cancer Treat. Rev. 2020, 90, 102086. [Google Scholar] [CrossRef]
- Spring, L.M.; Wander, S.A.; Andre, F.; Moy, B.; Turner, N.C.; Bardia, A. Cyclin-dependent kinase 4 and 6 inhibitors for hor-mone receptor-positive breast cancer: Past, present, and future. Lancet 2020, 395, 817–827. [Google Scholar] [CrossRef]
- Fasching, P.A.; Beck, J.T.; Chan, A.; De Laurentiis, M.; Esteva, F.J.; Jerusalem, G.; Neven, P.; Pivot, X.; Bianchi, G.V.; Martin, M.; et al. Ribociclib plus fulvestrant for advanced breast cancer: Health-related quality-of-life analyses from the MONALEESA-3 study. Breast 2020, 54, 148–154. [Google Scholar] [CrossRef]
- Rugo, H.; Diéras, V.; Gelmon, K.; Finn, R.; Slamon, D.; Martin, M.; Neven, P.; Shparyk, Y.; Mori, A.; Lu, D.; et al. Impact of palbociclib plus letrozole on patient-reported health-related quality of life: Results from the PALOMA-2 trial. Ann. Oncol. 2018, 29, 888–894. [Google Scholar] [CrossRef]
- Elfgen, C.; Montagna, G.; Schmid, S.M.; Bierbauer, W.; Güth, U. Noncompliance with palliative systemic therapy in pa-tients with distant metastatic breast cancer: A blind spot for oncologists? Breast Cancer Res. Treat. 2019, 176, 469–476. [Google Scholar] [CrossRef]
- Husinka, L.; Koerner, P.H.; Miller, R.T.; Trombatt, W. Review of cyclin-dependent kinase 4/6 inhibitors in the treatment of advanced or metastatic breast cancer. J. Drug Assess. 2021, 10, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, I.; Bonechi, M.; McCartney, A.; Guarducci, C.; Benelli, M.; Biganzoli, L.; Di Leo, A.; Malorni, L. CDK4/6 inhibitors: A focus on biomarkers of response and post-treatment therapeutic strategies in hormone receptor-positive HER2-negative breast cancer. Cancer Treat. Rev. 2011, 93, 102136. [Google Scholar] [CrossRef] [PubMed]
- Wander, S.A.; Zangardi, M.; Niermierko, A.; Kambadakone, A.; Kim, L.S.L.; Xi, J.; Pandey, A.K.; Spring, L.; Stein, C.; Juric, D.; et al. A multicenter analysis of abemaciclib after progression on palbociclib in patients with hormone receptor-positive (HR+)/HER2- metastatic breast cancer (MBC). J. Clin. Oncol. 2019, 37, 1057. [Google Scholar] [CrossRef]
- Costa, C.; Wang, Y.; Ly, A.; Hosono, Y.; Murchie, E.; Walmsley, C.S.; Huynh, T.; Healy, C.; Peterson, R.; Yanase, S.; et al. PTEN Loss Mediates Clinical Cross-Resistance to CDK4/6 and PI3Kα Inhibitors in Breast Cancer. Cancer Discov. 2020, 10, 72–85. [Google Scholar] [CrossRef]
- Fribbens, C.; O’Leary, B.; Kilburn, L.; Hrebien, S.; Garcia-Murillas, I.; Beaney, M.; Cristofanilli, M.; Andre, F.; Loi, S.; Loibl, S.; et al. Plasma ESR1 Mutations and the Treatment of Estrogen Receptor–Positive Advanced Breast Cancer. J. Clin. Oncol. 2016, 34, 2961–2968. [Google Scholar] [CrossRef]
- Wander, S.A.; Cohen, O.; Johnson, G.N.; Kim, D.; Luo, F.; Mao, P.; Nayar, U.; Helvie, K.; Marini, L.; Freeman, S.; et al. Whole exome sequencing (WES) in hormone-receptor positive (HR+) metastatic breast cancer (MBC) to identify mediators of re-sistance to cyclin-dependent kinase 4/6 inhibitors (CDK4/6i). J. Clin. Oncol. 2018, 36, 12016. [Google Scholar] [CrossRef]
- Brett, J.O.; Spring, L.M.; Bardia, A.; Wander, S.A. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021, 23, 1–15. [Google Scholar] [CrossRef]
- Schiavon, G.; Hrebien, S.; Garcia-Murillas, I.; Cutts, R.J.; Pearson, A.; Tarazona, N.; Fenwick, K.; Kozarewa, I.; Lopez-Knowles, E.; Ribas, R.; et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci. Transl. Med. 2015, 7, 313. [Google Scholar] [CrossRef]
- Raimondi, L.; Raimondi, F.M.; Pietranera, M.; Di Rocco, A.; Di Benedetto, L.; Miele, E.; Lazzeroni, R.; Cimino, G.; Spinelli, G.P. Assessment of Resistance Mechanisms and Clinical Implications in Patients with KRAS Mutated-Metastatic Breast Cancer and Resistance to CDK4/6 Inhibitors. Cancers 2021, 13, 1928. [Google Scholar] [CrossRef]
- Schoninger, S.F.; Blain, S.W. The ongoing search for biomarkers of CDK4/6 inhibitor responsiveness in Breast Cancer. Mol. Cancer 2020, 19, 3–12. [Google Scholar] [CrossRef]
- Vora, S.R.; Juric, D.; Kim, N.; Mino-Kenudson, M.; Huynh, T.; Costa, C.; Lockerman, E.; Pollack, S.F.; Liu, M.; Li, X.; et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014, 26, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metab-olism. Nat. Rev. Genet. 2020, 7, 606–619. [Google Scholar] [CrossRef]
- Martínez-Sáez, O.; Chic, N.; Pascual, T.; Adamo, B.; Vidal, M.; Gonzalez-Farre, B.; Sanfeliu, E.; Schettini, F.; Conte, B.; Bra-so-Maristany, F.; et al. Frequency and spectrum of PIK3CA somatic mutations in breast cancer. Breast Cancer Res. 2020, 22, 45. [Google Scholar] [CrossRef]
- Saal, L.H.; Holm, K.; Maurer, M.; Memeo, L.; Su, T.; Wang, X.; Yu, J.S.; Malström, P.-O.; Mansukhani, M.; Enoksson, J.; et al. PIK3CA muta-tions correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005, 65, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-R.; Jiang, Y.-Z.; Zuo, W.-J.; Yu, K.-D.; Shao, Z.-M. PIK3CA mutations define favorable prognostic biomarkers in operable breast cancer: A systematic review and meta-analysis. Oncotargets 2014, 7, 543–552. [Google Scholar]
- Mosele, F.; Stefanovska, B.; Lusque, A.; Dien, A.T.; Garberis, I.; Droin, N.; Tourneau, C.L.; Sablin, M.-P.; Lacroix, L.; Enrico, D.; et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann. Oncol. 2020, 31, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Tang, L.; Yang, F.; Jin, J.; Guan, X. PIK3CA mutations contribute to fulvestrant resistance in ER-positive breast cancer. Am. J. Transl. Res. 2019, 11, 6055–6065. [Google Scholar] [PubMed]
- Baselga, J.; Im, S.-A.; Iwata, H.; Cortes, J.; De Laurentiis, M.; Jiang, Z.; Arteaga, C.L.; Jonat, W.; Clemons, M.; Ito, Y.; et al. Bu-parlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 904–916. [Google Scholar] [CrossRef]
- Dent, S.; Cortes, J.; Im, Y.-H.; Dieras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovsky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Jang, H.; Nussinov, R. PI3K inhibitors: Review and new strategies. Chem. Sci. 2020, 11, 5855–5865. [Google Scholar] [CrossRef] [PubMed]
- Blow, T.; Hyde, P.N.; Falcone, J.N.; Neinstein, A.; Vasan, N.; Chitkara, R.; Hurd, M.A.; Sardesai, S.; Lustberg, M.B.; Flory, J.H.; et al. Treating Alpelisib-Induced Hyperglycemia with Very Low Carbohydrate Diets and Sodium-Glucose Co-Transporter 2 Inhibitors: A Case Series. Integr. Cancer 2021, 20, 15347354211032284. [Google Scholar] [CrossRef] [PubMed]
- Ciruelos, E.M.; Rugo, H.S.; Mayer, I.A.; Levy, C.; Forget, F.; Mingorance, J.I.D.; Safra, T.; Masuda, N.; Park, Y.H.; Juric, D.; et al. Patient-Reported Outcomes in Patients With PIK3CA-Mutated Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer From SOLAR-1. J. Clin. Oncol. 2021, 39, 2005–2015. [Google Scholar] [CrossRef]
- Hester, A.; Henze, F.; Travi, C.; Harbeck, N.; Wuerstlein, R. First Experiences with Alpelisib in Clinical Routine: Case Re-ports from a German Breast Center. Breast Care. 2021, 16, 129–134. [Google Scholar] [CrossRef]
- Jhaveri, K.; Drago, J.Z.; Shah, P.D.; Wang, R.; Pareja, F.; Ratzon, F.; Iasonos, A.; Patil, S.; Rosen, N.; Fornier, M.N.; et al. A Phase I Study of Alpelisib in Combination with Trastuzumab and LJM716 in Patients with PIK3CA-Mutated HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2021, 27, 3867–3875. [Google Scholar] [CrossRef]
- Martín, M.; Chan, A.; Dirix, L.; O’Shaughnessy, J.; Hegg, R.; Manikhas, A.; Shtivelband, M.; Krivorothko, P.; Lopez, N.B.; Campone, M.; et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2- advanced breast cancer (BELLE-4). Ann. Oncol. 2017, 28, 313–320. [Google Scholar] [CrossRef]
- Priedigkeit, N.; Ding, K.; Horne, W.; Kolls, J.K.; Du, T.; Lucas, P.C.; Blohmer, J.-U.; Denkert, C.; Machleidt, A.; Inhold-Heppner, B.; et al. Acquired mutations and transcriptional remodeling in long-term estrogen-deprived locoregional breast cancer recurrences. Breast Cancer Res. 2021, 23, 1. [Google Scholar] [CrossRef]
- Del Re, M.; Crucitta, S.; Lorenzini, G.; De Angelis, C.; Diodati, L.; Cavallero, D.; Bargagna, I.; Cinacchi, P.; Fratini, B.; Salva-dori, B.; et al. PI3K mutations detected in liquid biopsy are associated to reduced sensitivity to CDK4/6 inhibitors in meta-static breast cancer patients. Pharm. Res. 2021, 163, 105241. [Google Scholar] [CrossRef]
- Baselga, J.; Dent, S.F.; Cortes, J.; Im, Y.-H.; Dieras, V.; Harbeck, N.; Krop, I.E.; Verma, S.; Wilson, T.R.; Jin, H.; et al. Phase III study of taselisib (GDC-0032) + fulvestrant (FULV) v FULV in patients with estrogen receptor (ER)-positive, PIK3CA-mutant (MUT), locally advanced or metastatic breast cancer (MBC): Primary analysis from SANDPIPER. J. Clin. Oncol. 2018, 36, LBA1006. [Google Scholar] [CrossRef]
- Chang, D.-Y.; Ma, W.-L.; Lu, Y.-S. Role of Alpelisib in the Treatment of PIK3CA-Mutated Breast Cancer: Patient Selection and Clinical Perspectives. Clin. Risk Manag. 2021, 17, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Jerusalem, G.; Rorive, A.; Collignon, J. Use of mTOR inhibitors in the treatment of breast cancer: An evaluation of factors that influence patient outcomes. Breast Cancer Dove Med. Press. 2014, 6, 43–57. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gnant, M.; Baselga, J.; Rugo, H.S.; Noguchi, S.; Burris, H.A.; Piccart, M.; Hortobagyi, G.N.; Eakle, J.; Mukai, H.; Iwata, H.; et al. Effect of everolimus on bone marker levels and progressive disease in bone in BOLERO-2. J. Natl. Cancer Inst. 2013, 105, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.C.; Garcia, C.A.; Wu, S. Discontinuation of everolimus due to unrelated adverse events in cancer patients. J. Clin. Oncol. 2016, 34, e14020. [Google Scholar] [CrossRef]
- Huang, H.-W.; Huang, L.-S.; Xu, Q.-N.; Wang, H.-B.; Li, X.-Y.; Lin, J.-Z. CDK4/6 inhibition versus mTOR blockade as second-line strategy in postmenopausal patients with hormone receptor-positive advanced breast cancer. Medicine (Baltimore) 2019, 98, e13909. [Google Scholar] [CrossRef] [PubMed]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative ad-vanced breast cancer: Overall survival results from BOLERO-2. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, G.N.; Chen, D.; Piccart, M.; Rugo, H.S.; Burris, H.A.; Pritchard, K.I.; Campone, M.; Noguchi, S.; Perez, A.T.; Deleu, I.; et al. Correlative Analysis of Genetic Alterations and Everolimus Benefit in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From BOLERO-2. J. Clin. Oncol. 2016, 34, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Rozenblit, M.; Mun, S.; Soulos, P.; Adelson, K.; Pusztai, L.; Mougalian, S. Patterns of treatment with everolimus exemestane in hormone receptor-positive HER2-negative metastatic breast cancer in the era of targeted therapy. Breast Cancer Res. 2021, 23, 14. [Google Scholar] [CrossRef]
- Jones, R.H.; Casbard, A.; Carucci, M.; Cox, C.; Butler, R.; Alchami, F.; Madden, T.-A.; Bale, C.; Bezecny, P.; Joffe, J.; et al. Ful-vestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): A multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2020, 21, 345–357. [Google Scholar] [CrossRef]
- Rugo, H.S.; Rumble, R.B.; Macrae, E.; Barton, D.L.; Connolly, H.K.; Dickler, M.N.; Fallowfield, L.; Fowble, B.; Ingle, J.N.; Ja-hanzeb, M.; et al. Endocrine Therapy for Hormone Receptor–Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016, 34, 3069–3103. [Google Scholar] [CrossRef]
- Telli, M.L.; Gradishar, W.J.; Ward, J.H. NCCN Guidelines Updates: Breast Cancer. J. Natl. Compr. Canc. Netw. 2019, 17, 552–555. [Google Scholar] [PubMed]
- Mayer, E.L.; Dueck, A.C.; Martin, M.; Rubovsky, G.; Burstein, H.J.; Bellet-Eqquerra, M.; Miller, K.D.; Zdenkowski, N.; Winer, E.P.; Pfeiler, G.; et al. Palbociclib with adjuvant endocrine therapy in early breast cancer (PALLAS): Interim analysis of a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2021, 22, 212–222. [Google Scholar] [CrossRef]
- Loibl, S.; Marme, F.; Martin, M.; Untch, M.; Bonnefoi, H.; Kim, S.-B.; Bear, H.; McCarthy, N.; Melé Olivé, M.; Gelmon, K.; et al. Palbociclib for Residual High-Risk Inva-sive HR-Positive and HER2-Negative Early Breast Cancer-The Penelope-B Trial. J. Clin. Oncol. 2021, 39, 1518–1530. [Google Scholar] [CrossRef]
- Johnston, S.R.D.; Harbeck, N.; Hegg, R.; Toi, M.; Martin, M.; Shao, Z.M.; Zhang, Q.Y.; Rodriguez, J.L.M.; Campone, M.; Ham-ilton, E.; et al. Abemaciclib Combined With Endocrine Therapy for the Adjuvant Treatment of HR+, HER2-, Node-Positive, High-Risk, Early Breast Cancer (monarchE). J. Clin. Oncol. 2020, 38, 3987–3998. [Google Scholar] [CrossRef] [PubMed]
- Saura, C.; Hlauschek, D.; Oliveira, M.; Zardavas, D.; Jallitsch-Halper, A.; de la Pena, L.; Nuciforo, P.; Ballestrero, A.; Dubsky, P.; Lombard, J.M.; et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen re-ceptor-positive, HER2-negative, early-stage breast cancer (LORELEI): A multicentre, randomised, double-blind, place-bo-controlled, phase 2 trial. Lancet Oncol. 2019, 20, 1226–1238. [Google Scholar] [CrossRef]
| Clinical Trial | Patient Selection | n | Therapeutic Regimen | PFS (Months) | OS |
|---|---|---|---|---|---|
| PALOMA-1 | Postmenopausal women without systemic treatment for advanced disease | 165 | Palbociclib + letrozole versus letrozole alone | 20.2 versus 10.2 (HR 0.488, 95% CI 0.319–0.748; one-sided p = 0.0004) | Not significant |
| PALOMA-2 | Postmenopausal women without systemic treatment for advanced disease | 666 | Palbociclib + letrozole versus placebo + letrozole | 24.8 versus 14.5 (HR 0.58; 95% CI 0.46–0.72; p < 0.001) | Immature data |
| PALOMA-3 | Postmenopausal women with progress under endocrine therapy | 521 | Palbociclib + fulvestrant versus placebo + fulvestrant | 9.5 versus 4.6 (HR 0.46; 95% CI 0.36–0.59; p < 0.0001) | Not significant |
| MONARCH-2 | Pre- and postmenopausal women with progress under endocrine therapy | 669 | Abemaciclib + fulvestrant versus placebo + fulvestrant | 16.4 versus 9.3 (HR 0.553; 95% CI 0.449 to 0.681; p <0.001) | Not significant |
| MONARCH-3 | Postmenopausal women without systemic treatment for advanced disease | 493 | Abemaciclib + endocrine therapy versus placebo + endocrine therapy | 28.2 versus 14.8 (HR 95%; CI 0.418–0.698; p < 0.0001) | Immature data |
| MONALEESA-2 | Postmenopausal women without systemic treatment for advanced disease | 668 | Ribociclib + fulvestrant versus placebo + fulvestrant | 25.3 versus 16.0 (HR 0.568; 95% CI 0.457–0.704; p < 0.0001) | Immature data |
| MONALEESA-3 | Postmenopausal women without systemic treatment for advanced disease | 726 | Ribociclib + letrozole versus placebo + letrozole | 20.5 versus 12.8 (HR 0.59; 95% CI 0.48–0.73; p> 0.001) | 57.8% vs 45.9% at 42 months (HR 0.72; 95% CI 0.57–0.92; p = 0.00455) |
| MONALEESA-7 | Peri-/premenopausal | 672 | Ribociclib + endocrine therapy versus placebo + endocrine therapy | 23.8 versus 13.0 (HR 0.55; 95% CI 0.44–0.69; p > 0.001) | 70.2% vs 46.0% at 42 months (HR 0.71; 95% CI 0.54–0.95; p = 0.00973) |
| Clinical Trial | Patient Selection | Estimated Enrollment (n) | Therapeutic Regimen | Study Start Year | Estimated Completion Year | Clinical Trial Information |
|---|---|---|---|---|---|---|
| PRESERVE 2 | Pre- and postmenopausal women and men with metastatic TNBC | 250 | Trilaciclib + gemcitabine + carboplatin versus placebo + gemcitabine + carboplatin | 2021 | 2024 | NCT04799249 |
| Ribociclib with trastuzumab plus letrozole in postmenopausal HR+, HER2+ ABC Patients | Postmenopausal women with advanced HR+, HER2+ BC | 95 | Letrozole + trastuzumab + ribociclib | 2019 | 2021 | NCT03913234 |
| PATRICIA II | Pre- and postmenopausal women with metastatic HR+ or HR-, HER2+ BC | 232 | Trastuzumab + Palbociclib + letrozole (only in HR+ patients) | 2015 | 2020 | NCT02448420 |
| TOUCH | Pre- and postmenopausal women with early HR+, HER2+ BC | 144 | Paclitaxel + trastuzumab + pertuzumab versus Palbociclib + letrozole + trastuzumab + pertuzumab | 2019 | 2021 | NCT03644186 |
| DETECT V | Pre- and postmenopausal women with metastatic HR+, HER2+ BC | 270 | Ribociclib + trastuzumab + pertuzumab + endocrine therapy versus trastuzumab + pertuzumab + chemotherapy tailored by ribociclib | 2015 | 2021 | NCT02344472 |
| SONIA | Pre- and postmenopausal women with HR+, HER2− advanced/metastatic breast cancer. | 1050 | Aromatase inhibitor + a CDK4/6 inhibitor 1st line versus Fulvestrant + CDK4/6 inhibitor 2nd line | 2017 | 2022 | NCT03425838 |
| AMICA | Pre- and postmenopausal women with HR+, HER2− advanced/metastatic breast cancer and disease control after 1st line chemotherapy | 150 | Ribociclib + endocrine therapy versus endocrine therapy alone | 2018 | 2022 | NCT03555877 |
| MAINTAIN | Pre- and postmenopausal women and men with HR+, HER2− advanced/metastatic breast cancer after progression on anti-estrogen therapy plus CDK4/6 inhibitor | 132 | Ribociclib + fulvestrant versus placebo + fulvestrant | 2016 | 2021 | NCT02632045 |
| ABEMACARE | Pre- and postmenopausal women with HR+, HER2− advanced/metastatic breast cancer and symptomatic visceral metastases or high tumor burden | 120 | Abemaciclib + endocrine therapy | 2020 | 2024 | NCT04681768 |
| PALATINE | Pre- and postmenopausal women with HR+, HER2− advanced/metastatic breast cancer. | 200 | Palbociclib + endocrine therapy upfront | 2019 | 2023 | NCT03870919 |
| KENDO | Pre- and postmenopausal women and men with HR+, HER2− advanced/metastatic breast cancer. | 150 | A CDK4/6 inhibitor + endocrine therapy versus chemotherapy + endocrine therapy | 2017 | 2022 | NCT03227328 |
| FATIMA | Premenopausal women with HR+, HER2− advanced/metastatic breast cancer. | 160 | Palbociclib + exemestane + goserelin versus exemestane + goserelin alone | 2019 | 2021 | NCT02917005 |
| PACE | Pre- and postmenopausal women and men with HR+, HER2− advanced/metastatic breast cancer after CDK and endocrine therapy | 220 | Palbociclib + fulvestrant + avelumab versus Palbociclib + fulvestrant versus fulvestrant alone | 2017 | 2021 | NCT03147287 |
| Clinical Trial | Patient Selection | Estimated Enrollment (n) | Therapeutic Regimen | Study Start Year | Estimated Completion Year | Clinical Trial Information |
|---|---|---|---|---|---|---|
| Alpelisib with endocrine therapy following progression on endocrine therapy | Pre- and postmenopausal women and men with PIK3CA mutant HR+, HER2− MBC | 44 | Alpelisib + aromatase inhibitor or fulvestrant | 2021 | 2024 | NCT04762979 |
| Alpelisib with trastuzumab and pertuzumab as maintenance therapy | Pre- and postmenopausal women and men with HER2+, PIK3CA mutant ABC | 588 | Alpelisib + trastuzumab + pertuzumab versus placebo + trastuzumab + pertuzumab | 2020 | 2025 | NCT04208178 |
| Inavolisib in patients with PIK3CA-mutant, HR+, HER2− locally advanced or metastatic breast cancer | Pre- and postmenopausal women and men with HR+, HER2− PIK3CA mutant A/MBC | 400 | Inavolisib + palbociclib + fulvestrant versus placebo + palbociclib + fulvestrant | 2020 | 2025 | NCT04191499 |
| Alpelisib + nab-paclitaxel in subjects with advanced TNBC who carry either a PIK3CA mutation or have PTEN loss (EPIK-B3) | Pre- and postmenopausal women and men with advanced TNBC with PIK3CA mutation or PTEN loss | 566 | Alpelisib + nab-paclitaxel versus placebo + nab-paclitaxel | 2020 | 2023 | NCT04251533 |
| PERSEVERE | Pre- and postmenopausal women and men with post-neoadjuvant residual TNBC | 197 | Assignment due to the genomic target of ctDNA: talazoparib + capecitabine; atezolizumab + capecitabine; inavolisib + capecitabine; talazoparib + atezolizumab + capecitabine | 2021 | 2024 | NCT04849364 |
| Clinical Trial | Patient Selection | Estimated Enrollment (n) | Therapeutic Regimen | Study Start Year | Estimated Completion Year | Clinical Trial Information |
|---|---|---|---|---|---|---|
| NATALEE | Pre- and postmenopausal women and men | 5000 | Ribociclib + endocrine therapy versus endocrine therapy alone | 2018 | 2026 | NCT03701334 |
| APPALACHES | Women or men 70 years and older for whom chemotherapy is indicated | 366 | Palbociclib + endocrine therapy versus chemotherapy + endocrine therapy | 2019 | 2025 | NCT03609047 |
| POLAR | Pre- and postmenopausal women or men with local/regional recurrence of BC | 400 | Palbociclib + endocrine therapy versus endocrine therapy alone | 2019 | 2024 | NCT03820830 |
| POETIC-A | Postmenopausal woman with high 5-year risk of relapse | 2500 | Abemaciclib + endocrine therapy versus endocrine therapy alone | 2020 | 2026 | NCT04584853 |
| LEADER | Pre- and postmenopausal women | 120 | Ribociclib + endocrine therapy versus endocrine therapy alone | 2017 | 2022 | NCT03285412 |
| SAFIA | Pre- and postmenopausal women in the neoadjuvant setting | 400 | Palbociclib + fulvestrant versus placebo + fulvestrant alone | 2018 | 2023 | NCT03447132 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elfgen, C.; Bjelic-Radisic, V. Targeted Therapy in HR+ HER2− Metastatic Breast Cancer: Current Clinical Trials and Their Implications for CDK4/6 Inhibitor Therapy and beyond Treatment Options. Cancers 2021, 13, 5994. https://doi.org/10.3390/cancers13235994
Elfgen C, Bjelic-Radisic V. Targeted Therapy in HR+ HER2− Metastatic Breast Cancer: Current Clinical Trials and Their Implications for CDK4/6 Inhibitor Therapy and beyond Treatment Options. Cancers. 2021; 13(23):5994. https://doi.org/10.3390/cancers13235994
Chicago/Turabian StyleElfgen, Constanze, and Vesna Bjelic-Radisic. 2021. "Targeted Therapy in HR+ HER2− Metastatic Breast Cancer: Current Clinical Trials and Their Implications for CDK4/6 Inhibitor Therapy and beyond Treatment Options" Cancers 13, no. 23: 5994. https://doi.org/10.3390/cancers13235994
APA StyleElfgen, C., & Bjelic-Radisic, V. (2021). Targeted Therapy in HR+ HER2− Metastatic Breast Cancer: Current Clinical Trials and Their Implications for CDK4/6 Inhibitor Therapy and beyond Treatment Options. Cancers, 13(23), 5994. https://doi.org/10.3390/cancers13235994





